Abstract
Pentavalent antimonial (SbV) is the first treatment for cutaneous leishmaniasis (CL). Other drugs present similar side effects and higher cost. Oral miltefosine is effective to treat kala-azar. The aim of the present study was to compare the efficacy of glucamine (SbV) plus topical miltefosine with glucamine in the treatment of CL. Eighty isogenic C57BL/6 mice were inoculated with Leishmania (Leishmania) amazonensis and divided into two groups: one group was treated with SbV associated with miltefosine, and the other group received SbV plus saline solution. Groups were evaluated according to the diameter of the inoculated foot pad, the culture, and the parasite count using the limiting dilution assay. There was not statistical difference. The efficacy of glucamine in CL treatment did not increase when associated with topical miltefosine.
Cutaneous Leishmaniasis; Miltefosine; N-methyl glucamine
ORIGINAL PAPER
Inefficacy of the association N-methyl glucamine and topical miltefosine in the treatment of experimental cutaneous leishmaniasis by Leishmania (Leishmania) amazonensis
Sampaio R. N. R.; Lucas I. C.; Takami H. L.
Laboratory of Dermatomycology, University of Brasília, Brazil
Correspondence to Correspondence to: Raimunda Nonata Ribeiro Sampaio Universidade de Brasília, Campus Universitário Darcy Ribeiro Asa Norte Brasília, 70910-900, DF, Brasil Phone: +55 61 307 2502. Fax: +55 61 367 3825 Email: rsampaio@unb.br
ABSTRACT
Pentavalent antimonial (SbV) is the first treatment for cutaneous leishmaniasis (CL). Other drugs present similar side effects and higher cost. Oral miltefosine is effective to treat kala-azar. The aim of the present study was to compare the efficacy of glucamine (SbV) plus topical miltefosine with glucamine in the treatment of CL. Eighty isogenic C57BL/6 mice were inoculated with Leishmania (Leishmania) amazonensis and divided into two groups: one group was treated with SbV associated with miltefosine, and the other group received SbV plus saline solution. Groups were evaluated according to the diameter of the inoculated foot pad, the culture, and the parasite count using the limiting dilution assay. There was not statistical difference. The efficacy of glucamine in CL treatment did not increase when associated with topical miltefosine.
Key words: Cutaneous Leishmaniasis, Miltefosine, N-methyl glucamine.
INTRODUCTION
Cutaneous leishmaniasis (CL) is caused by the parasitism of vertebrate hosts macrophages by Leishmania. There is an annual incidence of 1,5 million human cases worldwide. Besides skin lesions, Leishmania may damage the mucous membrane (mucocutaneous leishmaniasis), causing high morbidity and mortality (10, 13). Cases of (CL) have been already notified in all states of Brazil.
The first drug for the treatment of leishmaniasis is pentavalent antimonial, whose mechanism of action is unknown (2, 3). It requires parenteral administration and presents high toxicity. In addition, this drug is not always effective, showing 20%45% recurrence (4, 10, 13-17). Amphotericin B and pentamidine (2, 9) constitute the second choice for leishmaniasis treatment; however, they are expensive and toxic, require parenteral administration, and present therapeutic fails as well.
Miltefosine (hexadecylphosphocholine) is a phosphorylcholine ester of hexadecanol, an acyl-phospholipid used in the treatment of cutaneous metastasis of breast cancer (8, 23). It was effective in the treatment of experimental visceral leishmaniasis, acting on the main enzymes of the lipid metabolism of parasites (6, 7, 12). There was a direct action of such drug on macrophages and T cells, stimulating the secretion of IFN-g and TNF-a and inducing respiratory burst as well as nitric oxide release, activating the main in vitro macrophages leishmanicidal mechanisms. In vivo, miltefosine leishmanicidal potential seems not to depend on the response of T cells nor on the activation of macrophages, since intracellular death of parasites was noticed in mice showing T cells deficiency (11, 12).
Oral miltefosine has been efficient in the treatment of visceral leishmaniasis, including cases of resistance to antimonial and immunossupression (11). Nowadays, miltefosine is used for the treatment of kala-azar in India. In a non-controlled study, cure was obtained in 94% of CL cases when miltefosine was used at the dose of 100150mg/day for 4 weeks (21). Topical miltefosine was also efficient in decreasing the number of parasites and in healing lesions in BALB/C and C57BL/6 mice infected with Leishmania (18).
The present study compared a group of leishmaniasis mice treated with N-methyl glucamine associated with topical miltefosine to another group treated with N-methyl glucamine only, aimed at verifying if the association of such two drugs is more efficient in CL treatment.
MATERIALS AND METHODS
The present study was controlled and randomized. It used isogenic male C57BL/6 mice and parasites of Leishmania (L.) amazonensis promastigotes (MHOM/BR/PH8) identified by isoenzymes and monoclonal antibodies.
Eighty mice were inoculated in their right foot pad with 3X106 promastigotes at the stationary growth phase (1, 25) and were randomly divided into two groups: Glucamine Group, treated with SbV (N-methyl glucamine), subcutaneously, at the dose of 400mg / kg body weight / day (26) for 28 days; and Miltefosine Group, treated with SbV, at the same dose, associated with topical miltefosine, for 28 days. Miltefosine in a 6% solution was administered as one drop once a day during the first week and every 12 hours from the second week (23). According to the drug use instructions, the application site was gently rubbed and kept uncovered.
Before treatment, the mice foot pads were measured using a Mitutoyo Pachymeter and no differences were noticed between groups. After treatment, they were measured again and material was aspirated for culture. The amount of amastigote in the inoculated paws was calculated using the limiting dilution assay (24): two mice of each group had their foot pads washed in alcohol, dissected, triturated in aseptic chamber, and cultured in ELISA well-plates using ten successive dilutions. The number of positive wells was subjected to statistical analysis using the software ELIDA® (24). Students t test (SPSS 9.0) and chi-square test (Epi Info 6.04) were used (a =0.05, p<0.05).
RESULTS
After treatment, Glucamine Group presented smaller average diameter of inoculated paws than Miltefosine Group (30.7mm and 33.15mm, respectively) (p>0.05; Figure 1).
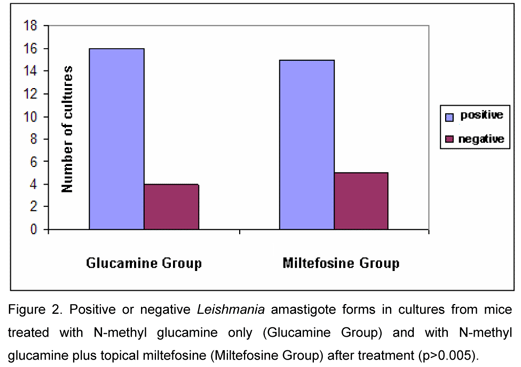
Analysis of cultures after treatment indicated 75% positivity in Glucamine Group and 80% in Miltefosine Group, without statistically significant difference (p>0.005; Figure 2).
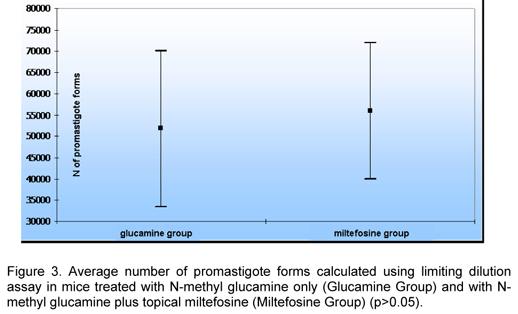
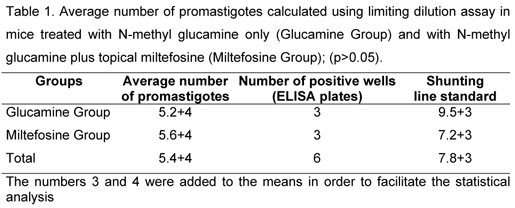
In a parasitological analysis using the limiting dilution assay, Miltefosine Group presented a higher average number of parasites, but there was not significant difference between groups (Table 1 and Figure 3).
DISCUSSION
Control of mucocutaneous leishmaniasis (MCL) and cutaneous leishmaniasis (CL) is very difficult since many factors are involved. The transmission types vary with the large number of different Leishmania species, vectors and natural reservoirs. A vaccine of confirmed efficacy has not been developed yet.
Currently, treatment is the unique effective measure against such disease. Antimonial, which is a drug of first line used since 1912 and administered by parenteral via, leads to frequent and severe side effects; also, the parasite can be resistant to it (2-4, 10, 13-17).
The Leishmania species tested in the present experiment, Leishmania (L.) amazonensis, is one of the three species that most frequently cause CL in Brazil. Generally, this parasite causes only cutaneous lesions, but 1% of the cases may manifest as diffuse cutaneous leishmaniasis (DCL), in which the patient has a specific deficiency of the immune response mediated by the host cells. A DCL patient presents negative Leishmania test, high number of parasites, and absence of response or cure with specific treatment. Thus, this species seems not to respond well to antimonials (2, 4, 17), making necessary the search for drugs that are efficient in the treatment of CL or DCL caused by such species.
Associations of drugs have helped to combat diseases caused by intracellular microorganisms that constitute worldwide public health problems like leprosy and tuberculosis; such associations represent a hope for the treatment of MCL. Therefore, the use of oral and topical drugs that cause fewer and less important side effects has to be investigated.
Due to its leishmanicidal and immunomodulating actions, miltefosine has been a promising drug for the treatment of leishmaniasis. It can be orally administered, was considered safe and efficient in the treatment of visceral leishmaniasis (5-7, 11, 12, 21, 22), and was efficient in experimental cutaneous leishmaniasis treatment when used topically (18).
In the present study, two evaluations (clinical and parasitological) were carried out to verify the efficacy of the therapeutic schemes used. Clinical evaluation is a method of low precision since the presence or absence of cutaneous lesions is not always associated with the presence and the quantity of parasites, e.g. big and disfiguring lesions of human mucous membrane may present small number of parasites whereas healed cutaneous lesions may present Leishmania forms (10, 13, 17).
As already mentioned, in a previous study using topical miltefosine for the treatment of experimental leishmaniasis caused by Leishmania major and Leishmania mexicana, good results were obtained (18). Therefore, it seems to be the first time this topical drug has been used for Leishmania (L.) amazonensis. The different results found in the current study could be justified by the different species studied. Some reports show good response to miltefosine in CL patients (20), which seems to vary according to the species: a study in humans infected with L (V.) panamensis and L (V.) braziliensis showed 91% cure of CL cases caused by L. (V.)panamensis; there was only 33% of response to treatment in cases caused by L. (V.) braziliensis (19).
There is not evident advantage in using the association of glucamine with topical miltefosine for the treatment of experimental leishmaniasis caused by Leishmania (L.) amazonensis. The data obtained in the present experiment suggested that further studies are needed to confirm such results as well as that other Leishmania species causing MCL have to be tested.
Received: August 28, 2006
Accepted: April 4, 2007
Abstract published online: April 5, 2007
Full paper published online: August 31, 2007
Conflicts of interest: There is no conflict.
- 1 ABOK KB., FREDRIKSSON A., BRUNK U. An experimental model system for leishmaniasis. Acta Pathol. Microbiol. Immunol. Scand., 1988, 96, 589-95.
- 2 BERMAN JD. Human leishmaniasis: clinical, diagnostic, and chemotherapeutic developments in the last 10 years. Clin. Infect. Dis, 1997, 24, 684-703.
- 3 DEPS PD., VIANA MC., FALQUETO A., DIETZ R. Avaliação comparativa da eficácia e toxicidade do antimoniato de N-methyl-glucamina e do Estibogluconato de sódio BP88® no tratamento da leishmaniose cutânea localizada. Rev. Soc. Bras. Med. Trop, 2000, 33, 535-43.
- 4 GROGL M., THOMASON TN., FRANKE ED. Drug resistance in Leishmaniasis: its implication in systemic chemotherapy of cutaneous and mucocutaneous disease. Am. J. Trop. Med. Hyg, 1992, 47, 117-26.
- 5 JHA TK., SUNDAR S., THAKUR CP., BACHMANN P., KARBWANG J., FISCHER C., VOSS A., BERMAN J. Miltefosine, an oral agent for the treatment of Indian visceral leishmaniasis. N. Engl. J. Med, 1999, 341, 1795-800.
- 6 KUBLENCORD A., MANIERA T., EIBL H., UNGER C. Hexadecylphosphocholine: oral treatment of visceral leishmaniasis in mice. Antimicrob. Agents Chemother, 1992, 36, 1630-4.
- 7 LE FICHOUX Y., ROUSSEAU D., FERRUA B., RUETTE S., LELIEVRE A., GROUSSO D., KUBAR J. Short- and long-term efficacy of hexadecylphosphocholine against established Leishmania infantum infection in BALB/c mice. Antimicrob. Agents Chemother, 1998, 42, 654-8.
- 8 LEONARD R., HARDY J. Randomized, double-blind, placebo-controlled, multicenter trial of 6% miltefosine solution, a topical chemotherapy in cutaneous metastases from breast cancer. J. Clin. Oncol, 2001, 19, 4150-9.
- 9 LUX H., HEISE N., KLENNER T., HART D., OPPERDOES FR. Ether-lipid (alkyl- phospholipid) metabolism and the mechanism of action of ether-lipid analogues in visceral leishmania. Mol. Biochem. Parasitol, 2000, 111, 1-14.
- 10 MARSDEN PD. Mucosal leishmaniasis due to Leishmania (Viannia) braziliensis L(V)b in Três Braços, Bahia, Brazil. Rev. Soc. Bras. Med. Trop, 1994, 27, 93-101.
- 11 MOHAN A., SETH S. Oral miltefosine in the treatment of kala-azar. Nat. Med. J. Ind, 2000, 13, 202-3.
- 12 MURRAY H., DELPH-ETIENE S. Visceral leishmanicidal activity of hexadecylphosphocoline (miltefosine) in mice deficient in T cells and activated macrophage microbicidas mechanisms. J. Infect Dis., 2000, 181, 795-9.
- 13 NETTO EM., MARSDEN PD., LLANOS-CUENTAS EA., COSTA JML., CUBA CC., BARRETO AC., BADARÓ R., JOHNSON WD., JONES TC. Long-term follow-up of patients with Leishmania (Viannia) brasiliensis infection and treated with Glucantime®. Trans. R. Soc. Trop. Med. Hyg, 1990, 84, 367-70.
- 14 ROMERO GAS., GUERRA MVF., PAES MG., MACEDO VO. Comparison of cutaneous leishmaniasis due to Leishmania (Viannia) braziliensis and L. (V) guyanensis in Brazil. Therapeutic response to meglumine antimoniate. Am. J. Trop. Med. Hyg, 2001, 65, 456-65.
- 15 SAMPAIO RNR., MARSDEN PD., FURTADO T., SAMPAIO JHD. Avaliação do tratamento da leishmaniose cutâneo-mucosa com três esquemas diferentes de antimoniais pentavalentes. An. Bras. Dermatol, 1989, 64, 201-5.
- 16 SAMPAIO RNR., NOGUEIRA LSC. Estudo hospitalar da leishmaniose tegumentar americana (LTA): epidemiologia e tratamento. An. Bras. Dermatol, 2001, 76, 51-62.
- 17 SAMPAIO RNR., SAMPAIO JHD., MARSDEN PD. Pentavalent antimonial treatment in mucosal leishmaniasis. Lancet, 1985, 1, 1097.
- 18 SCHMIDT-OTT R., KLENNER T., OVERATH P., AEBISCHER T. Topical treatment with Hexadecylphosphocoline (Miltex®) efficiently reduces parasite burden in experimental cutaneous leishmaniasis. Trans. R. Soc. Trop. Med. Hyg, 1999, 93, 85-90.
- 19 SOTO J., ARANA BA., TOLEDO J., RIZZO N., VEGA JC., DIAZ A., LUZ M., GUTIERREZ P., ARBOLETA M., BERMAN JD., JUNGE K., ENGEL J., SINDRMAN H. Miltefosine for New World cutaneous leishmaniasis. Clin. Infect Dis, 2004, 3, 1266-72.
- 20 SOTO J., TOLEDO P., GUTIERREZ RS., NICHOLLS J., PADILLA J., ENGEL C., FISCHER A., VOSS A., BERMAN J. Treatment of American cutaneous leishmaniasis with miltefosine, an oral agent. Clin. Infect Dis, 2001, 33, 57-61.
- 21 SUNDAR S., JHA TK., THAKUR CP., ENGEL J., SINDERMANN H., FISCHER C., JUNGE K., BRYCESON A., BERMAN J. Oral miltefosine for Indian visceral leishmaniasis. N. Engl. J. Med, 2002, 347, 1739-46.
- 22 SUNDAR S., ROSENKAIMER F., MAKHARIA M., GOYAL AK., MANDAL AK., VOSS A., HILGARD P., MURRAY HW. Trial of miltefosine for visceral leishmaniasis. Lancet, 1998, 352, 1821-3.
- 23 TERWOGT JM., MANDJES IA., SINDERMANN H., BEIJNEN JH., HUNINK WWW. Phase II trial of topically applied miltefosine solution in patients with skin-metastasized breast cancer. Br. J. Cancer, 1999, 79, 1158-61.
- 24 TITUS RG., MARCHAND M., BOON T., LOUIS JA. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol, 1985, 7, 545-55.
- 25 TRAVI B., OSORIO Y. Failure of albendazole as an alternative treatment of cutaneous leishmaniasis in the hamster model. Mem. Inst. Oswaldo Cruz, 1998, 93, 515.
- 26 VEIGA JPR., KHANAM R., ROSA TT., JUNQUEIRA JUNIOR LF., RAICK NA., FRIEDMAN H., MARSDEN PD. Pentavalent antimonial nephrotoxicity in the rat. Rev. Inst. Med. Trop. S. Paulo, 1990, 32, 304-9.
Publication Dates
-
Publication in this collection
14 Sept 2007 -
Date of issue
2007
History
-
Received
28 Aug 2006 -
Accepted
04 Apr 2007