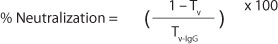Abstract
Cross-neutralization of Crotalus durissus terrificus venom coagulant activity was tested using bivalent horse antivenom against Bothrops alternatus and Bothrops diporus venoms. Our in vitro and in vivo experiments showed that bothropic antivenom neutralizes the thrombin-like activity of crotalic snake venom and this cross-reaction was demonstrated by immunoassays either with whole venom or a purified thrombin-like enzyme. These results suggest common antigenic properties and, consequently, similar molecular structure among venom thrombin-like enzymes. Besides, they provide information that could be further used in the development of new antivenom formulations.
thrombin-like enzyme; snake venom; antivenoms; immunological cross-reaction
ORIGINAL PAPERS
Cross-neutralization of the coagulant activity of Crotalus durissus terrificus venom from the northeast of Argentina by bivalent bothropic antivenom
Rodríguez JPI; Gay CCI; Fusco LSI; Gauna MCI; Acosta OCII; Leiva LCI
ILaboratory of Protein Research, School of Exact and Natural Sciences and Land Surveying, National University of the Northeast (UNNE), Corrientes, Argentina
IILaboratory of Pharmacology, School of Veterinary Sciences, National University of the Northeast (UNNE), Corrientes, Argentina
Correspondence to Correspondence to: Laura C. Leiva Laboratorio de Investigación en Proteínas (LabInPro) Facultad de Ciencias Exactas y Naturales y Agrimensura Universidad Nacional del Nordeste Av. Libertad 5400 (3400) Corrientes, Argentina. Phone: +54 379 4457996, ext. 112. Email: lcleiva@exa.unne.edu.ar.
ABSTRACT
Cross-neutralization of Crotalus durissus terrificus venom coagulant activity was tested using bivalent horse antivenom against Bothrops alternatus and Bothrops diporus venoms. Our in vitro and in vivo experiments showed that bothropic antivenom neutralizes the thrombin-like activity of crotalic snake venom and this cross-reaction was demonstrated by immunoassays either with whole venom or a purified thrombin-like enzyme. These results suggest common antigenic properties and, consequently, similar molecular structure among venom thrombin-like enzymes. Besides, they provide information that could be further used in the development of new antivenom formulations.
Key words: thrombin-like enzyme, snake venom, antivenoms, immunological cross-reaction.
INTRODUCTION
Snake venoms, particularly those belonging to Viperidae family, often induce disorders in the blood coagulation system, which represents a serious complication in snakebites (1, 2). Venom proteases, which are generally classified structurally as serine proteases and metalloproteinases, play a crucial role (3-5). Several snake venom proteases may activate blood coagulation factors such as factor V or X, or act as prothrombin convertases or as thrombin-like enzymes (TLE) (6). These latter have a direct action on fibrinogen (thrombin like activity); they transform fibrinogen into fibrin gel. Since TLE do not activate factor XIII, the resulting fibrin clot is not cross-linked, hence it is easily degraded by the fibrinolytic system and removed from circulation by mononuclear phagocytes causing acute defibrinogenating effects (7).
Crotalus durissus terrificus (C.d.t.) venom may cause complete and long lasting incoagulability of blood in patients, due to the hypofibrinogenemia or afibrinogenemia developed after the bite (8). Besides the substitution therapy with plasma or plasma derived products, the use of antivenoms is the only effective treatment to this disturbance of hemostasis (9). On the other hand, several TLE have been isolated from Bothrops venom, and they are endowed with similar toxic activities described for the crotalic TLE (cTLE).
Numerous authors evaluated cross-reactions against toxins and antivenoms, and found interesting results. Immunochemical investigations performed with antibodies raised against the phospholipase A2 (PLA2) neurotoxin agkistrodotoxin (AGTX), from Agkistrodon blomhoffii brevicaudus venom, and against the PLA2 subunit of crotoxin, from C.d.t. venom (cPLA2), indicated that they share some structural similarities. The majority of AGTX antigenic determinants are present on cPLA2 and on PLA2s from Viperidae venoms. Additionally, some of these determinants are involved in the neutralization of lethal potency and in the inhibition of enzymatic activity of AGTX and crotoxin (10).
On the other hand, Beghini et al. (11) demonstrated that it was possible to neutralize the neurotoxicity of C.d.t. and Bothrops jararacussu venoms and their major toxins, cdt-crotoxin and bothropstoxin-I respectively, with rabbit anti-sera produced against crotoxin and phospholipase A2 from C. durissus cascavela venom.
A previous work of our laboratory demonstrated a cross-reaction among bothropic PLA2 and IgG raised against cPLA2 (12). We showed that although an immunological cross-reaction exists, specific anti-cPLA2 antibodies do not have the appropriated pharmacological effect required for a complete neutralization of the PLA2 activity of whole bothropic venoms. These data corroborate the finding by Chippaux and Goyffon (13) that a cross-reaction does not necessarily means cross-protection.
In view of the similarities among Viperidae venoms and the possible presence of similar antigenic determinants between crotalic and bothropic toxins, in this work, the immunological cross-reactions between these venoms and their thrombin-like enzymes were studied. Therefore, we tested the neutralization capacity of the bivalent antivenom produced for Bothrops alternatus (B.a.) and Bothrops diporus (B.d.) bites - both species are responsible for most bothropic envenomations in northeastern Argentina - against C.d.t. venom. In order to further demonstrate that this cross-neutralization exits, a thrombin-like serine proteinase from C.d.t. was isolated and two immune and coagulant activity neutralization tests were assayed.
MATERIALS AND METHODS
Venoms and Antivenoms
Desiccated C.d.t. venom and bothropic venoms were obtained from adult specimens from the serpentarium of Corrientes city. Horse antivenoms were obtained after eleven injections of B.a. or B.d. venom per animal, performed over a period of eight months. Complete Freund's adjuvant (Sigma, USA) was used in the first injection and booster inoculations were prepared with incomplete Freund's adjuvant (Sigma, USA). The antibody levels in the sera were monitored by ELISA. Blood samples were collected from the jugular vein, sera were subsequently separated by centrifugation and antibodies were purified by affinity chromatography (HiTrap Protein G HP 1 mL, Amersham Biosciences, Sweden) in a FPLC System, according to the manufacturer's instructions to obtain IgG fraction. Equal amount of antibodies from each antisera (IgG anti B.a. venom or IgG anti B.d. venom) were mixed and the reactivity and the specificity of the mixture (IgGav) were tested by ELISA and dot blotting.
ELISA Assay
Briefly, microtiter plates (96 wells) were coated with 100 µL of C.d.t., B.a., B.d. venoms or TLE (5 µg.mL-1) in PBS for one hour at 37°C. The plates were washed and processed as described by Rodríguez et al. (14). Absorbance was read at 490 nm with a Multiskan® EX (Thermo Scientific, USA) multiwell plate reader.
Dot Blotting Tests
In order to provide highly specific results a dot blot test was performed. Antigens (bothropic venoms, C.d.t. venom and cTLE) were diluted to different concentrations, in a 20 mM Tris 500 mM NaCl buffer (TBS), pH 7.2. Different amount of antigen samples (3 to 12 µg) were pipetted into each dot in a vertical row of a nitrocellulose strip (Biorad, USA). The membranes were dried at room temperature for two hours. After that, the strips were washed once with TBS containing 0.1% (v/v) Tween 20 (TBS-Tween 20) and blocked with bovine serum albumin (1% in TBS-Tween 20) for one hour at 37°C, on plates on a shaking platform. Then, strips were washed once with TBS-Tween 20 and immediately incubated with IgGav for one hour at 37°C. Bound antibodies were detected with goat anti-horse IgG peroxidase conjugate (Sigma, USA; 1:1000 in TBS) incubating the strips in the same conditions. Finally, blots were washed, developed with 4-chloro-1-naphthol (Sigma, USA; 0.03% in 0.05 M Tris-HCl, pH 7.6, containing 0.03% H2O2/OPD) and documented. Horse pre-immune serum was employed as negative control.
cTLE Purification and TLE Activity
A crotalic thrombin-like enzyme (cTLE) was purified by a two-step chromatographic protocol as previously described (15, 16). Briefly, venom samples of 25 mg, obtained from adult specimens, were applied to a Sephadex G-75 (100 x 1 cm) column pre-equilibrated with 20 mM glycine, 150 mM NaCl buffer, pH 1.9. Fractions with high coagulant activity were pooled, concentrated and a cTLE was purified with a Benzamidine-Sepharose Fast Flow affinity chromatography column (10 x 1 cm; Amersham Biosciences, Sweden), previously equilibrated with 0.05 M Tris 0.4 M NaCl buffer. After elution of the unbound fraction, 0.1 M sodium acetate 0.3 M NaCl pH 3.0 buffer was applied to the column and the absorbance of the eluting material was monitored at 280 nm.
In Vitro TLE Activity Neutralization Tests
Neutralization of thrombin-like activity was tested using a Wiener Lab Fibrintimer 2® coagulometer (Germany). Different amount of venoms or cTLE (10 to 320 µg) were incubated with IgGav (35 mg/mL) for 30 minutes at 37°C and, then, 75 µL of the sample was added to 75 µL of human fibrinogen (4 mg/mL; Sigma, USA) or human plasma. The mixture of venom and IgGav (V-IgGav) was incubated at 37°C and the time until clot formation was measured (Tv-IgG). Venom solutions incubated with PBS were used as control samples (TV). Complete inhibition was assumed when no clot formation occurred within ten minutes and zero inhibition when the clotting time was identical to that of the control sample (TV). Percentage of neutralization was determinate using the ratio:
In Vivo Fibrinogen Consumption Neutralization Tests
Six groups of four mice each, weighing 18 to 22 g, were intraperitoneally (IP) injected with 0.5 µg/g of purified cTLE or cTLE preincubated with the amount of IgGav previously determined for completel neutralization of enzyme activity. Animals of the control group were injected with PBS. After the determined time of exposure (0, 1, 3, 6, 9 and 24 hours), animals were anesthetized and blood was collected from the cava vein and, then, trisodium citrate was added to prevent coagulation. Platelet-poor plasma was obtained after centrifugation of samples at 2500 rpm at room temperature for ten minutes. Fibrinogen content was determined on each sample by triplicate using Wiener Lab Fibrinogen® kit (Argentina). Fibrinogen values were expressed in percentages assuming as 100% the fibrinogen level at initial time.
RESULTS
ELISA and Dot Blotting Tests
The reactivity determined by ELISA of IgGav against bothropic venoms is shown in Figure 1 - A. As expected, the greatest reactivity was observed with B.a. and B.d. venoms, since they were used in the immunization process. On the other hand, C.d.t. venom and cTLE revealed similar behavior with absorbance values slightly over 50% of those obtained for bothropic venoms. The specificity of IgGav was demonstrated with dot blot test (Figure 1 - B). It showed that IgGav cross-reacted with C.d.t. venom and its cTLE, strong spots were noted in each case, even with 3 µg of protein.
In Vitro Neutralization Tests
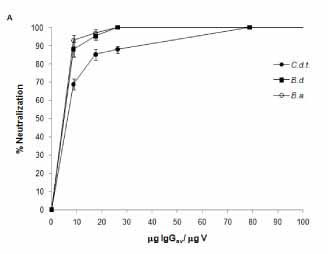
IgGav was able to neutralize the coagulant activity of bothropic venoms, and cross-neutralize crotalic venoms and cTLE. Figure 2 - A shows the neutralization of coagulant activity of bothropic and crotalic venoms by IgGav on human fibrinogen. The IgGav:venom ratio required to efficiently neutralize the bothropic coagulant activity was 26.25 µg IgGav/µg of venom. At the same IgGav:venom ratio, it was observed 80% of C.d.t. venom cross-neutralization. However, it was necessary a three-fold increase of IgGav:venom ratio to show 100% of C.d.t. venom neutralization.
In order to reproduce in vitro a more realistic scenario, similar results were obtained when the neutralization of plasma coagulant activity was tested with IgGav. Figure 2 - B shows that the IgGav:venom ratio needed to neutralize the activity of venoms is the same, and both bothropic venoms were efficiently neutralized at this ratio (100%).
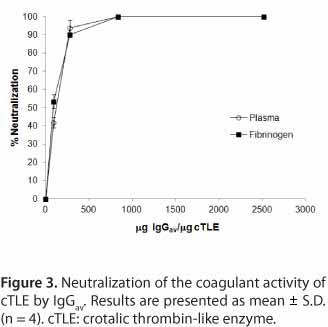
The results presented in Figure 3 demonstrate that cTLE serine proteinases from C.d.t. venom are cross-neutralized by IgGav. In that sense, similar neutralization tests were developed with cTLE, purified from whole venom, on plasma and fibrinogen. Since the same amount of enzyme was employed instead of whole venom (10 to 320 ¼g), the high clotting activity of this sample required a 10.7-fold increase of IgGav:TLE ratio to show 100% neutralization of enzymes, compared to IgGav:venom ratio, needed to cross-neutralize C.d.t. venom.
In Vivo Neutralization Tests
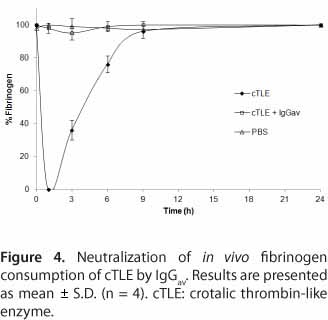
The effect of cTLE neutralized by IgGav was examined in vivo. Animals injected with cTLE showed rapid drop of plasma fibrinogen levels during the first hour, to undetectable values, then reaching its normal levels 10 hours after injection. On the other hand, cTLE (10 ¼g) preincubated with IgGav (according to the relation found in the in vitro assay), resulted in minimal decrease of the mouse plasma fibrinogen, and no significance differences was detected when these results were compared with those obtained in the control group (Figure 4).
Theoretical Structural Analysis
In order to explain the experimental results found, we have analyzed several sequenced toxins homologous to those used in the neutralization experiments of this work. Figure 5 details the structural aspects studied with MegAlignTM software ver. 5.01 (DNASTAR, USA).
DISCUSSION
In vitro evidence of immunological cross-reactivity is reported for many venoms and antivenoms as a way of detecting similarities among toxins and potential uses of antivenoms. Such cross-reactions have been studied by other authors using western blot and ELISA; however, they need to be individually verified with biological tests, according to the toxicological properties of venoms (13).
The present results indicated that IgGav was capable to cross-react with C.d.t. venom since sharp spots were obtained in a dot blot test and the ELISA test confirmed its reactivity.
Moreover, antibodies produced against bothropic venoms were able to block the coagulant activity of C.d.t. venom. These results are in opposition to those observed by de Roodt et al. (17), who stated that the coagulant activity of C.d.t. was not affected by preincubation with bothropic antivenoms. Furthermore, our results are supported by immunoassays and biological neutralization tests carried out with the isolated enzyme.
The finding that antibodies against bothropic venoms recognize crotalic TLE reveals structural similarities among viperid thrombins in spite of the large differences between the two genera. In order to explain this experimentally observed phenomenon, we analyzed some structural studies in the literature, with the aim of correlating the degree of conservation and homology of the molecules that cross-react with IgGav.
Some proteins, already sequenced and homologous to those studied here, were selected for analysis. Thus, gyroxin-like B2.1 (NCBI protein database accession number: Q58G94) - isolated by Brazilian authors from C.d.t. venom - and bhalternin (NCBI protein database accession number: P0CG03) - purified from B. alternatus venom from Brazilian specimens - were chosen (3). In addition, other three thrombins from venoms from other bothropic species - B. asper (NCBI protein database accession number: ABB76280), bothrombin from B. jararaca (NCBI Protein Database accession: P81661) and B. jararacussu (NCBI protein database accession number: ABC24687) - were included (18).
The homology analysis of protein sequences developed with MegAlignTM software ver. 5.01 (DNASTAR, USA) allowed us to detect over 63% of identity between gyroxin like B2.1 and bhalternin, and slightly lower percentages with other enzymes (Figure 5 - A). This would predict the existence of sequential epitopes shared. Figure 5 - B illustrates that gyroxin is the most phylogenetically separated compared with the other bothropic toxins analyzed. These observations corroborate our immunological cross-reaction tests between the crotalic venom and bothropic antivenom.
Furthermore, since antigenic sites are located within surface-exposed regions of a protein, ProteanTM software ver 5.01 (DNASTAR, USA) was employed to generate accurate antigenic profiles from the linear amino acid sequences with Jameson and Wolf algorithm (19). Figure 5 - C shows similar topological profiles between gyroxin and bhalternin that would explain potential conformational antigenic determinants shared, similar to those detected experimentally with IgGav. The other bothropic toxins also present a similar antigenic profile and it would not be surprising to find that they also show immunologic cross-reactivity; however, individual verification is required.
Clotting activity neutralization tests on plasma and on fibrinogen, of whole venom and cTLE, revealed that a higher amount of antibodies (approximately 10-fold) is required to neutralize 100% of the enzyme activity compared to that needed to neutralize whole venom. This is expected since whole venom is composed of a set of proteins among which thrombin-like enzymes, with serine protease structures, represent only 8% of it, as reported by Calvete et al. (20) in a venomic study of Crotalus species.
Additionally, an in vivo incoagulability assay was performed in order to provide further evidence of this immunological cross-reaction and render the work more conclusive. In a previous work by Maruñak et al. (15), the effects of cTLE and whole venom on mice plasmatic fibrinogen levels were characterized. It showed that fibrinogen concentrations decreased rapidly to incoagulability during the first hour after cTLE injection, and then returned to its normal levels ten hours later (15). In the present study, we demonstrated that it is possible to neutralize this incoagulability effect of cTLE preincubating with IgGav.
Current research on antiophidic therapy is focused on the production of new antivenoms with reduced protein content and high neutralization capacity. This can be achieved using either specific antibodies with the ability to inhibit the most toxic proteins from different venoms or antibodies that cross-react with them. Evidences of immunological cross-reactions between crotalic venoms and bothropic antivenoms presented herein reinforce the use of polyvalent antivenoms, which could have practical applications in the development of future new antitoxin formulations.
ACKNOWLEDGEMENTS
The authors thank Dr. Juan Pablo Zanin for bibliographic assistance.
Submission status
Received: July 1, 2011.
Accepted: November 3, 2011.
Abstract published online: November 3, 2011.
Full paper published online: February 28, 2012.
Conflicts of interest
The authors declare no conflicts of interest.
Financial source
This work was financially supported by the Agencia Nacional de Promoción Científica y Tecnológica (PICTO-2007-00143); Secretaría General de Ciencia y Técnica (PI B013-2010), Universidad Nacional del Nordeste and Proyecto Federal de Innovación Productiva 2005 (PEFIP-COFECyT). Moreover, J. P. Rodriguez and C. C. Gay are recipients of postdoctoral scholarships from CONICET - UNNE.
Ethics committee approval
The present study was approved by the Ethics and Biosafety Committee of the School of Veterinary Science, Northeast National University, Argentina.
- 1. Markland FS. Snake venoms and the hemostatic system. Toxicon. 1998;36(12):1749-800.
- 2. Gutierrez JM. Understanding snake venoms: 50 years of research in Latin America. Rev Biol Trop. 2002;50(2):377-94.
- 3. Costa J de O, Fonseca KC, Mamede CC, Beletti ME, Santos-Filho NA, Soares AM, et al. Bhalternin: functional and structural characterization of a new thrombin-like enzyme from Bothrops alternatus snake venom. Toxicon. 2010;55(7):1365-77.
- 4. de Oliveira DG, Murakami MT, Cintra AC, Franco JJ, Sampaio SV, Arni RK. Functional and structural analysis of two fibrinogen-activating enzymes isolated from the venoms of Crotalus durissus terrificus and Crotalus durissus collilineatus Acta Biochim Biophys Sin (Shanghai). 2009;41(1):21-9.
- 5. Gay CC, Leiva LC, Marunak S, Teibler P, Acosta de Perez O. Proteolytic, edematogenic and myotoxic activities of a hemorrhagic metalloproteinase isolated from Bothrops alternatus venom. Toxicon. 2005;46(5):546-54.
- 6. Kornalik F. The influence of snake venom enzymes on blood coagulation. Pharmacol Ther. 1985;29(3):353-405.
- 7. Gené JA, Roy A, Rojas G, Gutiérrez JM, Cerdas L. Comparative study on coagulant, defibrinating, fibrinolytic and fibrinogenolytic activities of Costa Rican crotaline snake venoms and their neutralization by a polyvalent antivenom. Toxicon. 1989;27(8):841-8.
- 8. Amaral CF, da Silva OA, Lopez M, Pedroso ER. Afibrinogenemia following snake bite (Crotalus durissus terrificus). Am J Trop Med Hyg. 1980;29(6):1453-5.
- 9. Claus I, Mebs D. Cross-neutralization of thrombin-like enzymes in snake venoms by polyvalent antivenoms. Toxicon. 1989;27(12):1397-9.
- 10. Choumet V, Jiang MS, Specker I, Bon C. Immunochemical cross-reactivity of two phospholipase A2 neurotoxins, agkistrodotoxin and crotoxin. Toxicon. 1991;29(4-5):441-51.
- 11. Beghini DG, da Cruz-Hofling MA, Randazzo-Moura P, Rodrigues-Simioni L, Novello JC, Hyslop S, et al. Cross-neutralization of the neurotoxicity of Crotalus durissus terrificus and Bothrops jararacussu venoms by antisera against crotoxin and phospholipase A2 from Crotalus durissus cascavella venom. Toxicon. 2005;46(6):604-11.
- 12. Rodríguez JP, De Marzi MC, Maruñak S, Teibler P, Acosta O, Malchiodi EL, et al. IgG antibodies against phospholipase A2 from Crotalus durissus terrificus: cross-reaction with venoms from Bothrops species from Argentina. J Venom Anim Toxins incl Trop Dis. 2009;15(3):460-78.
- 13. Chippaux JP, Goyffon M. Venoms, antivenoms and immunotherapy. Toxicon. 1998;36(6):823-46.
- 14. Rodríguez JP, De Marzi M, Maruñak S, Malchiodi EL, Leiva LC, Acosta O. Rabbit IgG antibodies against phospholipase A2 from Crotalus durissus terrificus neutralize the lethal activity of the venom. Medicina (B Aires). 2006;66(6):512-6.
- 15. Maruñak SL, Acosta OC, Leiva LC, Ruiz RM, Aguirre MV, Teibler P. Mice plasma fibrinogen consumption by thrombin-like enzyme present in rattlesnake venom from the north-east region of Argentina. Medicina (B Aires). 2004;64(6):509-17.
- 16. Ruiz de Torrent RM, Bongiovanni B, Leiva LC, Evangelista de Duffard AM, Rodríguez JP, Acosta de Pérez OC, et al. Neurotoxicological effects of a thrombin-like enzyme isolated from Crotalus durissus terrificus venom (preliminary study). Toxicon. 2007;50(1):144-52.
- 17. de Roodt AR, Dolab JA, Fernández T, Segre L, Hajos SE. Cross-reactivity and heterologous neutralization of crotaline antivenoms used in Argentina. Toxicon. 1998;36(7):1025-38.
- 18. Nishida S, Fujimura Y, Miura S, Ozaki Y, Usami Y, Suzuki M, et al. Purification and characterization of bothrombin, a fibrinogen-clotting serine protease from the venom of Bothrops jararaca Biochemistry. 1994;33(7):1843-9.
- 19. Jameson BA, Wolf H. The antigenic index: a novel algorithm for predicting antigenic determinants. Comput Appl Biosci. 1988;4(1):181-6.
- 20. Calvete JJ, Sanz L, Cid P, de la Torre P, Flores-Díaz M, Dos Santos MC, et al. Snake venomics of the Central American rattlesnake Crotalus simus and the South American Crotalus durissus complex points to neurotoxicity as an adaptive paedomorphic trend along Crotalus dispersal in South America. J Proteome Res. 2010;9(1):528-44.
Publication Dates
-
Publication in this collection
16 Mar 2012 -
Date of issue
2012
History
-
Received
01 July 2011 -
Accepted
03 Nov 2011