Abstracts
The authors describe the cases of two patients with pseudoaneurysms, discuss the difficulty in establishing diagnosis and treatment due to human immunodeficiency virus infection, and demonstrate the similarity with atherosclerotic saccular aneurysm of the abdominal aorta.
HIV; pseudoaneurysm; saccular aneurysm
Os autores descrevem os casos de dois pacientes que apresentaram pseudoaneurismas e ressaltam a dificuldade diagnóstica e terapêutica por apresentar associação com a infecção pelo vírus da imunodeficiência humana, e também demonstram a semelhança com aneurisma sacular aterosclerótico da aorta abdominal
HIV; pseudoaneurisma; aneurisma sacular
INTRODUCTION
Since it was first identified, human immunodeficiency virus infection has remained a pandemic that affects all social classes. The association of the acquired immunodeficiency syndrome (AIDS) and opportunistic infections has been clearly determined by patient immunosuppression. Also known is the fact that viral infections directly affect some organs: kidneys, liver, gastrointestinal tract and vascular system. In the vascular system, it may result in vasculitis, perivasculitis, arteritis, fibroproliferative diseases and aneurysms. However, the physiopathology of how HIV promotes vascular degeneration remains unclear. The diagnosis of mycotic aneurysms may be difficult when they mimic atherosclerotic aneurysms. The cases described in this study demonstrate the similarity of these aneurysms and atherosclerotic saccular aneurysm of the abdominal aorta, as well as a diagnostic suspicion of aneurysms at less common sites.
CASE REPORT
Case 1
A 67-year-old female patient was referred to the Outpatient Service of Vascular and Endovascular Surgery because she had been diagnosed with a saccular abdominal aortic aneurysm (AAA) during investigation of left lower back pain about one year before. During the review of systems, the patient denied intermittent claudication or pain at rest. She reported having hypertension, diabetes and hypothyroidism and HIV infection. She also had a clinical history of left kidney volume decrease and loss of renal function confirmed by scintigraphy findings, as well as a bacterial endocarditis treated in 2009. Physical examination revealed that the patient was in good physical condition, had no cardiac or pulmonary symptoms, but her abdomen, which had a hysterectomy scar, was enlarged and difficult to palpate. All pulses were palpable and normal in upper and lower extremities.
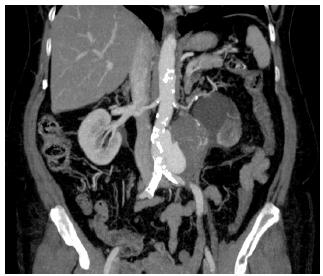
CT angiography two months before the procedure revealed a pseudoaneurysm of the infrarenal abdominal aorta measuring 8.4 cm in diameter and 9.2 cm in length (Figure 1). The patient was asymptomatic, and hypertension and diabetes were under control at the time of hospitalization. She was taking zidovudine, lamivudine and atazanavir/ritonavir to control HIV infection. Laboratory tests showed that viral load was undetectable (<50 copies/mL).
CT angiography scan shows infrarenal abdominal aortic pseudoaneurysm in patients with HIV infection.
As the patient was obese, had previous abdominal surgery and her anatomy was favorable, the plan was to use an endovascular approach to place an aortic endoprosthesis for aneurysm repair.
Right femoral dissection and left femoral puncture were used to place a 25 mm × 16 mm × 100 mm PowerlinkR endoprosthesis (Endologix, Irvine, CA) using a 9F introducer sheath, and the aneurysm was immediately repaired. Post-operative progression was good, and the patient was discharged on the second day after operation. Ten days after the operation, she presented with left lower back pain, diarrheic stools, vomiting and paresthesia of left lower extremity. Physical examination detected femoral and distal pulses and no sign of hyperemia in the surgical wound; laboratory tests were normal. Two days after hospitalization, her general state deteriorated and she had abdominal and lower extremity pain. Physical examination found that the lower extremities were cold, and no pulses should be detected. Ultrasound scanning confirmed the clinical hypothesis of endoprosthesis occlusion. She underwent an urgent surgery for an axillobifemoral bypass and placement of an 8-mm Dacron prosthesis, and revascularization of the lower extremities was successful. After operation in the ICU, she was administered broad spectrum antibiotics (vancomycin 1 g, 12h/12h and meropenem 1 g, 8h/8h), but had refractory septic shock and died.
Case 2
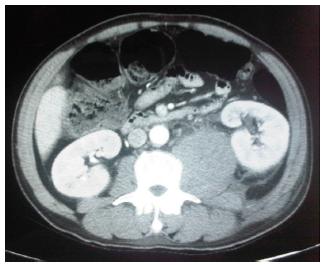
A previously healthy 47-year-old male smoking patient was transferred from another hospital ward with a history of daily low fever for 20 days. Nine days before, he felt a strong sudden pain in the proximal third of the left thigh, which radiated to the lower back. He was clinically stable and sweating profusely, his facial expression indicated pain, his blood pressure was normal, and his left lower extremity was flexed in pain. Initial laboratory tests showed electrolyte imbalance, leukocytosis with a left shift, hematocrit and hemoglobin of 23.4 and 8.5. CT angiography revealed a pseudoaneurysm in the left common iliac artery and a large retroperitoneal hematoma affecting the psoas muscle (Figures 2 and 3).
CT angiography scan shows pseudoaneurysm in left common iliac artery and retroperitoneal hematoma.
The patient underwent urgent surgery for a femorofemoral crossed bypass and placement of an 8-mm polytetrafluorethylene prosthesis because of the inadequate caliber of his magna saphenous vein, followed by exploration of the retroperitoneal region. During exploration, clamps and ligatures were used in the proximal and distal left common iliac artery segments, followed by draining of the pseudoaneurysm and psoas hematoma. There were no signs of purulent secretion, and material was collected for culture. On the first post-operative days in the ICU, the patient's general condition improved significantly, and laboratory tests were repeated. After patient agreement, material for hepatitis, syphilis and HIV tests was collected, and only HIV was positive. Culture of the material collected during surgery did not isolate any microorganisms.
After the seventh post-operative day, his general condition deteriorated, he had septic shock and had to be intubated and receive broader spectrum antibiotics and vasopressor support. Twenty days after the operation, an abdominal CT scan showed retroperitoneal liquid collection, which was drained surgically (Figure 4). Blood and urine were collected for culture, and a chest X-ray was obtained to investigate the presence of other infections. The tests were positive for carbapemenase-producing klebsiela and candida albicans, and there was opacification of the left lung base. Despite the use of broader spectrum antibiotics and antifungal agents, the patient had refractory septic shock and died on the 38th day after hospitalization.
CT scan shows retroperitoneal collection in region of left psoas muscle after pseudoaneurysm repair.
DISCUSSION
Systemic vasculitis may result from several types of bacterial, viral or fungal infection. These microorganisms may disturb endothelial functioning and cause direct lesions or the expansion of underlying inflammation. The immunological mediation of cytokines affects antigen expression in endothelial cells, which, in turn, activate T and B lymphocytes. Immune complexes damage cells on the vascular wall, with a subsequent increase of the subintimal and perivascular permeability and substance migration that may lead to several types of disorders, such as purpura, necrotizing angiitis, hives and diseases of small and large caliber vessels11. Calabrese LH, Estes M, Yen-Lieberman B, et al. Systemic vasculitis in association with human immunodeficiency virus infection. Arthritis Rheum. 1989;32:569-76. PMid:2655605. http://dx.doi.org/10.1002/anr.1780320509
http://dx.doi.org/10.1002/anr.1780320509...
,
22. Pitta GBB, Silva CRA, Medeiros JD, et al. Isquemia grave de membros inferiores por arterite por HIV. J Vasc Bras. 2011; 10:319-24.
,
33. Ando T, Makuuchi H, Kitanaka Y, Koizumi H. Rupture of a pseudo aneurysm of the abdominal aorta in a patient with human immunodeficiency virus infection. Ann Thorac Cardiovasc Surg. 2011;17(2):198-200. PMid:21597422. http://dx.doi.org/10.5761/atcs.cr.09.01485
http://dx.doi.org/10.5761/atcs.cr.09.014...
.
HIV infection is a growing public health problem, and current treatments have significantly extended patient life expectancy. HIV may affect the whole vascular system, either due to infection by other microorganisms, or as a result of direct lesions caused by the virus. Arterial aneurysms associated with HIV infection were first described in 1989 by Dupont et al.44. DuPont JR, Bonavita JA, Di Giovanni RJ, Spector HB, Nelson SC. Acquired immunodeficiency syndrome and mycotic abdominal aortic aneurysms: A new challenge? Report of a case. J Vasc Surg. 1989;10:254-7. PMid:2778888., for whom HIV defined a new frontier and a therapeutic challenge in the treatment of infectious aneurysms.
Twelve cases of aneurysms associated with HIV infection have been described by Marks and Kuscov55. Marks C, Kuskov S. Patterns of arterial aneurysms in acquired immunodeficiency disease. World J Surg. 1995;19:127-32. http://dx.doi.org/10.1007/BF00316996
http://dx.doi.org/10.1007/BF00316996 ...
. In their study, histology confirmed fibroproliferative periarteritis in their five patients; they also found inflammatory granulomas, and Staphylococcus aureus was detected in the surgical specimen of only one patient. Giant cell granuloma infiltrating the media of the aorta was described by Boggian et al.66. Boggian K, Leu HJ, Schneider J, Turina M, Oertle D. Aneurysma verum der aorta ascendens in Rahmeneiner HIVKrankheit. Schweiz Med Wochenschr. 1994;124:2083-7. PMid:7973546., who also found that the inflammatory infiltrate reached the vasa vasora.
The exact physiopathology of the lesion caused by HIV has not been clarified, but several mechanisms may be involved. Calabrese et al.11. Calabrese LH, Estes M, Yen-Lieberman B, et al. Systemic vasculitis in association with human immunodeficiency virus infection. Arthritis Rheum. 1989;32:569-76. PMid:2655605. http://dx.doi.org/10.1002/anr.1780320509
http://dx.doi.org/10.1002/anr.1780320509...
found an association between arterial wall weakening and HIV-induced immune complexes. There may be another mechanism, in which previous infections of ulcerated atherosclerotic plaque are amplified and destroy the arterial wall, resulting in aneurysms77. Ewart JM, Burke ML, Bunt TJ. Spontaneous abdominal aortic infections: Essentials of diagnosis and management. Am Surg. 1983;49:37-50. PMid:6337539.. Nair et al.88. Nair R, Abdool-Carrim ATO, Chetty R, Robbs JV. Arterial aneurysms in patients infected with human immunodeficiency virus: A distinct clinicopathology entity? J Vasc Surg. 1999; 29:600-7. http://dx.doi.org/10.1016/S0741-5214(99)70304-6
http://dx.doi.org/10.1016/S0741-5214(99)...
, however, found that culture was positive in only two cases of a series of ten patients with HIV and arterial aneurysms.
These aneurysms may be repaired by conventional surgery and grafts, preferably anatomic and autologous grafts, particularly in the case of visceral and peripheral aneurysms. The placement of prostheses, used as arterial grafts only when there is no adequate vein99.Clagett GP, Valentine RJ, Hagino RT. Autogenous aortoiliac/femoral reconstruction from superficial femoralpopliteal veins: Feasibility and durability. J Vasc Surg. 1997;25:255-66. http://dx.doi.org/10.1016/S0741-5214(97)70347-1
http://dx.doi.org/10.1016/S0741-5214(97)...
, should be preceded by debridement of infected and devitalized tissues and administration of broad spectrum antibiotics or specific antibiotics to fight the microorganism detected. When the extremity has to be revascularized before the affected site is approached, or when the area to be operated is markedly infected, extraanatomic bypasses should be the choice, as in our Case 2, in which the patient had clear signs of infection. An endoluminal approach may also be used in cases without clinical evidence of HIV activity and in high surgical risk patients, and that was our choice in Case 1. In similar cases, broad spectrum antibiotics should be used, although treatment duration has not been defined and may last months, years, or the rest of the patient's life1010. Kritpracha B, Premprabha D, Sungsiri J, Tantarattanapong W, Rookkapan S, Juntarapatin P. Endovascular therapy for infected aortic aneurysms. J Vasc Surg. 2011;54:1259-65. PMid:21802238. http://dx.doi.org/10.1016/j.jvs.2011.03.301
http://dx.doi.org/10.1016/j.jvs.2011.03....
,
77. Ewart JM, Burke ML, Bunt TJ. Spontaneous abdominal aortic infections: Essentials of diagnosis and management. Am Surg. 1983;49:37-50. PMid:6337539.
,
1111. Chello M, Tamburrini S, Mastroroberto P, Covino E. Pseudoaneurysm of the thoracic aorta in patients with human immunodeficiency virus infection. Eur J Cardiothorac Surg. 2002;22:454-6. http://dx.doi.org/10.1016/S1010-7940(02)00349-4
http://dx.doi.org/10.1016/S1010-7940(02)...
.
The surgical procedure for patients with HIV infection and arterial aneurysm should always be carefully evaluated and seen as a challenge, as there are several variables that may affect outcomes. In this case report, we found similarities between atherosclerotic saccular aneurysms and abdominal aortic pseudoaneurysms (Figure 5). The patient in Case 1 was an elderly woman in good clinical conditions, and her tests showed that infection was under control, which did not raise any concerns. In the second case, the suspicion of a possible etiological correlation with HIV infection was clearly defined because the patient was younger, had no atherosclerosis and the pseudoaneurysm affected the iliac artery, an unusual site for isolated aneurysms.
Studies in the literature and cases reported to this date led us to the conclusion that there should be a high level of suspicion of an etiological association between an arterial aneurysm and HIV infection when the aneurysm is found in an unusual site, is multiple or has an unusual presentation at clinical examination or on CT angiography scans.
REFERÊNCIAS
-
1Calabrese LH, Estes M, Yen-Lieberman B, et al. Systemic vasculitis in association with human immunodeficiency virus infection. Arthritis Rheum. 1989;32:569-76. PMid:2655605. http://dx.doi.org/10.1002/anr.1780320509
» http://dx.doi.org/10.1002/anr.1780320509 -
2Pitta GBB, Silva CRA, Medeiros JD, et al. Isquemia grave de membros inferiores por arterite por HIV. J Vasc Bras. 2011; 10:319-24.
-
3Ando T, Makuuchi H, Kitanaka Y, Koizumi H. Rupture of a pseudo aneurysm of the abdominal aorta in a patient with human immunodeficiency virus infection. Ann Thorac Cardiovasc Surg. 2011;17(2):198-200. PMid:21597422. http://dx.doi.org/10.5761/atcs.cr.09.01485
» http://dx.doi.org/10.5761/atcs.cr.09.01485 -
4DuPont JR, Bonavita JA, Di Giovanni RJ, Spector HB, Nelson SC. Acquired immunodeficiency syndrome and mycotic abdominal aortic aneurysms: A new challenge? Report of a case. J Vasc Surg. 1989;10:254-7. PMid:2778888.
-
5Marks C, Kuskov S. Patterns of arterial aneurysms in acquired immunodeficiency disease. World J Surg. 1995;19:127-32. http://dx.doi.org/10.1007/BF00316996
» http://dx.doi.org/10.1007/BF00316996 -
6Boggian K, Leu HJ, Schneider J, Turina M, Oertle D. Aneurysma verum der aorta ascendens in Rahmeneiner HIVKrankheit. Schweiz Med Wochenschr. 1994;124:2083-7. PMid:7973546.
-
7Ewart JM, Burke ML, Bunt TJ. Spontaneous abdominal aortic infections: Essentials of diagnosis and management. Am Surg. 1983;49:37-50. PMid:6337539.
-
8Nair R, Abdool-Carrim ATO, Chetty R, Robbs JV. Arterial aneurysms in patients infected with human immunodeficiency virus: A distinct clinicopathology entity? J Vasc Surg. 1999; 29:600-7. http://dx.doi.org/10.1016/S0741-5214(99)70304-6
» http://dx.doi.org/10.1016/S0741-5214(99)70304-6 -
9Clagett GP, Valentine RJ, Hagino RT. Autogenous aortoiliac/femoral reconstruction from superficial femoralpopliteal veins: Feasibility and durability. J Vasc Surg. 1997;25:255-66. http://dx.doi.org/10.1016/S0741-5214(97)70347-1
» http://dx.doi.org/10.1016/S0741-5214(97)70347-1 -
10Kritpracha B, Premprabha D, Sungsiri J, Tantarattanapong W, Rookkapan S, Juntarapatin P. Endovascular therapy for infected aortic aneurysms. J Vasc Surg. 2011;54:1259-65. PMid:21802238. http://dx.doi.org/10.1016/j.jvs.2011.03.301
» http://dx.doi.org/10.1016/j.jvs.2011.03.301 -
11Chello M, Tamburrini S, Mastroroberto P, Covino E. Pseudoaneurysm of the thoracic aorta in patients with human immunodeficiency virus infection. Eur J Cardiothorac Surg. 2002;22:454-6. http://dx.doi.org/10.1016/S1010-7940(02)00349-4
» http://dx.doi.org/10.1016/S1010-7940(02)00349-4
Publication Dates
-
Publication in this collection
Jul-Sep 2013
History
-
Received
10 Nov 2012 -
Accepted
15 July 2013