Abstract
Triatoma sordida is a species that transmits Trypanosoma cruzi to humans. In Brazil, T. sordida currently deserves special attention because of its wide distribution, tendency to invade domestic environments and vectorial competence. For the planning and execution of control protocols to be effective against Triatominae, they must consider its population structure. In this context, this study aimed to characterise the genetic variability of T. sordida populations collected in areas with persistent infestations from Minas Gerais, Brazil. Levels of genetic variation and population structure were determined in peridomestic T. sordida by sequencing a polymorphic region of the mitochondrial cytochrome b gene. Low nucleotide and haplotype diversity were observed for all 14 sampled areas; π values ranged from 0.002-0.006. Most obtained haplotypes occurred at low frequencies, and some were exclusive to only one of the studied populations. Interpopulation genetic diversity analysis revealed strong genetic structuring. Furthermore, the genetic variability of Brazilian populations is small compared to that of Argentinean and Bolivian specimens. The possible factors related to the reduced genetic variability and strong genetic structuring obtained for studied populations are discussed in this paper.
Triatominae; Triatoma sordida; cyt b diversity
In Brazil, four species currently deserve special attention in Trypanosoma cruzi transmission to humans: Triatoma brasiliensis, Panstrongylus megistus, Triatoma pseudomaculata and Triatoma sordida (MS 2015MS - Ministério da Saúde (BR). Informações técnicas sobre a doença de Chagas e seus vetores no Brasil referente ao ano de 2014 [Internet]. 2015 [cited 2015 May 10]. Available from: portalsaude.gov.br.
portalsaude.gov.br...
). The Reduviid bug T. sordida (Stal, 1859) is endemic in the Cerrado, the main biome of Central Brazil from which dispersion towards the southwest took place, and it is now widely distributed throughout Argentina, Bolivia, Paraguay and Uruguay (Forattini 1980Forattini OP. Biogeografia, origem e distribuição da domiciliação de triatomíneos no Brasil. Rev Saude Publica. 1980; 14: 265-99., Carcavallo et al. 1997Carcavallo RU, de Casas SIC, Sherlock I, Girón IG, Jurberg J, Galvão C, et al. Distribuição geográfica e dispersão altilatitudinal. In: Carcavallo RU, Girón IG, Jurberg J, Lent H, orgs. Atlas dos vetores da doença de Chagas nas Américas. Vol. III. Rio de Janeiro: Fiocruz; 1997. 350 pp., Galvão & Gurgel-Gonçalves 2015Galvão C, Gurgel-Gonçalves R. Vetores conhecidos no Brasil. In: Galvão C, org. Vetores da doença de Chagas no Brasil. Curitiba: Sociedade Brasileira de Zoologia; 2015. 232 pp.). A recent study of ecological niche modelling revealed the possibility that T. sordida is distributed over an area greater than initially thought, and it may be present in other biomes (e.g., Caatinga and Pantanal) (Gurgel-Gonçalves et al. 2012Gurgel-Gonçalves R, Galvão C, Costa J, Peterson AT. Geographic distribution of Chagas disease vectors in Brazil based on ecological niche modeling. J Trop Med. 2012; 15: 326-41., Galvão & Gurgel-Gonçalves 2015)Galvão C, Gurgel-Gonçalves R. Vetores conhecidos no Brasil. In: Galvão C, org. Vetores da doença de Chagas no Brasil. Curitiba: Sociedade Brasileira de Zoologia; 2015. 232 pp..
T. sordida is considered a ubiquitous species with high ecological potential that can live in various ecotopes and feed from different sources. This insect can withstand large environmental changes that cause its competitors to disappear and can widen its ecotopes to include dry trees and dead trees (Forattini et al. 1974Forattini OW, Ferreira OA, Rocha e Silva EO, Rabello EX. Aspectos ecológicos da Tripanossomíase americana. VI. Persistência do Triatoma sordida após alteração ambiental e suas possíveis relações com a dispersão da espécie. Rev Saude Publica. 1974; 8: 265-82.). The epidemiological importance of T. sordida is increasing due to its tendency to invade houses, particularly in areas where Triatoma infestans has been controlled. This process of domiciliation may merely reflect the invasion of habitats from which T. infestans has been eliminated, but it may also be primary, without any relation to the previous eradication of the main vector (Forattini et al. 1984Forattini OW, Rabello EX, Ferreira OA, Rocha e Silva EO, Santos JLF. Aspectos ecológicos da Tripanossomíase americana. XXI. Comportamento de espécies triatomíneas silvestres na reinfestação do intra e peridomicílio. Rev Saude Publica. 1984; 18: 185-208., Noireau et al. 1996Noireau F, Breniére F, Cardozo L, Bosseno MF, Vargas F, Peredo C, et al. Current spread of Triatoma infestans at the expense of Triatoma sordida in Bolivia. Mem Inst Oswaldo Cruz. 1996; 91(93): 271-2.).
In artificial environments, given the frequency with which it has been found outside the peridomicile and intradomicile, T. sordida is considered a semidomiciliar species (Coura 1993Coura JR. O falso dilema sobre a luta antivetorial e as perspectivas de controle da Doença de Chagas no Brasil. Cad Saude Publica. 1993; 9: 514-18.) and is present in some situations at high densities (Diotaiuti & Pinto 1991Diotaiuti L, Pinto CT. Suscetibilidade biológica do Triatoma sordida e Triatoma infestans a deltametrina e lambdacyalotrina em condições de campo. Rev Soc Bras Med Trop. 1991; 24: 151-5.). T. sordida is associated with the reinfestation of dwellings treated with insecticides (Pessoa et al. 2015a). The dispersal pattern of this triatomine is linked to increased infestations of dwellings closest to sylvatic environments and suggests that recolonisation flows to artificial environments from natural ecotopes (Forattini et al. 1974Forattini OW, Ferreira OA, Rocha e Silva EO, Rabello EX. Aspectos ecológicos da Tripanossomíase americana. VI. Persistência do Triatoma sordida após alteração ambiental e suas possíveis relações com a dispersão da espécie. Rev Saude Publica. 1974; 8: 265-82., Diotaiuti et al. 1998)Diotaiuti L, Azeredo BVM, Busek SCU, Fernandes AJ. Controle do Triatoma sordida no peridomicílio rural do Município de Porteirinha, Minas Gerais, Brasil. Rev Panam Salud Publica. 1998; 3: 21-5..
Considering the wide distribution of T. sordida, its tendency to invade domestic environments, and its vectorial competence in the laboratory, we consider it a triatomine that has potential epidemiological importance (Forattini et al. 1974Forattini OW, Ferreira OA, Rocha e Silva EO, Rabello EX. Aspectos ecológicos da Tripanossomíase americana. VI. Persistência do Triatoma sordida após alteração ambiental e suas possíveis relações com a dispersão da espécie. Rev Saude Publica. 1974; 8: 265-82., Diotaiuti 1995Diotaiuti L. Potencial vetorial do Triatoma sordida. Rev Soc Bras Med Trop. 1995; 28: 38-41., Rojas de Arias et al. 2012Rojas de Arias A, Abad-Franch F, Acosta N, López E, González N, Zerba E, et al. Post-control surveillance of Triatoma infestans and Triatoma sordida with chemically-baited sticky traps. PLoS Negl Trop Dis. 2012; 6: e1822.). In 2008, in Ibipitanga, Brazil, oral transmissions of Chagas disease occurred from the ingestion of sugarcane juice prepared in an abandoned sugarcane mill where specimens of T. sordida contaminated with T. cruzi were captured (Dias et al. 2008Dias JP, Bastos C, Araújo E, Mascarenhas AV, Martins Netto E, Grassi F, et al. Acute Chagas disease outbreak associated with oral transmission. Rev Soc Bras Med Trop. 2008; 41(1): 296-300.). These findings emphasise the necessity to evaluate the importance of vectors such as T. sordida in maintaining the endemicity of this disease. Thus, it is important to investigate aspects regarding the planning and execution of vector control initiatives including the assessment of levels of genetic variation, population structure and gene flow among insect populations (Costa et al. 1997Costa J, Freitas-Sibajev MGR, Marchon-Silva V, Pires MQ, Pacheco RS. Isoenzymes detect variation in populations of Triatoma brasiliensis (Hemiptera: Reduviidae: Triatominae). Mem Inst Oswaldo Cruz. 1997; 92(4): 459-64., Noireau et al. 1999Noireau F, Zegarra M, Ordoñez J, Gutierrez T, Dujardin J-P. Genetic structure of Triatoma sordida (Hemiptera: Reduviidae) domestic populations from Bolivia: application on control interventions. Mem Inst Oswaldo Cruz. 1999; 94(3): 347-51., Borges et al. 2000aBorges EC, Dujardin JP, Schofield CJ, Romanha AJ, Diotaiuti L. Genetic variability of Triatoma brasiliensis (Hemiptera: Reduviidae) populations. J Med Entomol. 2000a; 37(6): 872-7., bBorges E, Romanha AJ, Diotaiuti L. Use of random amplified polymorphic DNA (RAPD) in the population study of Triatoma brasiliensis Neiva, 1911. Cad Saude Publica. 2000b; 16: 97-100., Marcilla et al. 2002Marcilla A, Bargues MD, Abad-Franch F, Panzera F, Carcavallo RU, Noireau F, et al. Nuclear rDNA ITS-2 sequences reveal polyphyly of Panstrongylus species (Hemiptera: Reduviidae: Triatominae), vectors of Trypanosoma cruzi. Infect Genet Evol. 2002; 1: 225-235., Barbosa et al. 2003Barbosa SE, Dujardin JP, Soares RPP, Pires HHR, Margonari C, Romanha AJ, et al. Interpopulation variability among Panstrongylus megistus (Hemiptera: Reduviidae) from Brazil. J Med Entomol. 2003; 40: 411-20., 2006Barbosa SE, Belisário CJ, Souza RCM, Paula AX, Linardi PM, Romanha AJ, et al. Biogeography of Brazilian populations of Panstrongylus megistus (Hemiptera, Reduviidae, Triatominae) based on molecular marker and paleovegetational data. Acta Trop. 2006; 99: 144-54., Almeida et al. 2008Almeida CE, Pacheco RS, Haag K, Dupas S, Dotson, Costa J. Inferring from the Cyt B gene the Triatoma brasiliensis Neiva, 1911 (Hemiptera: Reduviidae: Triatominae) genetic structure and domiciliary infestation in the state of Paraíba, Brazil. Am J Trop Med. 2008; 78: 791-802., Kopp et al. 2009Kopp RL, Thomaz-Socool V, Klisiowicz DR, Membrive N, Barata MB, Jurberg J, et al. Phenetic analysis of Panstrongylus megistus Burmeister, 1835 (Hemiptera: Reduviidae: Triatominae) in the state of Paraná-Brazil. Braz Arch Biol Technol. 2009; 52(2): 349-57., Cavassin et al. 2014Cavassin FB, Kuehn CC, Kopp RL, Thomaz-Soccol V, da Rosa JA, Luz E, et al. Genetic variability and geographical diversity of the main Chagas’ disease vector Panstrongylus megistus (Hemiptera: Triatominae) in Brazil based on ribossomal DNA intergenic sequences. J Med Entomol. 2014; 51(3): 616-28., González-Brítez et al. 2014González-Brítez N, Carrasco HJ, Purroy CEM, Feliciangeli MD, Maldonado M, López E, et al. Genetic and morphometric variability of Triatoma sordida (Hemiptera: Reduviidae) from the eastern and western areas of Paraguay. Front Public Health. 2014; 2: 149., Panzera et al. 2015Panzera F, Pita S, Nattero J, Panzera Y, Galvão C, Chavez T, et al. Cryptic speciation in the Triatoma sordida subcomplex (Hemiptera, Reduviidae) revealed by chromosomal markers. Parasit Vectors. 2015; 8: 495-504.).
Therefore, this study aimed to characterise the genetic variability of T. sordida populations collected in persistently infested areas of Minas Gerais state, Brazil, using the mitochondrial (mt) cytochrome b gene (cyt b). To our knowledge, this is the first study to characterise the genetic diversity of insects from areas with reports of persistent triatomine reinfestations despite the chemical control activities existence in accordance with the Brazilian Ministry of Health.
MATERIALS AND METHODS
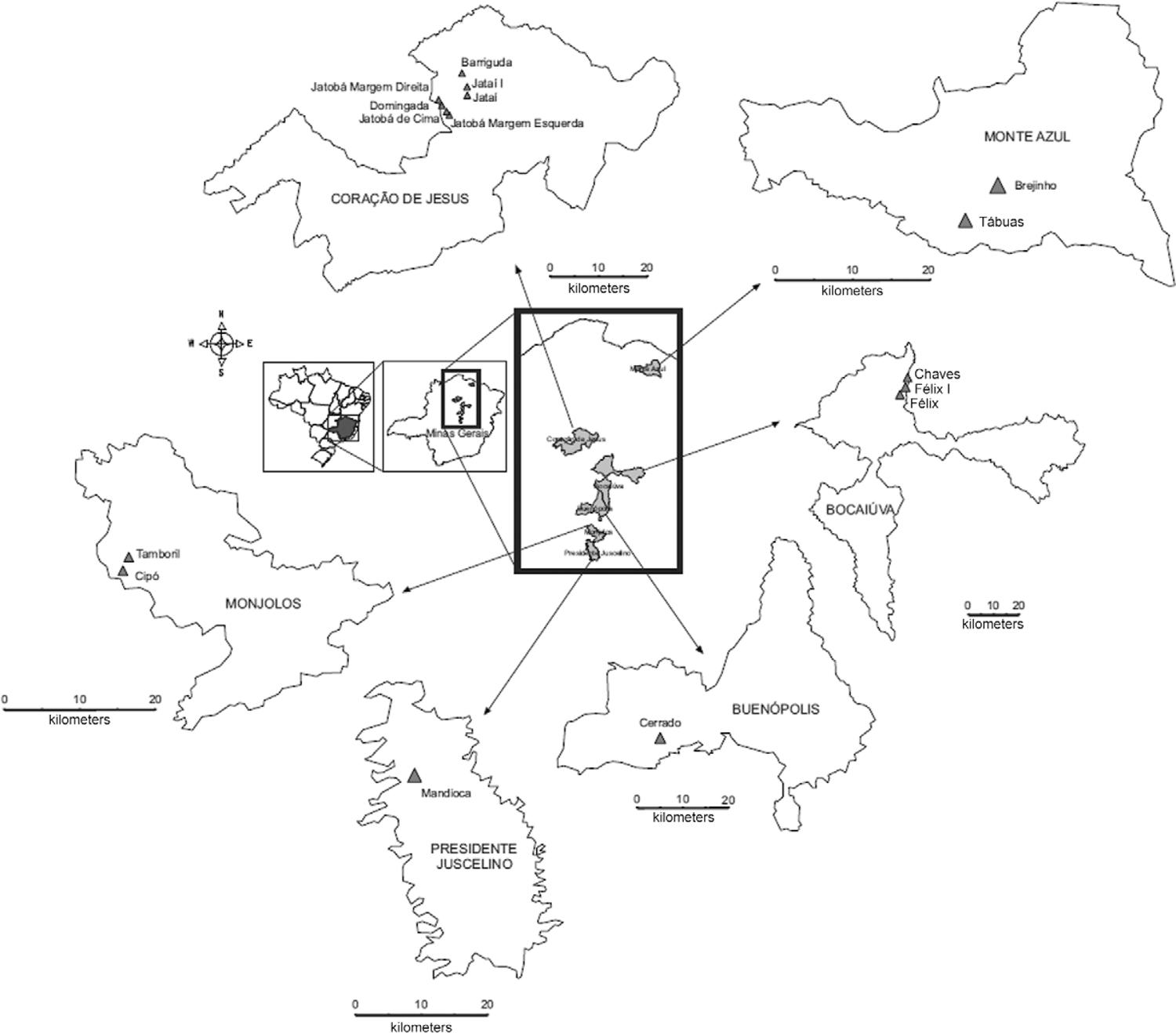
Insects’ origins - The studied populations were manually collected in 2007 with the assistance of technicians from Gerência Regional de Saúde de Montes Claros and Sete Lagoas, Minas Gerais (MG), Brazil, without using a dislodging agent. The insects came from peridomiciles in the central (Monjolos - 18º 19’ 30” S 44º 07’ 08” O; Presidente Juscelino - 18º 38’ 13” S 44º 03’ 28” O; Buenópolis - 17º 52’ 22” S 44º 10’ 48” O) and northern (Monte Azul - 15º 09’ 18” S 42º 52’ 30” O; Coração de Jesus - 16º 41’ 06” S 44º 21’ 54” O; and Bocaiúva - 17º 06’ 28” S 43º 48’ 54” O) areas of MG state, Brazil (Fig. 1). In these areas a Chagas Disease Control Programme was undertaken, and applied continuously and systematically over the last 30 years through applications of residual insecticides. Adults and nymphs of the parental generation were used to perform the experiments.
: map of Minas Gerais, Brazil, showing the study collection areas for Triatoma sordida populations.
DNA extraction, amplification and sequencing - Genomic DNA extractions from legs (two per specimen) were performed using the protocol described by Balbino et al. (2006)Balbino VQ, Abreu IVC, Sonoda IV, Melo MA, de Andrade PP, de Castro JA, et al. Genetic structure of natural populations of the sandfly Lutzomyia longipalpis (Diptera: Psychodidae) from the Brazilian northeastern area. Acta Trop. 2006; 98: 15-24.. The samples were subjected to polymerase chain reaction (PCR) using primers targeting the cyt b gene: CYT BF-5` GGACAAATATCATGAGGAGCAACAG 3` and CYT BR-5` ATTACTCCTCCTAGCTTATTAGGAATTG 3` (Lyman et al. 1999Lyman DF, Monteiro FA, Escalante AA, Cordon-Rosales C, Wesson DM, Dujardin JP, et al. Mitochondrial DNA sequences variation among triatomines vectors of Chagas’s disease. Am J Trop Med Hyg. 1999; 60(3): 377-86.). Briefly, all PCR reactions were performed in 20 μL volumes containing 1 pmol of each primer: 1.5 U Taq DNA Polymerase (Invitrogen, La Jolla, CA), 3 mM MgCl2, 0.2 mM of each dNTP and ~30 ng DNA. Amplifications were carried out in an Eppendorf Mastercycler Gradient (Eppendorf, Germany) using the following reaction conditions: 95ºC for 5 min, 30 cycles at 95ºC for 45 s, annealing at 50ºC for 45 s, 72ºC for 1 min, and a final extension step at 72ºC for 10 min. The amplified PCR products were purified using a GFX-96 PCR kit (Amersham Biosciences, Little Chalfont, UK) and directly sequenced with specific primers (CYT BF and CYT BR) using a DYEnamicTM ET dye terminator kit (Amersham Biosciences, Little Chalfont, UK). The products were analysed on a MegaBace 500 automated DNA sequencer (Amersham). The isolate sequences were sequenced at least twice for each strand from independent PCR amplifications.
Sequence analysis - Sequence alignment was performed using the MUSCLE multiple alignment program (Edgar 2004Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004; 32(5): 1792-7.). The number of segregating sites and haplotypes, as well estimates of nucleotide diversity (π, average number of substitutions between any two sequences, assuming that the sample is random) and their standard deviations were calculated using DnaSP 5.1 software (Librado & Rozas 2009Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009; 25: 1451-2.). Between-population differentiations were measured using the pairwise fixation index (FST) (Wright 1951)Wright S. The genetical structure of populations. Ann Eugen. 1951; 15(4): 323-54. with Arlequin 3.5 software (Excoffier & Lischer 2010)Excoffier L, Lischer HEL. Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Resour. 2010; 10(3): 564-7.. The correlation between pairwise population genetic distances (FST) and geographical distances was estimated by nonparametric Spearman’s rank correlation. Phylogenetic trees were reconstructed by the maximum likelihood method in PhyML 3.0 (Guindon et al. 2010)Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 2010; 59: 307-21. using the Hasegawa-Kishino-Yano model with gamma distributed rate variation among sites (Hasegawa et al. 1985)Hasegawa M, Kishino H, Yano T. Dating the human-ape split by a molecular clock of mitochondrial DNA. J Mol Evol. 1985; 22: 160-74.. jModeltest was used to assess the best fit model of nucleotide substitution (Posada 2008)Posada D. jModelTest: phylogenetic model averaging. Mol Biol Evol. 2008; 25: 1253-6.. The reliability of clustering patterns in trees was assessed by 1,000 bootstrap replicates (Felsenstein 1985)Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985; 39: 783-91.. We used the cyt b sequences of P. megistus (GenBank accession number: AF045722.1) and Rhodnius prolixus (EF011726.1) as outgroups. A sequence of Bolivian isolate (AF045730.1) was used as a reference in this study (Monteiro et al. 1999)Monteiro FA, Pérez R, Panzera F, Dujardin J-P, Galvão C, Rocha D, et al. Mitochondrial DNA variation of Triatoma infestans populations and its implication on the specific status of T. melanosoma. Mem Inst Oswaldo Cruz. 1999; 94(Suppl. 1): 229-38.. The complete description of the sequences analysed here is shown in Supplementary Table SUPPLEMENTARY TABLE Description of cytochrome b haplotypes of Triatoma sordida isolates Haplotype identification Number of isolates Isolates description (Accession number)b Geographical origin Country Municipalityc H1a 77 KR822185; KC249289; KC249292; KC249294 Brazil Buenópolis/Cerrado (3), Coração de Jesus/Jatobá (1)/Jatobá de Cima (9)/Jataí (4), Bocaiúva/Chaves (10)/Félix (8)/Félix I (7), Monte Azul/Brejinho (9)/Tábuas (9), Monjolos/Cipó (1)/Tamboril (6), Presidente Juscelino/Mandioca (7), Others regions H2 11 KR822186; KC608980 Buenópolis/Cerrado (1), Coração de Jesus/Domingada (7)/Jatobá de Cima (1), Monte Azul/Brejinho (1), Others regions H3 1 KR822187 Buenópolis/Cerrado H4 4 KR822188 Buenópolis/Cerrado (2), Monte Azul/Tábuas (2) H5 9 KR822189 Coração de Jesus/Barriguda (7), Monte Azul/Tábuas (2) H6 1 KR822190 Coração de Jesus/Barriguda H7 1 KR822191 Coração de Jesus/Barriguda H8 13 KR822192 Coração de Jesus/Domingada (5)/Jatobá (1)/Jataí (3), Monte Azul/Brejinho (2)/Tábuas (1), Monjolos/Tamboril (1) H9 7 KR822193 Coração de Jesus/Jatobá H10 1 KR822194 Coração de Jesus/Jatobá de Cima H11 1 KR822195 Coração de Jesus/Jatobá de Cima H12 1 KR822196 Coração de Jesus/Jataí H13 1 KR822197 Monjolos/Cipó H14 1 KR822198 Monjolos/Cipó H15 1 KR822199 Monjolos/Tamboril H16 1 KC249295 Argentina H17 1 KC249293 Bolivia H18 1 KC249291 Bolivia H19 1 HQ333243 Bolivia H20 1 HQ333242 Bolivia H21 1 AF045730 Bolivia a: the haplotypes were reconstructed from a sequence of 233 bp (from nt 86-318) analysed from the cyt b gene and correspond to the haplotypes used to infer the phylogenetic relationships among T. sordida isolates; b: GenBank accession numbers of the haplotype sequences of cyt b from Brazilian isolates and isolate sequences available from GenBank from other regions used in our analysis; c: the number of Brazilian isolates characterised by the haplotype is indicated in parentheses. . This study was approved by the Animal Ethics Committee of Fundação Oswaldo Cruz (number 29/14-1).
RESULTS
Nucleotide and haplotype diversity of cyt b among Brazilian isolates - We sequenced a fragment consisting of 233 bp of the cyt b gene from 126 isolates of T. sordida originating from 14 different areas of MG state in southwestern Brazil. The sequenced region (from nt 86-318) corresponds to the most polymorphic portion of the gene. 13 polymorphic sites (six synonymous substitutions and seven nonsynonymous substitutions) were identified, with an overall nucleotide diversity of 0.004 (Table I). For all 14 sampled areas of MG, low nucleotide diversity was estimated with π values ranging from 0.002-0.006. We also analysed five sequences of cyt b available in GenBank of isolates from Bolivia. 28 polymorphic sites were identified in the same 233 bp. We observed extensive variability with an overall nucleotide diversity of 0.053 in the Bolivian samples.
The polymorphisms obtained were arranged in 15 haplotypes (Table I, Fig. 2), corresponding to a mean haplotype diversity of 0.633. Among these haplotypes, only five (H1, H2, H5, H8 and H9) had frequencies above five percent. A single haplotype (H1) was predominant in 60% of the isolates, which were distributed across all but two of the sampled areas (Fig. 2). With few exceptions, H1 was the most frequent haplotype in the areas where it occurred. Different unique patterns of haplotypic variation with other predominant haplotypes were observed in Jatobá, Domingada and Barriguda (municipality of Coração de Jesus). The cyt b haplotype sequences obtained here were deposited in GenBank with accession numbers KR822185, KR822186, KR822187, KR822188, KR822189, KR822190, KR822191, KR822192, KR822193, KR822194, KR822195, KR822196, KR822197, KR822198 and KR822199.
: haplotype frequencies of cytochrome b in Minas Gerais state, Brazil. The scale bar shows the frequency of each haplotype per area indicated in the horizontal boxes.
Interpopulation genetic diversity - Comparative analyses of genetic variability between populations of T. sordida from the 14 areas in MG state showed that three populations (Jatobá, Barriguda and Domingada) are distinctly different from all other studied populations (Table II). The FST values of these populations ranged from 0.29-0.80. On the other hand, for the majority of populations, we observed overall low genetic differentiation and FST values were not significant (p > 0.05). Moreover, the FST values did not correlate with the geographic distance between populations (Spearman correlation coefficient = -0.160, p = 0.129).
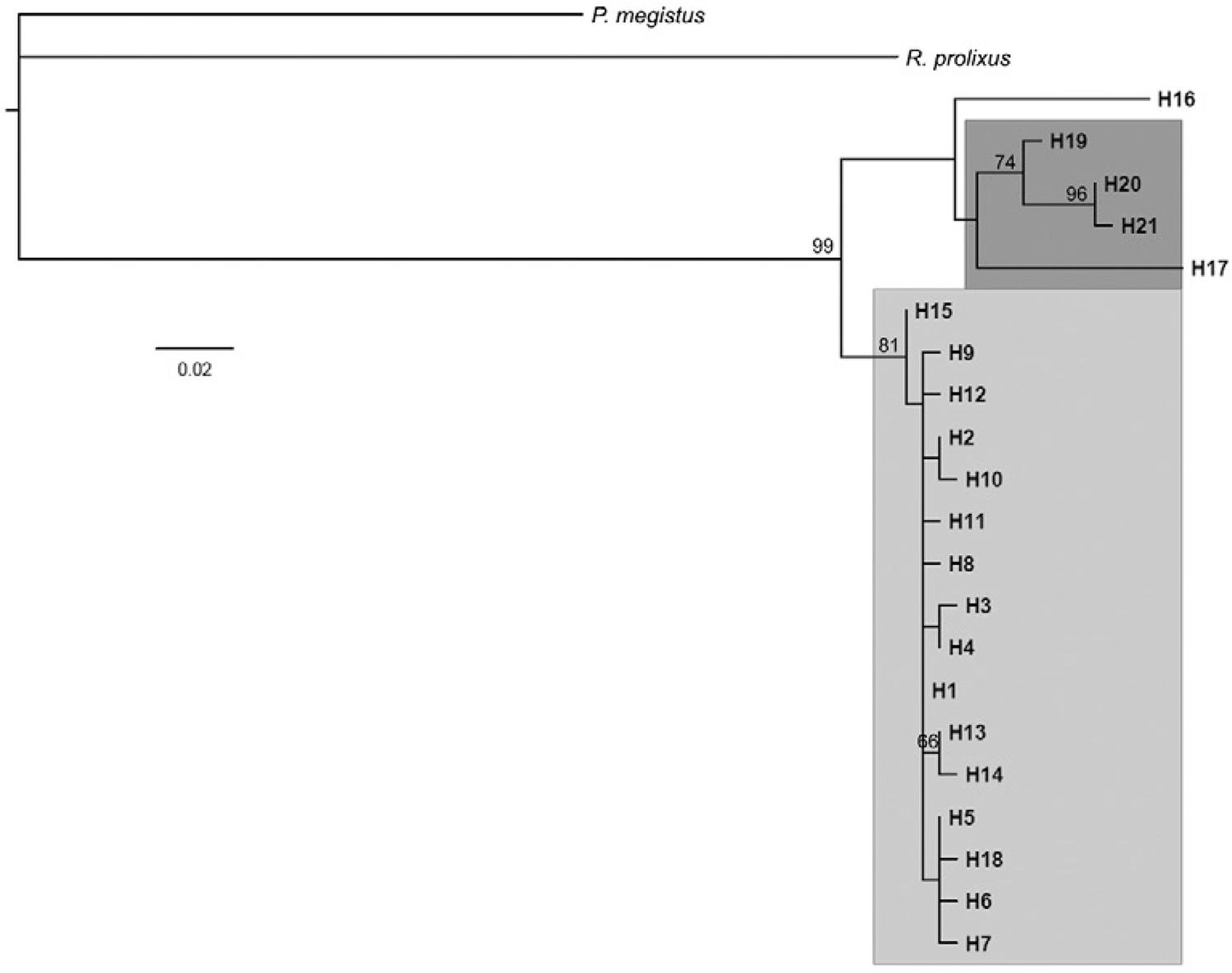
Phylogenetic analysis - The phylogenetic relationships among T. sordida isolates from different areas including Brazil, Argentina and Bolivia were inferred from the cyt b sequences (Fig. 3). The Brazilian isolates felt into a group of isolates separated from those from the other countries with high support (bootstrap value of 81%). Only one sample from Bolivia (haplotype 18) clustered with the same group of Brazilian isolates. The other Bolivian isolates, represented by haplotypes 19, 20 and 21, were placed in a separate group with high support.
: best maximum likelihood tree reconstructed. The numbers above the branches represent clade support higher than 50. The description of haplotypes is shown in Supplementary Table. The cytochrome b sequences of Panstrongylus megistus and Rhodnius prolixus were used as an outgroup. Clades were highlighted according to the geographic origin of haplotypes: dark grey = Bolivia; light grey = Brazil.
DISCUSSION
There is no doubt that chemical control of triatomine populations was successful in most Southern Cone countries, resulting in Uruguay, Chile and Brazil being certified as free of vector transmission by T. infestans (Dias 2006Dias JCP. Doença de Chagas: sucessos e desafios. Cad Saude Publica. 2006; 22: 2020-1.). Parallel T. infestans elimination effort in Brazil, was also reduced the density of other triatomine species (Dias et al. 2002Dias JCP, Machado EMM, Borges EC, Moreira EF, Gontijo C, Azeredo BVM. Doença de Chagas em Lassance, MG. Reavaliação clínico-epidemiológica 90 anos após a descoberta de Carlos Chagas. Rev Soc Bras Med Trop. 2002; 35: 167-76.). However, reinfestation by native triatomines, with a high capacity for invasion/colonisation of domestic units persists in Brazil in substantially different epidemiological settings, requiring individual evaluations of the various scenarios. Persistent reinfestations that particularly stand out include those by T. brasiliensis in semiarid areas, by P. megistus in areas associated with residual forests and by T. sordida in the Cerrado (Pessoa et al. 2015bPessoa GCD, Viñaes PA, Rosa AC, Diotaiuti L. History of insecticide resistance of Triatominae vectors. Rev Soc Bras Med Trop. 2015b; 48: 380-9.).
In this sense, despite the importance of knowing the genetic structure and dynamics of triatomine infestation/reinfestation for the design of chemical control activities, there are few studies in the literature. Studies with Brazilian populations of P. megistus using isoenzymes (Kopp et al. 2009Kopp RL, Thomaz-Socool V, Klisiowicz DR, Membrive N, Barata MB, Jurberg J, et al. Phenetic analysis of Panstrongylus megistus Burmeister, 1835 (Hemiptera: Reduviidae: Triatominae) in the state of Paraná-Brazil. Braz Arch Biol Technol. 2009; 52(2): 349-57.), Random Amplified Polymorphic DNA (RAPD) (Barbosa et al. 2003Barbosa SE, Dujardin JP, Soares RPP, Pires HHR, Margonari C, Romanha AJ, et al. Interpopulation variability among Panstrongylus megistus (Hemiptera: Reduviidae) from Brazil. J Med Entomol. 2003; 40: 411-20., 2006Barbosa SE, Belisário CJ, Souza RCM, Paula AX, Linardi PM, Romanha AJ, et al. Biogeography of Brazilian populations of Panstrongylus megistus (Hemiptera, Reduviidae, Triatominae) based on molecular marker and paleovegetational data. Acta Trop. 2006; 99: 144-54.) and ribosomal intergenic sequences (ITS1 and ITS2) (Cavassin et al. 2014Cavassin FB, Kuehn CC, Kopp RL, Thomaz-Soccol V, da Rosa JA, Luz E, et al. Genetic variability and geographical diversity of the main Chagas’ disease vector Panstrongylus megistus (Hemiptera: Triatominae) in Brazil based on ribossomal DNA intergenic sequences. J Med Entomol. 2014; 51(3): 616-28.) revealed populations with strong population structure and reduced genetic diversity that was directly related to the geographic distance between the studied areas. This same pattern was observed in Brazilian populations of T. brasiliensis using isoenzymes (Costa et al. 1997Costa J, Freitas-Sibajev MGR, Marchon-Silva V, Pires MQ, Pacheco RS. Isoenzymes detect variation in populations of Triatoma brasiliensis (Hemiptera: Reduviidae: Triatominae). Mem Inst Oswaldo Cruz. 1997; 92(4): 459-64.), RAPD (Borges et al. 2000aBorges EC, Dujardin JP, Schofield CJ, Romanha AJ, Diotaiuti L. Genetic variability of Triatoma brasiliensis (Hemiptera: Reduviidae) populations. J Med Entomol. 2000a; 37(6): 872-7., bBorges E, Romanha AJ, Diotaiuti L. Use of random amplified polymorphic DNA (RAPD) in the population study of Triatoma brasiliensis Neiva, 1911. Cad Saude Publica. 2000b; 16: 97-100.) and the cyt b gene (Monteiro et al. 2004Monteiro FA, Donnelly MJ, Beard CB, Costa J. Nested clade and phylogeographic analyses of the Chagas disease vector Triatoma brasiliensis in Northeast Brazil. Mol Phylogenet Evol. 2004; 32(1): 46-56., Almeida et al. 2008Almeida CE, Pacheco RS, Haag K, Dupas S, Dotson, Costa J. Inferring from the Cyt B gene the Triatoma brasiliensis Neiva, 1911 (Hemiptera: Reduviidae: Triatominae) genetic structure and domiciliary infestation in the state of Paraíba, Brazil. Am J Trop Med. 2008; 78: 791-802.).
To date, there has been only one study that investigated the genetic diversity of natural Brazilian populations of T. sordida (Monteiro et al. 2009Monteiro FA, Jurberg J, Lazoski C. Very low levels of genetic variation in natural peridomestic populations of the Chagas disease vector Triatoma sordida (Hemiptera: Reduviidae) in Southeastern Brazil. Am J Trop Med Hyg. 2009; 81: 223-7.). Monteiro et al. (2009)Monteiro FA, Jurberg J, Lazoski C. Very low levels of genetic variation in natural peridomestic populations of the Chagas disease vector Triatoma sordida (Hemiptera: Reduviidae) in Southeastern Brazil. Am J Trop Med Hyg. 2009; 81: 223-7. determined the genetic variation levels and the population structure for 181 specimens of T. sordida collected from four municipalities of MG state (Espinosa, Mamonas, Januária and Corinto) by analysing 28 allozyme loci. None of these loci presented fixed differences between any pair of populations, and only two revealed polymorphisms, accounting for extremely low levels of heterozygosity (He = 0.027). Regardless of the low levels of polymorphism obtained, the results indicated the existence of genetic structure among the populations analysed (FST = 0.214). In turn, the studies using isoenzymes (Noireau et al. 1999Noireau F, Zegarra M, Ordoñez J, Gutierrez T, Dujardin J-P. Genetic structure of Triatoma sordida (Hemiptera: Reduviidae) domestic populations from Bolivia: application on control interventions. Mem Inst Oswaldo Cruz. 1999; 94(3): 347-51.), RAPD (González-Brítez et al. 2014González-Brítez N, Carrasco HJ, Purroy CEM, Feliciangeli MD, Maldonado M, López E, et al. Genetic and morphometric variability of Triatoma sordida (Hemiptera: Reduviidae) from the eastern and western areas of Paraguay. Front Public Health. 2014; 2: 149.) showed similar results for T. sordida populations in Bolivia and Paraguay, respectively.
Corroborating Monteiro et al. (2009)Monteiro FA, Jurberg J, Lazoski C. Very low levels of genetic variation in natural peridomestic populations of the Chagas disease vector Triatoma sordida (Hemiptera: Reduviidae) in Southeastern Brazil. Am J Trop Med Hyg. 2009; 81: 223-7., we report that the genetic structure of natural Brazilian populations of T. sordida has low levels of genetic variability. Overall, the sequencing of a polymorphic fragment of the cyt b gene of T. sordida from 14 areas of MG, Brazil, showed low genetic diversity in the 126 isolates analysed. The nucleotide diversity of isolates from Bolivia was approximately 13 times greater than that of isolates from Brazil. The analysis of haplotypic data confirmed these results. A predominant haplotype (H1) was obtained in 60% of samples. Most of the 15 identified haplotypes were observed at low frequency, and some were exclusive to only one of the study populations. It is noteworthy that we obtained a very different haplotype profile for the three populations from Coração de Jesus municipality (Barriguda, Domingada and Jatobá). The interpopulation genetic diversity analysis also revealed strong genetic structuring - particularly in populations from Coração de Jesus - compared with other study populations of this insect vector (FST > 0.3).
The low genetic diversity and strong genetic structuring of the population samples from MG may be related to different factors, alone or in combination, as follows: (i) possible geographical isolation due to an obstacle to triatomine flow between neighbouring localities (Noireau et al. 1998Noireau F, Gutierrez T, Zegarra M, Flores R, Breniere F, Cardoso R, et al. Cryptic speciation in Triatoma sordida (Hemiptera: Reduviidae) from the Bolivian Chaco. Trop Med Int Health. 1998; 3: 364-72., González-Brítez 2013González-Brítez N. Population dynamics of triatomines (Hemiptera: Reduviidae) related to Trypanosoma cruzi in Paraguay with emphasis on Triatoma sordida. Mem Inst Investig Cienc Salud. 2013; 11(2): 105-11., González-Brítez et al. 2014González-Brítez N, Carrasco HJ, Purroy CEM, Feliciangeli MD, Maldonado M, López E, et al. Genetic and morphometric variability of Triatoma sordida (Hemiptera: Reduviidae) from the eastern and western areas of Paraguay. Front Public Health. 2014; 2: 149.); (ii) focal distribution of insects in small colonies usually comprised of a few individuals, thereby limiting gene flow between them; (iii) the low dispersal capacity of T. sordida (Monteiro et al. 2009Monteiro FA, Jurberg J, Lazoski C. Very low levels of genetic variation in natural peridomestic populations of the Chagas disease vector Triatoma sordida (Hemiptera: Reduviidae) in Southeastern Brazil. Am J Trop Med Hyg. 2009; 81: 223-7.); and (iv) the long Triatominae life cycle, which ensures that contributions from young adults able to reproduce (and consequently exchange genetic material) occurs over long intervals that differ among triatominic species (Forattini et al. 1974Forattini OW, Ferreira OA, Rocha e Silva EO, Rabello EX. Aspectos ecológicos da Tripanossomíase americana. VI. Persistência do Triatoma sordida após alteração ambiental e suas possíveis relações com a dispersão da espécie. Rev Saude Publica. 1974; 8: 265-82.). Moreover, considering that this area has suffered continuous pressure from insecticides used since the 1950s to triatomines control (Silveira 1994Silveira AC. Controle da transmissão vetorial da doença de Chagas no Brasil. Taller sobre evaluación de efecto insecticida sobre Triatominos. Acta Toxicol Argent. 1994; 2: 29-58., Mendes 2008Mendes PC. Aspectos ecológicos e sociais da doença de Chagas no Município de Uberlândia, Minas Gerais - Brasil [Tese de Doutorado]. Uberlândia; Universidade Federal de Uberlândia; 2008. 249 pp., Pessoa 2012Pessoa GCD. Perfil da suscetibilidade a deltametrina em populações de Triatoma sordida (Hemiptera: Reduviidae) do estado de Minas Gerais procedentes de áreas com infestação persistente [Tese de Doutorado]. Belo Horizonte: Universidade Federal de Minas Gerais; 2012. 179 pp.), it could be expected that the studied populations would show a low genetic variability. There is evidence that genetic diversity is reduced in areas treated with insecticides compared with untreated areas due to bottleneck events (Pérez de Rosas et al. 2007Pérez de Rosas AR, Segura EL, García BA. Microssatellite analysis of genetic structure in natural Triatoma infestans (Hemiptera: Reduviidae) populations from Argentina: its implication in assessing the effectiveness of Chagas disease vector control programmes. Mol Ecol. 2007; 16(7): 1401-12., 2008Pérez de Rosas AR, Segura EL, Fichera L, Garcia BA. Macrogeographic and microgeographic genetic structure of the Chagas disease vector Triatoma infestans (Hemiptera: Reduviidae) from Catamarca, Argentina. Genetica. 2008; 133(3): 247-60.). In this context, the study area of this work overlaps with the dengue and leishmaniasis endemics. These programmes execute their vector chemical control activities simultaneously and independently. In addition, the utilisation of agricultural and domestic insecticides exacerbates the chemical pressure on triatomine populations of the area, which can contribute to indiscriminate and unwanted increases in insecticide resistance (Pessoa 2012Pessoa GCD. Perfil da suscetibilidade a deltametrina em populações de Triatoma sordida (Hemiptera: Reduviidae) do estado de Minas Gerais procedentes de áreas com infestação persistente [Tese de Doutorado]. Belo Horizonte: Universidade Federal de Minas Gerais; 2012. 179 pp.). Pessoa et al. (2015a)Pessoa GCD, Obara MT, Rezende JC, de Mello BV, Ferraz ML, Diotaiuti L. Deltamethrin toxicological profile of peridomestic Triatoma sordida in the North of Minas Gerais, Brazil. Parasit Vectors. 2015a; 8: 263-9. - for the same populations studied in this work - revealed the largest resistance ratios ever identified for populations of T. sordida (RR50 2.5-7.2). Of the 14 studied populations, all populations presented equal or higher slopes compared to the Susceptibility Reference Lineage (SRL), suggesting low genetic variation when compared to SRL. Moreover, different deltamethrin susceptibility profiles were identified in populations from distinct locations that, nevertheless, belong to the same municipalities (ex. Localities of Coração de Jesus), reinforcing the complexity of the resistance phenotype, not only at the macrogeographical level, but at the microgeographical level. It should be noted that the insecticide used in the field does not appear to have homogeneous effects over different populations; consequently, it applies different selection pressures to different populations, which is a reflection of the genetic variability among those same populations. The complexity of the peridomicile itself cannot be neglected, either. The large variety of ecotopes that exist in peridomiciles makes spraying these ecotopes an exhaustive job. Separating all the material accumulated there for spraying is operationally impossible for the responsible health agent. Consequently, T. sordida (eggs, nymphs and adults) remain even after the application of the insecticide, hidden deep in piles of firewood, under barn roofs and in a variety of other nearly inaccessible places, staying free from contact with the active chemical and/or in contact only with sublethal doses, which favours their multiplication in these ecotopes (Diotaiuti et al. 1998Diotaiuti L, Azeredo BVM, Busek SCU, Fernandes AJ. Controle do Triatoma sordida no peridomicílio rural do Município de Porteirinha, Minas Gerais, Brasil. Rev Panam Salud Publica. 1998; 3: 21-5.). In this case, triatomine populations may be formed from the surviving specimens and could found a new colony - with reduced genetic variability.
For populations with greater relative haplotype diversity, one should consider possible flows of insects from wild to domestic environments that occur in response to environmental changes caused by human action in the study area. In agricultural areas and livestock operations, substantial modifications to the natural environment have led to the displacement or disappearance of ref- uges and natural food sources of T. sordida. As a result, the insect seeks artificial alternative environments in which it can survive. It appears as if changes in vegetation coverage, at least to some extent, cause dispersion of T. sordida. Wide infestation in households closer to wild environments suggests a triatomine (nymph to adult) recolonisation flow to artificial environments from natural ecotopes (Forattini et al. 1971Forattini OP, Rocha e Silva EO, Ferreira AO, Rabello EX, Patolli DGB. Aspectos ecológicos da tripanossomíase Americana. III. Dispersão local de triatomíneos, com especial referência ao Triatoma sordida. Rev Saude Publica. 1971; 5: 193-205.), thus contributing to the observed persistent reinfestations in the area.
The phylogenetic analysis performed in this study showed that T. sordida specimens from Argentina and Bolivia are grouped into a separate cluster than the Brazilian populations. Although only one Argentinean sample and five Bolivian samples were compared, there was a closer relationship between the Argentinian specimen and most Bolivian samples of T. sordida. However, one Bolivian isolate was grouped with the Brazilian samples. Cytogenetic studies combined with isoenzymes using Argentinean and Brazilian populations of T. sordida corroborate the results of the present study and also showed high levels of genetic differentiation (Panzera et al. 1997Panzera F, Hornos S, Pereira J, Cestau R, Canale D, Diotaiuti L, et al. Genetic variability and geographic differentiation among three species of triatomine bugs (Hemiptera: Reduviidae). Am J Trop Med Hyg. 1997; 57: 732-9.). In addition, Justi et al. (2014)Justi AS, Russo CA, Mallet JRS, Obara MT, Galvão C. Molecular phylogeny of Triatomini (Hemiptera: Reduviidae: Triatominae). Parasit Vectors. 2014; 7: 149-57. compared the genetic variability among eight specimens of T. sordida from Brazil, Bolivia and Argentina using different mt markers (16S, cyt oxidase I, cyt oxidase II and cyt b) and two nuclear markers (18S and 28S), showing that the T. sordida from group two were restricted to Chaco, while those from group one were restricted to Bolivia and Brazil (Forattini 1980Forattini OP. Biogeografia, origem e distribuição da domiciliação de triatomíneos no Brasil. Rev Saude Publica. 1980; 14: 265-99., Noireau et al. 1996Noireau F, Breniére F, Cardozo L, Bosseno MF, Vargas F, Peredo C, et al. Current spread of Triatoma infestans at the expense of Triatoma sordida in Bolivia. Mem Inst Oswaldo Cruz. 1996; 91(93): 271-2.).
SUPPLEMENTARY TABLE
ACKNOWLEDGEMENTS
To Secretaria de Saúde do Estado de Minas Gerais, for the collection of Triatominae, and Dr João Victor Leite Dias, for a map and suggestions.
REFERENCES
- Almeida CE, Pacheco RS, Haag K, Dupas S, Dotson, Costa J. Inferring from the Cyt B gene the Triatoma brasiliensis Neiva, 1911 (Hemiptera: Reduviidae: Triatominae) genetic structure and domiciliary infestation in the state of Paraíba, Brazil. Am J Trop Med. 2008; 78: 791-802.
- Balbino VQ, Abreu IVC, Sonoda IV, Melo MA, de Andrade PP, de Castro JA, et al. Genetic structure of natural populations of the sandfly Lutzomyia longipalpis (Diptera: Psychodidae) from the Brazilian northeastern area. Acta Trop. 2006; 98: 15-24.
- Barbosa SE, Belisário CJ, Souza RCM, Paula AX, Linardi PM, Romanha AJ, et al. Biogeography of Brazilian populations of Panstrongylus megistus (Hemiptera, Reduviidae, Triatominae) based on molecular marker and paleovegetational data. Acta Trop. 2006; 99: 144-54.
- Barbosa SE, Dujardin JP, Soares RPP, Pires HHR, Margonari C, Romanha AJ, et al. Interpopulation variability among Panstrongylus megistus (Hemiptera: Reduviidae) from Brazil. J Med Entomol. 2003; 40: 411-20.
- Borges E, Romanha AJ, Diotaiuti L. Use of random amplified polymorphic DNA (RAPD) in the population study of Triatoma brasiliensis Neiva, 1911. Cad Saude Publica. 2000b; 16: 97-100.
- Borges EC, Dujardin JP, Schofield CJ, Romanha AJ, Diotaiuti L. Genetic variability of Triatoma brasiliensis (Hemiptera: Reduviidae) populations. J Med Entomol. 2000a; 37(6): 872-7.
- Carcavallo RU, de Casas SIC, Sherlock I, Girón IG, Jurberg J, Galvão C, et al. Distribuição geográfica e dispersão altilatitudinal. In: Carcavallo RU, Girón IG, Jurberg J, Lent H, orgs. Atlas dos vetores da doença de Chagas nas Américas. Vol. III. Rio de Janeiro: Fiocruz; 1997. 350 pp.
- Cavassin FB, Kuehn CC, Kopp RL, Thomaz-Soccol V, da Rosa JA, Luz E, et al. Genetic variability and geographical diversity of the main Chagas’ disease vector Panstrongylus megistus (Hemiptera: Triatominae) in Brazil based on ribossomal DNA intergenic sequences. J Med Entomol. 2014; 51(3): 616-28.
- Costa J, Freitas-Sibajev MGR, Marchon-Silva V, Pires MQ, Pacheco RS. Isoenzymes detect variation in populations of Triatoma brasiliensis (Hemiptera: Reduviidae: Triatominae). Mem Inst Oswaldo Cruz. 1997; 92(4): 459-64.
- Coura JR. O falso dilema sobre a luta antivetorial e as perspectivas de controle da Doença de Chagas no Brasil. Cad Saude Publica. 1993; 9: 514-18.
- Dias JCP, Machado EMM, Borges EC, Moreira EF, Gontijo C, Azeredo BVM. Doença de Chagas em Lassance, MG. Reavaliação clínico-epidemiológica 90 anos após a descoberta de Carlos Chagas. Rev Soc Bras Med Trop. 2002; 35: 167-76.
- Dias JCP. Doença de Chagas: sucessos e desafios. Cad Saude Publica. 2006; 22: 2020-1.
- Dias JP, Bastos C, Araújo E, Mascarenhas AV, Martins Netto E, Grassi F, et al. Acute Chagas disease outbreak associated with oral transmission. Rev Soc Bras Med Trop. 2008; 41(1): 296-300.
- Diotaiuti L, Azeredo BVM, Busek SCU, Fernandes AJ. Controle do Triatoma sordida no peridomicílio rural do Município de Porteirinha, Minas Gerais, Brasil. Rev Panam Salud Publica. 1998; 3: 21-5.
- Diotaiuti L, Pinto CT. Suscetibilidade biológica do Triatoma sordida e Triatoma infestans a deltametrina e lambdacyalotrina em condições de campo. Rev Soc Bras Med Trop. 1991; 24: 151-5.
- Diotaiuti L. Potencial vetorial do Triatoma sordida Rev Soc Bras Med Trop. 1995; 28: 38-41.
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004; 32(5): 1792-7.
- Excoffier L, Lischer HEL. Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Resour. 2010; 10(3): 564-7.
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985; 39: 783-91.
- Forattini OP, Rocha e Silva EO, Ferreira AO, Rabello EX, Patolli DGB. Aspectos ecológicos da tripanossomíase Americana. III. Dispersão local de triatomíneos, com especial referência ao Triatoma sordida. Rev Saude Publica. 1971; 5: 193-205.
- Forattini OP. Biogeografia, origem e distribuição da domiciliação de triatomíneos no Brasil. Rev Saude Publica. 1980; 14: 265-99.
- Forattini OW, Ferreira OA, Rocha e Silva EO, Rabello EX. Aspectos ecológicos da Tripanossomíase americana. VI. Persistência do Triatoma sordida após alteração ambiental e suas possíveis relações com a dispersão da espécie. Rev Saude Publica. 1974; 8: 265-82.
- Forattini OW, Rabello EX, Ferreira OA, Rocha e Silva EO, Santos JLF. Aspectos ecológicos da Tripanossomíase americana. XXI. Comportamento de espécies triatomíneas silvestres na reinfestação do intra e peridomicílio. Rev Saude Publica. 1984; 18: 185-208.
- Galvão C, Gurgel-Gonçalves R. Vetores conhecidos no Brasil. In: Galvão C, org. Vetores da doença de Chagas no Brasil. Curitiba: Sociedade Brasileira de Zoologia; 2015. 232 pp.
- González-Brítez N, Carrasco HJ, Purroy CEM, Feliciangeli MD, Maldonado M, López E, et al. Genetic and morphometric variability of Triatoma sordida (Hemiptera: Reduviidae) from the eastern and western areas of Paraguay. Front Public Health. 2014; 2: 149.
- González-Brítez N. Population dynamics of triatomines (Hemiptera: Reduviidae) related to Trypanosoma cruzi in Paraguay with emphasis on Triatoma sordida Mem Inst Investig Cienc Salud. 2013; 11(2): 105-11.
- Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 2010; 59: 307-21.
- Gurgel-Gonçalves R, Galvão C, Costa J, Peterson AT. Geographic distribution of Chagas disease vectors in Brazil based on ecological niche modeling. J Trop Med. 2012; 15: 326-41.
- Hasegawa M, Kishino H, Yano T. Dating the human-ape split by a molecular clock of mitochondrial DNA. J Mol Evol. 1985; 22: 160-74.
- Justi AS, Russo CA, Mallet JRS, Obara MT, Galvão C. Molecular phylogeny of Triatomini (Hemiptera: Reduviidae: Triatominae). Parasit Vectors. 2014; 7: 149-57.
- Kopp RL, Thomaz-Socool V, Klisiowicz DR, Membrive N, Barata MB, Jurberg J, et al. Phenetic analysis of Panstrongylus megistus Burmeister, 1835 (Hemiptera: Reduviidae: Triatominae) in the state of Paraná-Brazil. Braz Arch Biol Technol. 2009; 52(2): 349-57.
- Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009; 25: 1451-2.
- Lyman DF, Monteiro FA, Escalante AA, Cordon-Rosales C, Wesson DM, Dujardin JP, et al. Mitochondrial DNA sequences variation among triatomines vectors of Chagas’s disease. Am J Trop Med Hyg. 1999; 60(3): 377-86.
- Marcilla A, Bargues MD, Abad-Franch F, Panzera F, Carcavallo RU, Noireau F, et al. Nuclear rDNA ITS-2 sequences reveal polyphyly of Panstrongylus species (Hemiptera: Reduviidae: Triatominae), vectors of Trypanosoma cruzi Infect Genet Evol. 2002; 1: 225-235.
- Mendes PC. Aspectos ecológicos e sociais da doença de Chagas no Município de Uberlândia, Minas Gerais - Brasil [Tese de Doutorado]. Uberlândia; Universidade Federal de Uberlândia; 2008. 249 pp.
- Monteiro FA, Donnelly MJ, Beard CB, Costa J. Nested clade and phylogeographic analyses of the Chagas disease vector Triatoma brasiliensis in Northeast Brazil. Mol Phylogenet Evol. 2004; 32(1): 46-56.
- Monteiro FA, Jurberg J, Lazoski C. Very low levels of genetic variation in natural peridomestic populations of the Chagas disease vector Triatoma sordida (Hemiptera: Reduviidae) in Southeastern Brazil. Am J Trop Med Hyg. 2009; 81: 223-7.
- Monteiro FA, Pérez R, Panzera F, Dujardin J-P, Galvão C, Rocha D, et al. Mitochondrial DNA variation of Triatoma infestans populations and its implication on the specific status of T. melanosoma Mem Inst Oswaldo Cruz. 1999; 94(Suppl. 1): 229-38.
- MS - Ministério da Saúde (BR). Informações técnicas sobre a doença de Chagas e seus vetores no Brasil referente ao ano de 2014 [Internet]. 2015 [cited 2015 May 10]. Available from: portalsaude.gov.br
» portalsaude.gov.br - Noireau F, Breniére F, Cardozo L, Bosseno MF, Vargas F, Peredo C, et al. Current spread of Triatoma infestans at the expense of Triatoma sordida in Bolivia. Mem Inst Oswaldo Cruz. 1996; 91(93): 271-2.
- Noireau F, Gutierrez T, Zegarra M, Flores R, Breniere F, Cardoso R, et al. Cryptic speciation in Triatoma sordida (Hemiptera: Reduviidae) from the Bolivian Chaco. Trop Med Int Health. 1998; 3: 364-72.
- Noireau F, Zegarra M, Ordoñez J, Gutierrez T, Dujardin J-P. Genetic structure of Triatoma sordida (Hemiptera: Reduviidae) domestic populations from Bolivia: application on control interventions. Mem Inst Oswaldo Cruz. 1999; 94(3): 347-51.
- Panzera F, Hornos S, Pereira J, Cestau R, Canale D, Diotaiuti L, et al. Genetic variability and geographic differentiation among three species of triatomine bugs (Hemiptera: Reduviidae). Am J Trop Med Hyg. 1997; 57: 732-9.
- Panzera F, Pita S, Nattero J, Panzera Y, Galvão C, Chavez T, et al. Cryptic speciation in the Triatoma sordida subcomplex (Hemiptera, Reduviidae) revealed by chromosomal markers. Parasit Vectors. 2015; 8: 495-504.
- Pérez de Rosas AR, Segura EL, Fichera L, Garcia BA. Macrogeographic and microgeographic genetic structure of the Chagas disease vector Triatoma infestans (Hemiptera: Reduviidae) from Catamarca, Argentina. Genetica. 2008; 133(3): 247-60.
- Pérez de Rosas AR, Segura EL, García BA. Microssatellite analysis of genetic structure in natural Triatoma infestans (Hemiptera: Reduviidae) populations from Argentina: its implication in assessing the effectiveness of Chagas disease vector control programmes. Mol Ecol. 2007; 16(7): 1401-12.
- Pessoa GCD, Obara MT, Rezende JC, de Mello BV, Ferraz ML, Diotaiuti L. Deltamethrin toxicological profile of peridomestic Triatoma sordida in the North of Minas Gerais, Brazil. Parasit Vectors. 2015a; 8: 263-9.
- Pessoa GCD, Viñaes PA, Rosa AC, Diotaiuti L. History of insecticide resistance of Triatominae vectors. Rev Soc Bras Med Trop. 2015b; 48: 380-9.
- Pessoa GCD. Perfil da suscetibilidade a deltametrina em populações de Triatoma sordida (Hemiptera: Reduviidae) do estado de Minas Gerais procedentes de áreas com infestação persistente [Tese de Doutorado]. Belo Horizonte: Universidade Federal de Minas Gerais; 2012. 179 pp.
- Posada D. jModelTest: phylogenetic model averaging. Mol Biol Evol. 2008; 25: 1253-6.
- Rojas de Arias A, Abad-Franch F, Acosta N, López E, González N, Zerba E, et al. Post-control surveillance of Triatoma infestans and Triatoma sordida with chemically-baited sticky traps. PLoS Negl Trop Dis. 2012; 6: e1822.
- Silveira AC. Controle da transmissão vetorial da doença de Chagas no Brasil. Taller sobre evaluación de efecto insecticida sobre Triatominos. Acta Toxicol Argent. 1994; 2: 29-58.
- Wright S. The genetical structure of populations. Ann Eugen. 1951; 15(4): 323-54.
-
Financial support: CNPq, CPqRR, FIOCRUZ, SVS-MS, WHO.
Publication Dates
-
Publication in this collection
03 May 2016 -
Date of issue
May 2016
History
-
Received
17 Nov 2015 -
Accepted
17 Mar 2016