Abstract
The clay minerals have characteristics and properties that allow their applicability in the cosmetic area, being incorporated into formulations as an active principle. Therefore, the aim of this work is characterizing and evaluating the influence of a clay from Miracema do Norte, Tocantins, Brazil, named Clay V, on the physicochemical characteristics of a hydrophilic gel of Aristoflex®, a copolymer of the neutralized acryloyldimethyltaurate and vinylpyrrolidone sulphonic acid. Clay V was decontaminated and characterized through microbiological evaluation, crystalline phases present by X-Ray Diffraction (XRD), chemical composition by X-Ray Fluorescence (XRF), thermogravimetric analysis (TGA) and granulometric distribution by sieving. Gels were prepared using Clay V with particles in the range ≤ 180 μm in three different concentrations: 1%, 3% and 5% (w/w) and a standard gel without clay. The formulations were evaluated according to organoleptic characteristics, pH, viscosity, spreadability and the centrifugation test. The microbiological evaluation showed that the clay sample is in compliance with the parameters established by the legislation. The following mineral phases were identified by XRD: kaolinite, illite, vermiculite and quartz, mainly composed of silica and alumina according to XRF. Thermal analysis showed that the clay has two thermal decomposition reactions, the largest being 547.6°C. The granulometric analysis identified that the largest fraction (63.22%) was of particles with sizes greater than 710 μm. The organoleptic characteristics presented by the formulations were suitable, with characteristic gel odor, homogeneous appearance, soft and refreshing texture and staining based on the concentration of Clay V used. The obtained pH values were within the range between 5.5 and 6.5, and it was verified that the gel has high spreadability, distributing evenly on the skin. The values obtained for viscosity showed that the formulations are non-Newtonian fluids with pseudoplastic behavior. The centrifugation test showed that the formulations are stable, with no phase separation. The results obtained with the tests showed that the natural clay V material is beneficial in cosmetic products and can be used for incorporation in cosmetic gel formulations of Aristoflex® type.
Keywords:
clay minerals; cosmetics; formulation; gel
1. Introduction
Due to their compositions, unique properties and applications, clays are attractive materials in several areas, including cosmetology11 López-Galindo A, Viseras C, Cerezo P. Compositional, technical and safety specifications of clays to be used as pharmaceutical and cosmetic products. Appl Clay Sci. 2007;36:51-63. http://dx.doi.org/10.1016/j.clay.2006.06.016.
http://dx.doi.org/10.1016/j.clay.2006.06...
. In the segment of cosmetology, clays, according to their chemical and mineralogical composition, are used because of their moisturizing, skin oiliness reducing, anti-aging and makeup properties22 Gomes CSF, Silva JBP. Minerals and clay in medical geology. Appl Clay Sci. 2007;36:4-21. http://dx.doi.org/10.1016/j.clay.2006.08.006.
http://dx.doi.org/10.1016/j.clay.2006.08...
,33 Carretero MI, Pozo M. Clay and non-clay minerals in the pharmaceutical and cosmetic industries Part II. Active ingredients. Appl Clay Sci. 2010;47:171-81. http://dx.doi.org/10.1016/j.clay.2009.10.016.
http://dx.doi.org/10.1016/j.clay.2009.10...
.
Therefore, it is paramount to be informed about their composition, both mineralogical and physicochemical. characteristics, by the means of different techniques available for this purpose44 Carretero MI, Pozo M, Legido JL, Fernández-González MV, Delgado R, Gómez I, et al. Assessment of three Spanish clays for their use in pelotherapy. Appl Clay Sci. 2014;99:131-43. http://dx.doi.org/10.1016/j.clay.2014.06.022.
http://dx.doi.org/10.1016/j.clay.2014.06...
,55 Khiari I, Mefteh S, Sánchez-Espejo R, Cerezo P, Aguzzi C, López-Galindo A, et al. Study of traditional Tunisian medina clays used in therapeutic and cosmetic mud-packs. Appl Clay Sci. 2014;101:141-8. http://dx.doi.org/10.1016/j.clay.2014.07.029.
http://dx.doi.org/10.1016/j.clay.2014.07...
.
In the cosmetic industry, clay minerals are often used as excipients to stabilize emulsions or suspensions and to modify the rheological behavior of these systems. They also play an important role as adsorbents or absorbents, not only in cosmetics but also in other industries, such as pharmaceuticals66 Carretero MI, Pozo M. Clay and non-clay minerals in the pharmaceutical industry: part I. Excipients and medical applications. Appl Clay Sci. 2009;46(1):73-80. http://dx.doi.org/10.1016/j.clay.2009.07.017.
http://dx.doi.org/10.1016/j.clay.2009.07...
.
In general, minerals clays contain Si, Al, Fe, Ti, Mg, Ca, K, Na, as phyllosilicates, oxides, carbonates, kaolinite, chlorides, etc. The importance of these elements in the cosmetics field is based on the assumed role of various compounds on the skin, such as hematite (Fe2O3) as pigment, opacifier, antiseptic and as a cell renewal catalyst., Phyllosilicates, vermiculite (MgFe,Al)3(Al,Si)4O10(OH)2∙4H2O, kaolinite – Al2Si2O5(OH)4, as a diluent and binder, emulsifying, thickening and anticaking agent, flavor corrector, carrier releaser of active principles, providing reconstruction of skin tissues, hydration and a soothing effect, ZnO and MgO that are invigorating22 Gomes CSF, Silva JBP. Minerals and clay in medical geology. Appl Clay Sci. 2007;36:4-21. http://dx.doi.org/10.1016/j.clay.2006.08.006.
http://dx.doi.org/10.1016/j.clay.2006.08...
. Titanium oxide (rutile) is a further compound of interest in cosmetology, chiefly employed in photo protection formulations because it provides reflection of UV radiation66 Carretero MI, Pozo M. Clay and non-clay minerals in the pharmaceutical industry: part I. Excipients and medical applications. Appl Clay Sci. 2009;46(1):73-80. http://dx.doi.org/10.1016/j.clay.2009.07.017.
http://dx.doi.org/10.1016/j.clay.2009.07...
.
Minerals are widely used in the pharmaceutical industry as lubricants, desiccants, disintegrating, diluents, binders, pigments, and opacifiers, as well as emulsifying, thickening, isotonic, agents, and anticaking agents, and flavor correctors and carriers of active ingredients66 Carretero MI, Pozo M. Clay and non-clay minerals in the pharmaceutical industry: part I. Excipients and medical applications. Appl Clay Sci. 2009;46(1):73-80. http://dx.doi.org/10.1016/j.clay.2009.07.017.
http://dx.doi.org/10.1016/j.clay.2009.07...
. In addition, also clays have been employed as carriers for organic molecules in cosmetics and drugs, as a catalyst support77 Zhang D, Zhou C, Lin C, Tong D, Yu W. Synthesis of clay minerals. Appl Clay Sci. 2010;50:1-11. http://dx.doi.org/10.1016/j.clay.2010.06.019.
http://dx.doi.org/10.1016/j.clay.2010.06...
and as excipient in solid, liquid, and semi-solid pharmaceutical, such as gels, for example11 López-Galindo A, Viseras C, Cerezo P. Compositional, technical and safety specifications of clays to be used as pharmaceutical and cosmetic products. Appl Clay Sci. 2007;36:51-63. http://dx.doi.org/10.1016/j.clay.2006.06.016.
http://dx.doi.org/10.1016/j.clay.2006.06...
,66 Carretero MI, Pozo M. Clay and non-clay minerals in the pharmaceutical industry: part I. Excipients and medical applications. Appl Clay Sci. 2009;46(1):73-80. http://dx.doi.org/10.1016/j.clay.2009.07.017.
http://dx.doi.org/10.1016/j.clay.2009.07...
,88 Carretero MI. Clay minerals and their beneficial effects upon human health. a review. Appl Clay Sci. 2002;21:155-63. http://dx.doi.org/10.1016/S0169-1317(01)00085-0.
http://dx.doi.org/10.1016/S0169-1317(01)...
,99 Carretero MI, Legaly G. Clays and health: an introduction. Appl Clay Sci. 2007;36:1-3. http://dx.doi.org/10.1016/j.clay.2006.09.001.
http://dx.doi.org/10.1016/j.clay.2006.09...
. Gels are semisolid systems composed of two phases: the liquid dispersing phase and the solid dispersed phase1010 Viseras C, Aguzzi C, Cerezo P, Lopez-Galindo A. Uses of clay minerals in semisolid health care and therapeutic products. Appl Clay Sci. 2007;36:37-50. http://dx.doi.org/10.1016/j.clay.2006.07.006.
http://dx.doi.org/10.1016/j.clay.2006.07...
. There is a growing interest in the use of clays based on the search for abundant and low-priced materials that when disposed of do not harm the environment1111 Bergaya F, Lagaly G. General introduction: clays, clay minerals, and clay science. In: Bergaya F, Theng BKG, Lagaly G. Handbook of clay science. Amsterdam: Elsevier; 2006. p. 1-18.. A variety of minerals are used as excipients in cosmetic preparations because they have certain desirable physical and physicochemical properties, such as high adsorption capacity, specific surface area, swelling capacity, and reactivity to acids. Other important properties are water solubility and dispersivity, hygroscopicity, unctuosity, thixotropy, slightly alkaline reaction (pH), plasticity, opacity, and color1212 Moraes JDD, Bertolino SRA, Cuffini SL, Ducart DF, Bretzke PE, Leonardi GR. Clay minerals: properties and applications to dermocosmetic products and perspectives of natural raw materials for therapeutic purposes - a review. Int J Pharm. 2017;534:213-9. http://dx.doi.org/10.1016/j.ijpharm.2017.10.031.
http://dx.doi.org/10.1016/j.ijpharm.2017...
.
This work aims to characterize and decontaminate the clay from Tocantins/Brazil, here named Clay V, and to evaluate the incorporation viability of this substance in different concentrations into cosmetic formulations in gel form.
2. Materials and Methods
2.1 Materials
The clay sample (named Clay V) was collected in Tocantins (Brazil) in coordinates -9°32’48.04”S, 48°24’01.49”W. According to the geological map (Figure 1), the collection region located in Folha Miracema do Norte identified as rocks of the Archaean basement belonging to the Rio do Coco Group, Neoproterozoic supracrustal rocks associated with the Cinturão Araguaia and Phanerozoic coverage related to the Paleozoic sedimentary rocks of the Parnaíba Basin1313 Ribeiro PSE, Alves CL. Geology and Mineral Resources of the Region of Palmas. Folha Miracema do Norte (SC.22-X-D), National Port (SC.22-Z-B) and Santa Teresinha (SC.22-Z-a) [map]. Goiânia: CPRM; 2017. Scale 1: 250,000..
TO map addapted from Ribeiro and Alves1313 Ribeiro PSE, Alves CL. Geology and Mineral Resources of the Region of Palmas. Folha Miracema do Norte (SC.22-X-D), National Port (SC.22-Z-B) and Santa Teresinha (SC.22-Z-a) [map]. Goiânia: CPRM; 2017. Scale 1: 250,000..
The Pimenteiras Formation is represented by Devonian sediments of the Basin of the Parnaíba Basin and is constituted in a succession of fine and coarse sandstones, laminated siltstones and siltstones, argillaceous and conglomeratic and microconglomerate secondary levels. This formation is of marine origin, predominantly of shallow water and subordinated to two different environments. In the studied region, these rocks comprise partially reddish, fine-grained and also better sandstones1313 Ribeiro PSE, Alves CL. Geology and Mineral Resources of the Region of Palmas. Folha Miracema do Norte (SC.22-X-D), National Port (SC.22-Z-B) and Santa Teresinha (SC.22-Z-a) [map]. Goiânia: CPRM; 2017. Scale 1: 250,000..
2.1.1 Clay characterization
2.1.1.1 X-ray diffraction analysis
Mineralogical analysis was carried out by X-ray diffraction (XRD). XRD patterns were obtained with a Siemens (BRUKER-AXS) D-5000 diffractometer (Germany) operating at 40 kV and 40 mA using Cu-Kα monochromatic radiation (λ = 1.5406 Å), divergence and anti-scattering slits of 1° and 0.2mm detector slit. It was used for total rock analysis the angular range from 2 to 72° 2θ, scan speed of 0.02°/1s. For the oriented slides (< 2µm fraction) the angular range from 2 to 28° 2θ was chosen, scan speed of 0.02°/2s for natural (N) and heated (H) samples and 0.02°/3s for glycolated sample (G). The samples were prepared as it follows: unprocessed (raw), clay V was disaggregated by hand grinding to a fine powder with the use of agate mortar and pestle. Then, the disaggregated material was sieved through 74 mμ sieve. Finally, the obtained powder was mounted on a sample holder for subsequent analysis. After primary disaggregation of raw sample, the preparation procedure was followed by: (1) powdered material was dispersed in deionized water and stirred for continued 14 hours; (2) for further disaggregation ultrasonic probe was used for approximately 6 minutes; (3) the clay fraction < 2 µm was separated following the Stokes's law which relates the settling velocity of a particle to its size and specific gravity. The sample was placed in a settling vessel and filled up with deionized water to complete the settling zone, then the temperature of the dispersion was measured (24°C) and the vessel agitated. According to the calculation of the fluid viscosity, the sample was decanted for 4 hours and 21 minutes; at the end of the settlement time, the supernatant clay particles was quickly syphoned out to a beaker; (4) the supernatant material obtained from decantation was pipetted onto glass slides. It was used only enough sample to cover each slide; (5) the glass slides were allowed to dry at room temperature overnight. After dried the slides were ready for the XRD analysis.
2.1.1.2 Chemical analysis
Elemental chemical analysis was performed by X-ray fluorescence spectrometry (XRF) on a Rigaku RIX2000 (Japan) sequential spectrometer equipped with Rh X-ray Tube. Loss on ignition (LOI) was determined in accordance with ASTM D7348-08 method1414 ASTM: American Society for Testing Materials. ASTM D7348-08: Standard Test Methods for Loss on Ignition (LOI) of Solid Combustion Residues. West Conshohocken: ASTM; 2008..
2.1.1.3 Thermal analysis
Thermo gravimetric analysis (TGA/DTA) of Clay V was performed on a Netzsch, STA 449 F3 (Germany) thermo balance from ambient temperature (25°C) until 800°C, using a heating rate 10°C ∙ min-1 under N2 atmosphere.
2.1.1.4 Fourier Transform Infrared Spectroscopy (FTIR)
Infrared spectra were obtained using a Nicolet IS10, Thermo Scientific spectrophotometer (USA) in the Attenuated Total Reflectance (ATR) mode, in the interval of 400-4,000 cm-1, 32 scans and 4 cm-1 resolution.
2.1.1.5 Particle Size Distribution
The granulometric analysis of the clay sample was performed, after decontamination, by the method of sieving, in order to determine the particle size distribution. The procedure consisted of firstly defragmenting the larger clusters of the sample using gral and pistil, and subsequently inserting the solid material to be analyzed in a sieve (Bertel brand) for 25 minutes with vibration 6 (medium speed). Six sieves and the collection bottom were used. The sieves used were of 707 mu (710 µm), 500 mu (500 µm), 420 mu (425 µm), 354 mu (355 µm), 250 mu (250 µm) and 177mu (180 µm).
2.1.1.6 Raw sample preparation and microbial sterilization
Before being used in the preparation of formulations, Clay V samples were oven dried (Tecnal, model TE-394/2) at 120ºC during 24 hours. This sterilization process was based on the World Health Organization hand hygiene observation method1515 Sax H, Allegranzi B, Chraıti M, Boyce J, Larson E, Pittet D. The World Health Organization hand hygiene observation method. Am J Infect Control. 2009;37(10):827-34. http://dx.doi.org/10.1016/j.ajic.2009.07.003.
http://dx.doi.org/10.1016/j.ajic.2009.07...
.
2.1.2 Preparation and Characterization of Gel
2.1.2.1 Clay-gel preparation
A hydrophilic gel of Aristoflex® AVC (Ammonium Acryloyldimethyltaurate/VP Copolymer) was prepared in concentrations of 1%, 3% and 5% (w/w) of clay V. In parallel, a clay less gel was prepared, which was used as standard. The qualitative and quantitative composition of the gel is shown in Table 1.
Aristoflex® AVC was weighed and dispersed in a part of distilled water. Methylparaben was dissolved in another portion of heated water (50°C) under stirring. In another vessel, the oil, butylhydroxytoluene (BHT) and propylparaben were mixed under stirring. Both aqueous and oily solutions were incorporated into the gel and the final blend was allowed to rest for 24 hours. The granulometric range of Clay V used was ≤ 180 μm. This granulometry was selected because it is a size range that provides greater adhesiveness and better cutaneous sensorial. Clay V was dispersed in glycerin, and the pre-prepared gel was slowly added over this dispersion. 100 g of each formulation were divided into three 20 g units, packed at room temperature (20°C ± 2) in polyethylene bottles.
2.1.2.2 Control quality of clay-gel formulations
All the tests were done in triplicate, and the results corresponded to the average of the three verifications, except the organoleptic analyzes and the centrifugation because they were visual.
2.1.2.3 Organoleptic Analysis
Sensory analysis was performed with visual verification of color, odor, appearance and texture of the gel1616 Lange MK, Heberlé G, Milán D. Evaluation of the stability and antioxidant activity of a non-ionic base emulsion containing resveratrol. Braz J Pharm Sci. 2009;45(1):145-51. http://dx.doi.org/10.1590/S1984-82502009000100018.
http://dx.doi.org/10.1590/S1984-82502009...
,1717 Dário GM, Silva GG, Gonçalves DL, Silveira P, Teixeria A Jr, Angioletto E, et al. Evaluation of the healing activity of therapeuticclay in rat skin wounds. Mater Sci Eng C. 2014;(43):109-16. http://dx.doi.org/10.1016/j.msec.2014.06.024.
http://dx.doi.org/10.1016/j.msec.2014.06...
.
2.1.2.4 pH determination
The pH of the samples was read by dispersing the sample in distilled water (10%, v/v) at 25ºC in potentiometer (Micronal/model B474), calibrated with pH 4.0 and 7.0 solutions1818 Davis HM. Analysis of creams and lotions. In: Sensel AJ, editor. Newburger’s manual of cosmetic analysis. Washington: Association Official Analytical Chemists; 1977. p. 32..
2.1.2.5 Viscosity Measurements
Viscosity was determined by measurements on a digital viscometer (Mars /model MVD-20) using spindle 4. The spindle rotational speeds were 3, 6 and 9 rpm, increasing and decreasing, and the temperature of work was 20°C ± 2.
2.1.2.6 Spreadability determination
The spreadability determination was performed according to the methodology described by the literature1919 Knorst MT. Technological development of plastic pharmaceutical form containing concentrated extract of Achyrocline satureioides (Lam.) DC. Compositae (marcela) [dissertation]. Porto Alegre: Universidade Federal do Rio Grande do Sul; 1991.. The results were expressed as spreadability of the sample as a function of the applied weight, according to Equation 1.
Considering: Ei = spreadability of the sample for a given weight i (mm2); d = average diameter (mm).
2.1.2.7 Centrifugation Test
The centrifugation procedure consisted of inserting 1 g of the sample into a graduated conical test tube and introducing the tube in the centrifuge (ALC brand, model PK131) to the cycle of 3000 rpm for 30 minutes at room temperature.
3. Results and Discussion
3.1 Mineralogical composition
3.1.1 X-ray diffraction
Clay minerals are phyllosilicates containing two types of sheets, structurally organized in units named tetrahedral sheet (T) and octahedral sheet (O). The cations present in each sheet and the substitutions in them may lead to a net charge deficit that can depend on the type of sheet (T or O) and on the type of substituting cations (the substitutions are basically driven by the ion size, charge and other atomic properties). The type of substitution affects the behavior of the clay in regard to adsorption capacity and rheological properties11 López-Galindo A, Viseras C, Cerezo P. Compositional, technical and safety specifications of clays to be used as pharmaceutical and cosmetic products. Appl Clay Sci. 2007;36:51-63. http://dx.doi.org/10.1016/j.clay.2006.06.016.
http://dx.doi.org/10.1016/j.clay.2006.06...
. The utility of a clay mineral in specific applications are due to their physical and chemical properties, which are mainly dependent on two factors: (a) their crystal structure, which can be either a 1:1 structure (one tetrahedral sheet bound to another octahedral sheet) or a 2:1 structure (one octahedral sheet between two tetrahedral sheet) and (b) their chemical composition33 Carretero MI, Pozo M. Clay and non-clay minerals in the pharmaceutical and cosmetic industries Part II. Active ingredients. Appl Clay Sci. 2010;47:171-81. http://dx.doi.org/10.1016/j.clay.2009.10.016.
http://dx.doi.org/10.1016/j.clay.2009.10...
,1010 Viseras C, Aguzzi C, Cerezo P, Lopez-Galindo A. Uses of clay minerals in semisolid health care and therapeutic products. Appl Clay Sci. 2007;36:37-50. http://dx.doi.org/10.1016/j.clay.2006.07.006.
http://dx.doi.org/10.1016/j.clay.2006.07...
.
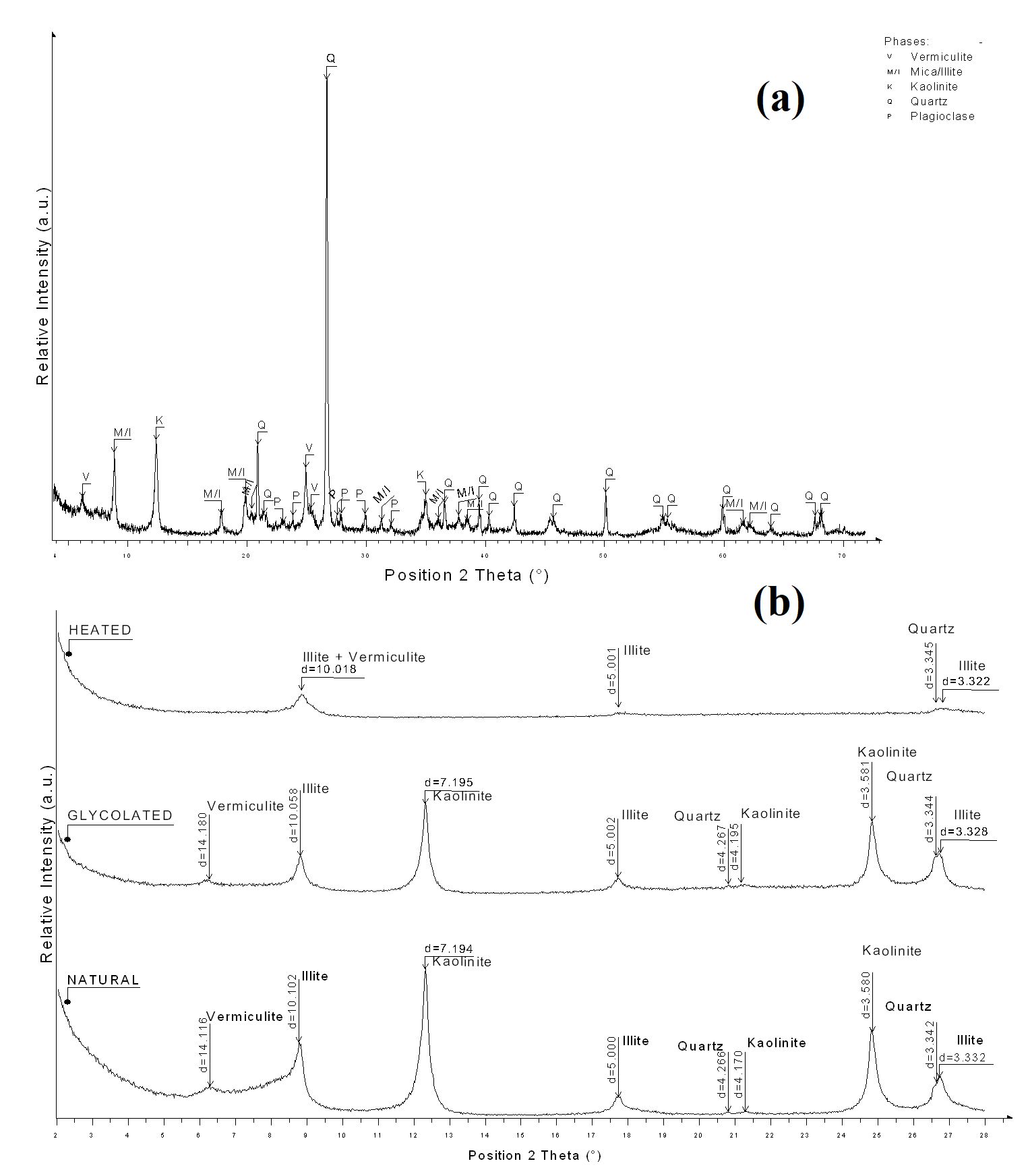
The determination of the crystalline phases of clay minerals present in a sample is very important before their application as raw material constituting an end product. Figure 2 shows the XRD patterns of Clay V. It can be seen on diffractogram, Figure 2 (a), the presence of vermiculite (peak at 14.15° in the Handbook of Mineralogy), mica/illite (K,H3O)(Al,Mg,Fe)2(Si,Al)4O10[(OH)2,H2O], kaolinite [Al2Si2O5(OH)4] reflections were observed2020 Hammami-Ben Zaied F, Abidi R, Slim-Slim N, Somarin AK. Potentiality of clay raw materials from Gram area (Northern Tunisia) in the ceramic industry. Appl Clay Sci. 2015;112-113:1-9. http://dx.doi.org/10.1016/j.clay.2015.03.027.
http://dx.doi.org/10.1016/j.clay.2015.03...
, quartz2121 Mattioli M, Giardini L, Roselli C, Desideri D. Mineralogical characterization of commercial clays used in cosmetics and possible risk for health. Appl Clay Sci. 2016;119:449-54. http://dx.doi.org/10.1016/j.clay.2015.10.023.
http://dx.doi.org/10.1016/j.clay.2015.10...
and plagioclase. In Figure 2 (b) the oriented slides is shown. It is possible to verify that the peak of vermiculite after heating shifts to the same position of illite. No expandable clay minerals were observed on glycolated analysis. Mica is no longer visible on oriented slides due the < 2µm fraction that was analyzed. It is known that clays with kaolinite and illite have already been used in the cosmetic composition55 Khiari I, Mefteh S, Sánchez-Espejo R, Cerezo P, Aguzzi C, López-Galindo A, et al. Study of traditional Tunisian medina clays used in therapeutic and cosmetic mud-packs. Appl Clay Sci. 2014;101:141-8. http://dx.doi.org/10.1016/j.clay.2014.07.029.
http://dx.doi.org/10.1016/j.clay.2014.07...
.
3.1.2 Chemical Composition
Table 2 shows the components of the Clay V sample as well as their percentages in relation to the total mass obtained by XRF. The results found show that the clay is composed mainly of silica (55.80) and alumina 22.66% in the composition. The other compounds present in the sample are in very small proportions. The composition found is confirmed by the presence of kaolinite, illite, vermiculite and quartz phases according to the XRD results.
The chemical composition of a mineral directly influences the function it will perform in a product. A clay containing silica has as main cosmetic functions moisturizing, reconstitution of tissues and reduction of inflammations. Silica may be in crystalline form (quartz) or non-crystalline form (amorphous)33 Carretero MI, Pozo M. Clay and non-clay minerals in the pharmaceutical and cosmetic industries Part II. Active ingredients. Appl Clay Sci. 2010;47:171-81. http://dx.doi.org/10.1016/j.clay.2009.10.016.
http://dx.doi.org/10.1016/j.clay.2009.10...
. In case of crystalline form, which was identified in Clay V, studies describing the toxic potential of this component can be found in the literature. Long-term inhalation studies of rats and mice have shown that quartz particles produce cellular proliferation, nodule formation, suppressed immune functions, and alveolar proteinosis. Experimental studies of rats reported the occurrence of adenocarcinomas and squamous cell carcinomas after the inhalation or intratracheal instillation of quartz2222 WHO: World Health Organization. Crystalline silica, quartz. Geneva: WHO; 2008. Concise International Chemical Assessment Document, no. 24. .
Problems regarding to quartz toxicity are related to the inhalation of this substance, and it should still be taken into consideration the inhaled dose and the degree of exposure of the quartz user. In case of applying Clay V in cosmetic products, the risk of toxicity to the user may occur through an accidental inhalation of products such as talcum powder, makeup and other presentations in the form of powder. In the case of gel, a semi-solid pharmaceutical form (there is no aerosol release) where the application form is topical the risk of inhalation is non-existent. However, studies that evaluate the degree of quartz penetration on the skin and a possible absorption of this element by the cutaneous via are interesting, but they are not part of the scope of this work. Alumina has a moisturizing, antiseptic, astringent and healing1212 Moraes JDD, Bertolino SRA, Cuffini SL, Ducart DF, Bretzke PE, Leonardi GR. Clay minerals: properties and applications to dermocosmetic products and perspectives of natural raw materials for therapeutic purposes - a review. Int J Pharm. 2017;534:213-9. http://dx.doi.org/10.1016/j.ijpharm.2017.10.031.
http://dx.doi.org/10.1016/j.ijpharm.2017...
. Therefore, the presence of this element in the composition of Clay 5 may suggest these resources for this raw material.
Previous studies have already analyzed chemical composition of clay minerals used in cosmetic formulations. In one of these studies, 4 clays containing kaolinite and illite were evaluated and had silica content of 48.1%, 53.4%, 55.8% and 54.6%, respectively, and alumina content of 28.0% 27.5%, 26.0 and 26.0%, respectively. Clay V, for having higher silica content, has cosmetic functions of moisturizing, reconstitution of tissues and reduction of inflammations more notable2323 Favero JS, Parisotto-Peterle J, Weiss-Angeli V, Brandalise RN, Gomes LB, Bergmann CP, et al. Physical and chemical characterization and method for the decontamination of clays for application in cosmetics. Appl Clay Sci. 2016;124-125:252-9. http://dx.doi.org/10.1016/j.clay.2016.02.022.
http://dx.doi.org/10.1016/j.clay.2016.02...
.
3.1.3 Thermal characteristics
The Figure 3 shows the thermal characteristics of Clay V.
Thermogravimetric curves of the samples: Thermogravimetric Analysis (TG) and Differential Thermal Analysis (DTG).
TGA/DTG curves of Clay V sample showed mass loss in the temperature range of 60°C and 220°C of the order of 1.55%, suggesting that this loss was due to release of water molecules trapped at clay surface. An inflection point occurred at 278.4°C in the thermal balance curve (0.65%) followed by dehydroxylation at 547.6°C (5.99%)2424 Qtaitat MA, Al-Trawneh IN. Characterization of kaolinite of the Baten El- Ghoul region/south Jordan by infrared spectroscopy. Spectrochimica Acta Part A. 2005;61:1519-23. http://dx.doi.org/10.1016/j.saa.2004.11.008.
http://dx.doi.org/10.1016/j.saa.2004.11....
.
3.1.4 Fourier Transform Infrared Spectroscopy (FTIR)
The infrared spectra (Figure 44b and Table 3) contained kaolinite, illite, vermiculite and quartz, according to XRD.
Infrared spectra for clay V: (a) region between 4000 and 3300 cm-1; (b) region below 1900 to 400 cm-1.
The O─H asymmetric stretching occurs around 3700 cm-1, probably as a result of the presence of the surface and internal OH groups of the Al─OH in the octahedral sheets. The band at 3660 cm-1 is attributed to water OH bending vibrations3232 Boulingui JE, Nkoumbou C, Njoya D, Thomas F, Yvon J. Characterization of clays from Mezafe and Mengono (Ne-Libreville, Gabon) for potential uses in fired products. Appl Clay Sci. 2015;115:132-44. http://dx.doi.org/10.1016/j.clay.2015.07.029.
http://dx.doi.org/10.1016/j.clay.2015.07...
. Bands at 3700 cm-11 López-Galindo A, Viseras C, Cerezo P. Compositional, technical and safety specifications of clays to be used as pharmaceutical and cosmetic products. Appl Clay Sci. 2007;36:51-63. http://dx.doi.org/10.1016/j.clay.2006.06.016.
http://dx.doi.org/10.1016/j.clay.2006.06...
and 3652 cm-11 López-Galindo A, Viseras C, Cerezo P. Compositional, technical and safety specifications of clays to be used as pharmaceutical and cosmetic products. Appl Clay Sci. 2007;36:51-63. http://dx.doi.org/10.1016/j.clay.2006.06.016.
http://dx.doi.org/10.1016/j.clay.2006.06...
can be assigned to the stretching vibration of vermiculite structural O─H bonds. Both these bands are related to vibration of the O─H bond associated to interlayer cations3333 Chmielarz L, Wojciechowska M, Rutkowska M, Adamski A, Węgrzyn A, Kowalczyk A, et al. Acid-activated vermiculites as catalysts of the DeNO(x) process. Catal Today. 2012;191:25-31. http://dx.doi.org/10.1016/j.cattod.2012.03.042.
http://dx.doi.org/10.1016/j.cattod.2012....
. The broad band at 3400 cm-11 López-Galindo A, Viseras C, Cerezo P. Compositional, technical and safety specifications of clays to be used as pharmaceutical and cosmetic products. Appl Clay Sci. 2007;36:51-63. http://dx.doi.org/10.1016/j.clay.2006.06.016.
http://dx.doi.org/10.1016/j.clay.2006.06...
is attributed to stretching vibrations of O-H bonds of water. The last band related to vibration of O─H bond is spectral band at 1638 cm-11 López-Galindo A, Viseras C, Cerezo P. Compositional, technical and safety specifications of clays to be used as pharmaceutical and cosmetic products. Appl Clay Sci. 2007;36:51-63. http://dx.doi.org/10.1016/j.clay.2006.06.016.
http://dx.doi.org/10.1016/j.clay.2006.06...
(deformation vibration of O─H bonds of water). Intense bands in 1029 and 1006 cm-11 López-Galindo A, Viseras C, Cerezo P. Compositional, technical and safety specifications of clays to be used as pharmaceutical and cosmetic products. Appl Clay Sci. 2007;36:51-63. http://dx.doi.org/10.1016/j.clay.2006.06.016.
http://dx.doi.org/10.1016/j.clay.2006.06...
is due to stretching vibration of Si─O bonds of vermiculites, this band can be assigned to stretching vibration of Si─O bonds of amorphous silica. The weak bands at 780 cm-11 López-Galindo A, Viseras C, Cerezo P. Compositional, technical and safety specifications of clays to be used as pharmaceutical and cosmetic products. Appl Clay Sci. 2007;36:51-63. http://dx.doi.org/10.1016/j.clay.2006.06.016.
http://dx.doi.org/10.1016/j.clay.2006.06...
, 695 cm-11 López-Galindo A, Viseras C, Cerezo P. Compositional, technical and safety specifications of clays to be used as pharmaceutical and cosmetic products. Appl Clay Sci. 2007;36:51-63. http://dx.doi.org/10.1016/j.clay.2006.06.016.
http://dx.doi.org/10.1016/j.clay.2006.06...
and 538 cm-11 López-Galindo A, Viseras C, Cerezo P. Compositional, technical and safety specifications of clays to be used as pharmaceutical and cosmetic products. Appl Clay Sci. 2007;36:51-63. http://dx.doi.org/10.1016/j.clay.2006.06.016.
http://dx.doi.org/10.1016/j.clay.2006.06...
are related to in plane deformation vibration of Al─O─Si bonds of vermiculites; band at 538 cm-11 López-Galindo A, Viseras C, Cerezo P. Compositional, technical and safety specifications of clays to be used as pharmaceutical and cosmetic products. Appl Clay Sci. 2007;36:51-63. http://dx.doi.org/10.1016/j.clay.2006.06.016.
http://dx.doi.org/10.1016/j.clay.2006.06...
is seen as shoulder in intensive band at 430 cm-11 López-Galindo A, Viseras C, Cerezo P. Compositional, technical and safety specifications of clays to be used as pharmaceutical and cosmetic products. Appl Clay Sci. 2007;36:51-63. http://dx.doi.org/10.1016/j.clay.2006.06.016.
http://dx.doi.org/10.1016/j.clay.2006.06...
. Intense band at 430 cm-11 López-Galindo A, Viseras C, Cerezo P. Compositional, technical and safety specifications of clays to be used as pharmaceutical and cosmetic products. Appl Clay Sci. 2007;36:51-63. http://dx.doi.org/10.1016/j.clay.2006.06.016.
http://dx.doi.org/10.1016/j.clay.2006.06...
can be assignment to deformation vibration of Si─O─Si bonds of vermiculites3131 Ritz M, Zdrálková J, Valásková M. Vibrational spectroscopy of acid treated vermiculites. Vib Spectrosc. 2014;70:63-9. http://dx.doi.org/10.1016/j.vibspec.2013.11.007.
http://dx.doi.org/10.1016/j.vibspec.2013...
.
For kaolinite the band at 3621 cm-1 is the inner OH vibrational mode. Between 1100 and 400 cm-1 there were kaolinite Si─O bands. Bands at 934 and 913 cm-1 corresponded to bending modes of the Al–OH–Al2424 Qtaitat MA, Al-Trawneh IN. Characterization of kaolinite of the Baten El- Ghoul region/south Jordan by infrared spectroscopy. Spectrochimica Acta Part A. 2005;61:1519-23. http://dx.doi.org/10.1016/j.saa.2004.11.008.
http://dx.doi.org/10.1016/j.saa.2004.11....
,2525 Dontosova KM, Norton LD, Johnston CT, Bigham JM. Influence of exchangeable cations on water adsoption on water by soil clays. Soil Sci Soc Am J. 2004;68:1218-27. http://dx.doi.org/10.2136/sssaj2004.1218.
http://dx.doi.org/10.2136/sssaj2004.1218...
,2727 Madejová J. FTIR techniques in clay mineral studies. Vib Spectrosc. 2003;31:1-10. http://dx.doi.org/10.1016/S0924-2031(02)00065-6.
http://dx.doi.org/10.1016/S0924-2031(02)...
,3030 Worasith N, Goodman BA, Neampan J, Jeyachoke N, Thiravetyan P. Characterization of modified kaolin from the Ranong deposit Thailand by XRD, XRF, SEM, FTIR and EPR techniques. Clay Miner. 2011;46:539-59. http://dx.doi.org/10.1180/claymin.2011.046.4.539.
http://dx.doi.org/10.1180/claymin.2011.0...
. The illite shows bands 3652 and 3621 cm-1, at 3652 cm-1 region occurred overlapping bands from both the kaolinite and illite2020 Hammami-Ben Zaied F, Abidi R, Slim-Slim N, Somarin AK. Potentiality of clay raw materials from Gram area (Northern Tunisia) in the ceramic industry. Appl Clay Sci. 2015;112-113:1-9. http://dx.doi.org/10.1016/j.clay.2015.03.027.
http://dx.doi.org/10.1016/j.clay.2015.03...
,2929 Oinuma K, Hayashi H. Infrared study of mixed-layer clay minerals. Am Mineral. 1965;50:1213-27.. The absorption band at 1638 cm-1 was related to the water OH bending mode2626 Frost R, Mendelovici E. Modification of fibrous silicates surfaces with organic derivatives: an infrared spectroscopic study. J Colloid Interface Sci. 2006;294:47-52. http://dx.doi.org/10.1016/j.jcis.2005.07.014.
http://dx.doi.org/10.1016/j.jcis.2005.07...
,2727 Madejová J. FTIR techniques in clay mineral studies. Vib Spectrosc. 2003;31:1-10. http://dx.doi.org/10.1016/S0924-2031(02)00065-6.
http://dx.doi.org/10.1016/S0924-2031(02)...
,2828 Makó E, Senkár Z, Kristóf J, Vágvölgyi V. Surfacemodification of mechanochemically activated kaolinites by selective leaching. J Colloid Interface Sci. 2006;294:362-70. http://dx.doi.org/10.1016/j.jcis.2005.07.033.
http://dx.doi.org/10.1016/j.jcis.2005.07...
,2929 Oinuma K, Hayashi H. Infrared study of mixed-layer clay minerals. Am Mineral. 1965;50:1213-27.. The bands at 797 cm-1, 780 cm-1 and 695 cm-1 are typical for quartz3434 Akyuz S, Akyuz T, Basaran S, Bolcal C, Gulec A. Analysis of ancient potteries using FT-IR, micro-Raman and EDXRF spectrometry. Vib Spectrosc. 2008;48(2):276-80. http://dx.doi.org/10.1016/j.vibspec.2008.02.011.
http://dx.doi.org/10.1016/j.vibspec.2008...
.
3.1.5 Particle size distribution
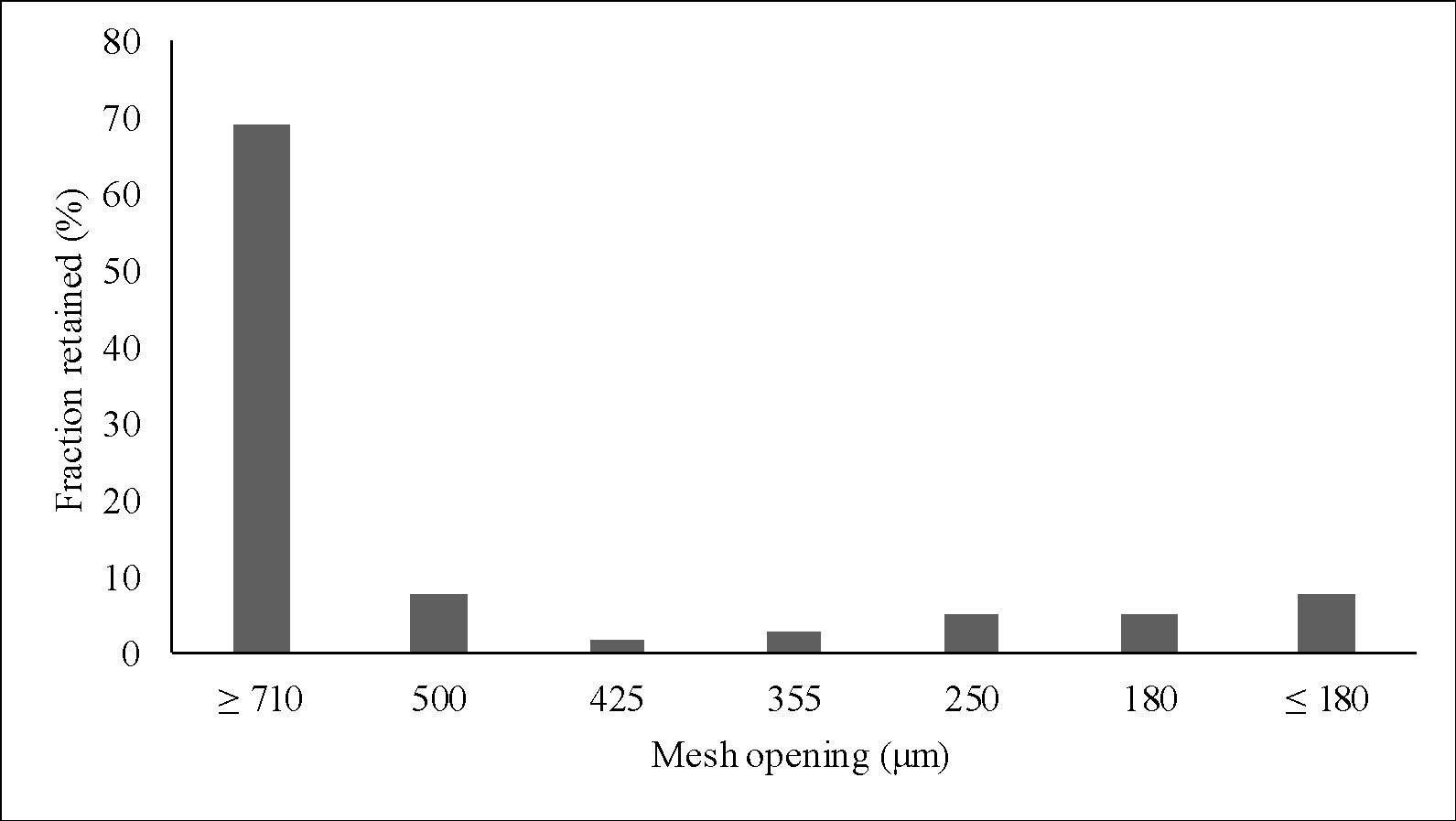
Figure 5 shows the particle size distribution of clay particles. The largest fraction retained in the sieves was of particles larger than 710 μm (63.22%). The second largest fraction was of particles smaller than 180 μm (9.70%) – which was the fraction used for incorporation into the gel. This size range (0 – 180 μm) was chosen since small size particles provide better sensory to the product and adhesiveness to the skin.
The granulometry of a material is a very important property that must be known for the application in cosmetics, since according to the size of the particles, the purpose of the product is different. Raw materials with high granulometry are usually exploited by their exfoliating action, while those with fine granulometry are used for skin hydration because they have greater adhesiveness and more pleasant sensory to the skin3535 Poensin D, Carpentier P, Féchoz C, Gasparini S. Effects of mud pack treatment on skin microcirculation. Joint Bone Spine. 2003;70:367-70. http://dx.doi.org/10.1016/S1297-319X(03)00064-2.
http://dx.doi.org/10.1016/S1297-319X(03)...
. Therefore considering the above described the clays in the cosmetology area can be used as exfoliating or moisturizing. In the present work it was chosen to work with the lower particle size, although the retained fraction of the clay in the 0 - 180 μm range was low (9.70%). It was decided to work with the lowest particle size and not with the largest retained fraction as the aim was to evaluate the incorporation of clays in cosmetic formulations for moisturizing use. In addition, products currently on the market, such as bentonite, consist of fine-grained powder (no more than 0.5% particles larger than 75 μm), aggregates are usually 50-300 μm, although the individual particles making them up is not over 2 μm11 López-Galindo A, Viseras C, Cerezo P. Compositional, technical and safety specifications of clays to be used as pharmaceutical and cosmetic products. Appl Clay Sci. 2007;36:51-63. http://dx.doi.org/10.1016/j.clay.2006.06.016.
http://dx.doi.org/10.1016/j.clay.2006.06...
.
It is known that in terms of process, choosing to work with the smallest particle size range will involve a particle size reduction step that may imply higher cost of production. However, in this first moment, the optimization of the granulometric separation process of clay was not the focus of study in this work, but in later studies with this raw material.
3.1.6 Microbiological assessment
In Table 4, the results of the microbiological analysis obtained after the sample sterilization process by dry heat are described. The sample, in all analyzed items, is in compliance with the microbiological parameters for Personal Hygiene Products, Cosmetics and Perfumes established by the Brazilian cosmetics legislation, Resolution 481/193636 Brasil. Agência Nacional de Vigilância Sanitária – ANVISA. Resolução RDC n° 481, de 23 de setembro de 1999. Estabelece os parâmetros de controle microbiológico para os produtos de higiene pessoal, cosméticos e perfumes. Diário Oficial da União; Brasília; 27 setembro 1999., which are the same as those defined by the British Pharmacopoeia3737 British Pharmacopeia. London: Her Majesty's Stationery Office; 2008..
Facing these results, it was verified that the procedure used for the sterilization of the sample was effective. The dry heat method allowed the sterilization of the sample by the high temperature. Previous studies3838 Hong E, Kang D. Effect of sequential dry heat and hydrogen peroxide treatment on inactivation of Salmonella typhimurium on alfalfa seeds and seeds germination. Food Microbiol. 2016;53:9-14. http://dx.doi.org/10.1016/j.fm.2015.08.002.
http://dx.doi.org/10.1016/j.fm.2015.08.0...
point out that dry heat methodology (physical method) is more indicated than ethanol sterilization (chemical method), mainly because it does not use solvents, avoiding the generation of effluents and waste.
In a previous work2323 Favero JS, Parisotto-Peterle J, Weiss-Angeli V, Brandalise RN, Gomes LB, Bergmann CP, et al. Physical and chemical characterization and method for the decontamination of clays for application in cosmetics. Appl Clay Sci. 2016;124-125:252-9. http://dx.doi.org/10.1016/j.clay.2016.02.022.
http://dx.doi.org/10.1016/j.clay.2016.02...
, clay samples were microbiologically evaluated right after being collected from the environment. The results obtained by this research indicated high concentration of microorganisms in the samples, therefore, the need for decontamination of clays became mandatory.
3.2 Characterization of gels containing clay
3.2.1 Control quality of clay-gel formulations
Gels are vehicles widely used in cosmetology for the incorporation of assets. Hydrophilic gels as the one prepared in this study make the product sensibly pleasant in the application and are suitable for people who have oily skin. The gels are characterized by the fact that they can be distributed readily on the skin. After the product application only very little residue should remain on the skin. Gels generally comprise a relatively high proportion of hydrophilic gelling substance and the gelling has a significant influence on the sensory properties of the product. The common gelling systems either cannot be readily distributed on the skin, do not give a feeling of freshness or leave behind too sticky a residue on the skin. To improve these sensory problems related to traditional gellings, new generation thickeners such as Aristoflex AVC, are being used in the preparation of gels. Aristoflex AVC not contain carbohydrates and aromatic solvents. It is used as gelling substance in aqueous systems and thickening agent in emulsions of “o/w” . The stabilizing effect of “Aristoflex AVC” is explained by the structure of the polymer: Gel coats droplets or solids (e.g., pigments). “Aristoflex AVC” is a copolymer, which due to its hydrophobic linkers is used as an effective combining agent. In addition, due to the hydrophilic sulfo groups the polymer interacts with biologically active substances formulations and prolongs their action retaining them on the skin surface longer3939 Baranova I, Kovalenko SM, Khokhlenkova NV, Martyniuk TV, Kutsenko SA. Prospects of using synthetic and semi- synthetic gelling substances in development of medicinal and cosmetic gels. Asian Journal of Pharmaceutic. 2017;11:2-9. http://dx.doi.org/10.22377/ajp.v11i02.1267.
http://dx.doi.org/10.22377/ajp.v11i02.12...
.
Therefore, it is recommended to use “Aristoflex AVC” in products for aging skin, as well as for treating blepharitis and demodicosis. Recommended amount of“Aristoflex AVC” is 0.5-1.5%4040 Fairclough JP, Norman J. Structure and rheology of aqueous gels. Annu Rep Prog Chem. 2003;99:243-76..
Thus, for the advantages that the Aristoflex AVC present, they were the gelling substance chosen in this study to prepare the gel and to evaluate the potential of incorporation of the clay (Clay V) in cosmetic vehicles. It is known that there is often the impossibility of associating assets in vehicles, as these can generate destabilization of the system, for example, increasing or decreasing the viscosity, cause agglomeration and coagulation of gels, turbidity, among other changes that can be technological limitations on the use of an asset. The concentrations of clay tested (1, 3 and 5%) were chosen based on the products currently on the market that contain clay in their composition and that have a clay concentration range of 1-5%.
3.2.2 Organoleptic evaluation
The organoleptic characteristics of a cosmetic product are important because they can help detecting instabilities in formulations. Table 5 represents the organoleptic characteristics observed for the different formulations prepared. For all organoleptic parameters tested, the gels showed characteristics compatible with those of dispersed systems. The coloring intensities presented by the samples corresponded to the amount of clay added. The higher the concentration of clay, the darker the formulation.
It is observed that the incorporation of Clay V in the cosmetic gel formulation did not change macroscopic aspects of the product, which is a positive point, since any modification could make the cosmetic less attractive to the consumer.
3.2.3 pH determination
The skin of the facial region presents pH values ranging from 5.5 to 6.5, defined by internal and external factors4141 Modabberi S, Namayandeh A, López-Galindo A, Viseras C, Setti M, Ranjbaran M. Characterization of Iranian bentonites to be used as pharmaceutical materials. Appl Clay Sci. 2015;116-117:193-201. http://dx.doi.org/10.1016/j.clay.2015.03.013.
http://dx.doi.org/10.1016/j.clay.2015.03...
. Therefore, cosmetic formulations of facial skin application must present a pH within this range.
The averages of the results obtained for the pH determination of the formulations, performed in triplicate, are shown in Table 6. It was observed that all gels presented pH values are within the range suitable for facial application.
3.2.4 Viscosity assessment
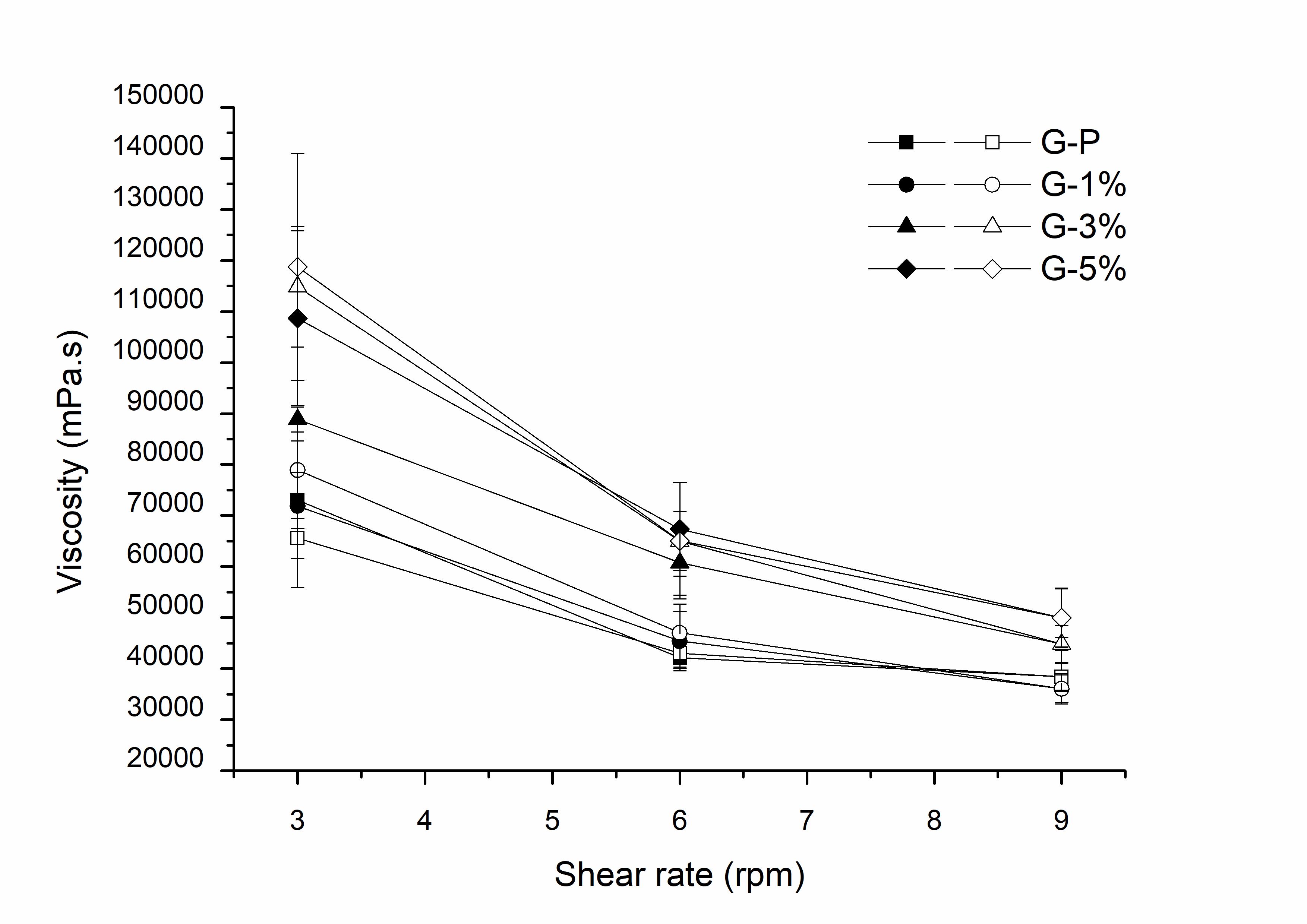
Figure 6 shows the average viscosity of the gels, determined in triplicate. By the analysis of the viscosity graph as a function of the shear rate, it was verified that all formulations showed a pseudoplastic behavior, which is evidenced by the reduction of the viscosity values as the shear increases. Thus, it can be said that the clay did not modify the rheological behavior of the Aristoflex® gel (G-P). The gels prepared can be classified as non-Newtonian fluids, since they have viscosity variation according to the shear rate, but without a linear relationship4343 Viseras C, Cultrone G, Cerezo P, Aguzzi P, Baschini MT, Valles J, et al. Characterisation of northern Patagonian bentonite for pharmaceutical uses. Appl Clay Sci. 2006;31:272-81. http://dx.doi.org/10.1016/j.clay.2005.11.002.
http://dx.doi.org/10.1016/j.clay.2005.11...
. The pseudoplastic characteristic is ideal in cosmetic formulations, since it prevents the dispersed phase from moving4444 Buhse L, Kolinski R, Westenberger B, Wokovich A, Spencer J, Chenb CW, et al. Topical drug classification. Int J Pharm. 2005;295:101-12. http://dx.doi.org/10.1016/j.ijpharm.2005.01.032.
http://dx.doi.org/10.1016/j.ijpharm.2005...
. The addition of Clay V did not cause rheological changes in the product, which ensures that it can be associated with the vehicle in question without hindering its use. It was observed, Figure 7, an increase in the viscosity of the samples as the clay concentration was increased, 1%, 3% and 5% (w/w). According to the literature, this phenomenon has already been observed in other studies1010 Viseras C, Aguzzi C, Cerezo P, Lopez-Galindo A. Uses of clay minerals in semisolid health care and therapeutic products. Appl Clay Sci. 2007;36:37-50. http://dx.doi.org/10.1016/j.clay.2006.07.006.
http://dx.doi.org/10.1016/j.clay.2006.07...
. Clays that present kaolinite generate a viscosity increase in aqueous dispersions4141 Modabberi S, Namayandeh A, López-Galindo A, Viseras C, Setti M, Ranjbaran M. Characterization of Iranian bentonites to be used as pharmaceutical materials. Appl Clay Sci. 2015;116-117:193-201. http://dx.doi.org/10.1016/j.clay.2015.03.013.
http://dx.doi.org/10.1016/j.clay.2015.03...
. This occurs due to the dilating flux caused by interactions between the dispersed particles during the shear.
Viscosity of the gels (G-P: standard gel; G-1%: gel within clay 1%; G-3%: gel within clay 3%; G-5%: gel within clay 5%. Filled in symbol: going; Blank symbol: return).
Gels spreadability (G-P: standard gel; G-1%: gel within clay 1%; G-3%: gel within clay 3%; G-5%: gel within clay 5%).
3.2.5 Spreadability determination
Figure 7 shows the behavior of the gels regarding to the spreadability, with the average values obtained from the triplicates. The larger the diameter reached by the sample in the application of the technique, the greater the spreadability. All gels showed maximum spreadability greater than 2000 mm2.
The spreadability test of a cosmetic product evaluates its expansion content on a contact surface. Good spreadability is essential for a formulation since it facilitates application and ensures even distribution, which influences the penetration of the active principle and the effectiveness of the product. In addition, the result is important for the market acceptance, since the consumer searches for cosmetics that present easy distribution on the skin4545 Estanqueiro M, Amaral MH, Lobo SJM. Comparison between sensory and instrumental characterization of topical formulations: impact of thickening agents. Int J Cosmet Sci. 2016;38:389-98. http://dx.doi.org/10.1111/ics.12302.
http://dx.doi.org/10.1111/ics.12302...
.
3.2.6 Centrifugation test
The incorporation of assets in cosmetic vehicles can often cause the destabilization of the system, causing product heterogeneity and its instability. Centrifugation is a physical method to prompt evaluates possible changes that may occur with the product, since the conditions created simulate an increase in the force of gravity, allowing and anticipating possible changes in stability. These changes can be observed in the form of precipitates, occurrence of phase separation, formation of compact sediments (caking), and coalescence, among others. In the case of the gels under study, the aim was to verify the presence of precipitates of clay or phase separation of the system4646 Bastos-Leal L, Sousa GD, Seixas CB, Souza PHN, Davi Pereira de Santana DP. Determination of the critical hydrophile-lipophile balance of licuri oil from Syagrus coronata: application for topical emulsions and evaluation of its hydrating function. Brazilian of Pharmaceutical Sciences. 2013;49:167-73. http://dx.doi.org/10.1590/S1984-82502013000100018.
http://dx.doi.org/10.1590/S1984-82502013...
.
The gels developed, when submitted to centrifugation, proved the stability of the formulation. No formulation presented sediments formed by the clay, and the dispersed systems do not experience phase separation. All formulations behaved with no flocculation, precipitation and phase separation. With the result observed, the non-existence of physical instabilities of these systems was confirmed.
Opposite results were found by previous research, in which centrifugation tests were performed with 3 samples of facial masks containing clay, and all showed phase separation, showing to be visually unstable4747 Silva PSC, Oliveira SMB, Farias L, Fávaro DIT, Mazzilli BP. Chemical and radiological characterization of clay minerals used in pharmaceutics and cosmetics. Appl Clay Sci. 2011;52:145-9. http://dx.doi.org/10.1016/j.clay.2011.02.013.
http://dx.doi.org/10.1016/j.clay.2011.02...
. Therefore, the formulation developed in this study with Clay V is more stable and has better interactions between the components.
4. Conclusion
Regarding the chemical composition, the clay can be used as a moisturizer, inflammations reducer, antiseptic, astringent and healer.
The results obtained for the characterization of the gel, under the conditions tested, containing the different concentrations of Clay V showed that regardless of the concentration used the physical characteristics of the gels were not altered. This information is important in the development of a cosmetic because it allows indicating the maximum concentration of Clay V that can be added to the gel without harm to the final product.
The vehicle used is traditional in the cosmetic and pharmaceutical fields, what is innovative is the clay. Therefore, it was proven that Clay V, which was characterized by some techniques, can be incorporated into the tested vehicle. The concern is that often these clays are difficult to be associated with vehicles, as they can cause rheological changes that hinder use, or alter macroscopic aspects that do not become attractive to the consumer.
Thus, it was confirmed that the clay under study can be incorporated into formulations as gels, as a great option to be used as an active principle. In addition, Clay V has potential for continuity regarding research and its application in other cosmetic vehicles.
5. Acknowledgements
Authors are beholden to the University of Caxias do Sul (UCS), to Federal University of Rio Grande do Sul (UFRGS) and to the Research Support Foundation of the Rio Grande do Sul State (FAPERGS) for financial support.
6. References
-
1López-Galindo A, Viseras C, Cerezo P. Compositional, technical and safety specifications of clays to be used as pharmaceutical and cosmetic products. Appl Clay Sci. 2007;36:51-63. http://dx.doi.org/10.1016/j.clay.2006.06.016
» http://dx.doi.org/10.1016/j.clay.2006.06.016 -
2Gomes CSF, Silva JBP. Minerals and clay in medical geology. Appl Clay Sci. 2007;36:4-21. http://dx.doi.org/10.1016/j.clay.2006.08.006
» http://dx.doi.org/10.1016/j.clay.2006.08.006 -
3Carretero MI, Pozo M. Clay and non-clay minerals in the pharmaceutical and cosmetic industries Part II. Active ingredients. Appl Clay Sci. 2010;47:171-81. http://dx.doi.org/10.1016/j.clay.2009.10.016
» http://dx.doi.org/10.1016/j.clay.2009.10.016 -
4Carretero MI, Pozo M, Legido JL, Fernández-González MV, Delgado R, Gómez I, et al. Assessment of three Spanish clays for their use in pelotherapy. Appl Clay Sci. 2014;99:131-43. http://dx.doi.org/10.1016/j.clay.2014.06.022
» http://dx.doi.org/10.1016/j.clay.2014.06.022 -
5Khiari I, Mefteh S, Sánchez-Espejo R, Cerezo P, Aguzzi C, López-Galindo A, et al. Study of traditional Tunisian medina clays used in therapeutic and cosmetic mud-packs. Appl Clay Sci. 2014;101:141-8. http://dx.doi.org/10.1016/j.clay.2014.07.029
» http://dx.doi.org/10.1016/j.clay.2014.07.029 -
6Carretero MI, Pozo M. Clay and non-clay minerals in the pharmaceutical industry: part I. Excipients and medical applications. Appl Clay Sci. 2009;46(1):73-80. http://dx.doi.org/10.1016/j.clay.2009.07.017
» http://dx.doi.org/10.1016/j.clay.2009.07.017 -
7Zhang D, Zhou C, Lin C, Tong D, Yu W. Synthesis of clay minerals. Appl Clay Sci. 2010;50:1-11. http://dx.doi.org/10.1016/j.clay.2010.06.019
» http://dx.doi.org/10.1016/j.clay.2010.06.019 -
8Carretero MI. Clay minerals and their beneficial effects upon human health. a review. Appl Clay Sci. 2002;21:155-63. http://dx.doi.org/10.1016/S0169-1317(01)00085-0
» http://dx.doi.org/10.1016/S0169-1317(01)00085-0 -
9Carretero MI, Legaly G. Clays and health: an introduction. Appl Clay Sci. 2007;36:1-3. http://dx.doi.org/10.1016/j.clay.2006.09.001
» http://dx.doi.org/10.1016/j.clay.2006.09.001 -
10Viseras C, Aguzzi C, Cerezo P, Lopez-Galindo A. Uses of clay minerals in semisolid health care and therapeutic products. Appl Clay Sci. 2007;36:37-50. http://dx.doi.org/10.1016/j.clay.2006.07.006
» http://dx.doi.org/10.1016/j.clay.2006.07.006 -
11Bergaya F, Lagaly G. General introduction: clays, clay minerals, and clay science. In: Bergaya F, Theng BKG, Lagaly G. Handbook of clay science. Amsterdam: Elsevier; 2006. p. 1-18.
-
12Moraes JDD, Bertolino SRA, Cuffini SL, Ducart DF, Bretzke PE, Leonardi GR. Clay minerals: properties and applications to dermocosmetic products and perspectives of natural raw materials for therapeutic purposes - a review. Int J Pharm. 2017;534:213-9. http://dx.doi.org/10.1016/j.ijpharm.2017.10.031
» http://dx.doi.org/10.1016/j.ijpharm.2017.10.031 -
13Ribeiro PSE, Alves CL. Geology and Mineral Resources of the Region of Palmas. Folha Miracema do Norte (SC.22-X-D), National Port (SC.22-Z-B) and Santa Teresinha (SC.22-Z-a) [map]. Goiânia: CPRM; 2017. Scale 1: 250,000.
-
14ASTM: American Society for Testing Materials. ASTM D7348-08: Standard Test Methods for Loss on Ignition (LOI) of Solid Combustion Residues. West Conshohocken: ASTM; 2008.
-
15Sax H, Allegranzi B, Chraıti M, Boyce J, Larson E, Pittet D. The World Health Organization hand hygiene observation method. Am J Infect Control. 2009;37(10):827-34. http://dx.doi.org/10.1016/j.ajic.2009.07.003
» http://dx.doi.org/10.1016/j.ajic.2009.07.003 -
16Lange MK, Heberlé G, Milán D. Evaluation of the stability and antioxidant activity of a non-ionic base emulsion containing resveratrol. Braz J Pharm Sci. 2009;45(1):145-51. http://dx.doi.org/10.1590/S1984-82502009000100018
» http://dx.doi.org/10.1590/S1984-82502009000100018 -
17Dário GM, Silva GG, Gonçalves DL, Silveira P, Teixeria A Jr, Angioletto E, et al. Evaluation of the healing activity of therapeuticclay in rat skin wounds. Mater Sci Eng C. 2014;(43):109-16. http://dx.doi.org/10.1016/j.msec.2014.06.024
» http://dx.doi.org/10.1016/j.msec.2014.06.024 -
18Davis HM. Analysis of creams and lotions. In: Sensel AJ, editor. Newburger’s manual of cosmetic analysis. Washington: Association Official Analytical Chemists; 1977. p. 32.
-
19Knorst MT. Technological development of plastic pharmaceutical form containing concentrated extract of Achyrocline satureioides (Lam.) DC. Compositae (marcela) [dissertation]. Porto Alegre: Universidade Federal do Rio Grande do Sul; 1991.
-
20Hammami-Ben Zaied F, Abidi R, Slim-Slim N, Somarin AK. Potentiality of clay raw materials from Gram area (Northern Tunisia) in the ceramic industry. Appl Clay Sci. 2015;112-113:1-9. http://dx.doi.org/10.1016/j.clay.2015.03.027
» http://dx.doi.org/10.1016/j.clay.2015.03.027 -
21Mattioli M, Giardini L, Roselli C, Desideri D. Mineralogical characterization of commercial clays used in cosmetics and possible risk for health. Appl Clay Sci. 2016;119:449-54. http://dx.doi.org/10.1016/j.clay.2015.10.023
» http://dx.doi.org/10.1016/j.clay.2015.10.023 -
22WHO: World Health Organization. Crystalline silica, quartz. Geneva: WHO; 2008. Concise International Chemical Assessment Document, no. 24.
-
23Favero JS, Parisotto-Peterle J, Weiss-Angeli V, Brandalise RN, Gomes LB, Bergmann CP, et al. Physical and chemical characterization and method for the decontamination of clays for application in cosmetics. Appl Clay Sci. 2016;124-125:252-9. http://dx.doi.org/10.1016/j.clay.2016.02.022
» http://dx.doi.org/10.1016/j.clay.2016.02.022 -
24Qtaitat MA, Al-Trawneh IN. Characterization of kaolinite of the Baten El- Ghoul region/south Jordan by infrared spectroscopy. Spectrochimica Acta Part A. 2005;61:1519-23. http://dx.doi.org/10.1016/j.saa.2004.11.008
» http://dx.doi.org/10.1016/j.saa.2004.11.008 -
25Dontosova KM, Norton LD, Johnston CT, Bigham JM. Influence of exchangeable cations on water adsoption on water by soil clays. Soil Sci Soc Am J. 2004;68:1218-27. http://dx.doi.org/10.2136/sssaj2004.1218
» http://dx.doi.org/10.2136/sssaj2004.1218 -
26Frost R, Mendelovici E. Modification of fibrous silicates surfaces with organic derivatives: an infrared spectroscopic study. J Colloid Interface Sci. 2006;294:47-52. http://dx.doi.org/10.1016/j.jcis.2005.07.014
» http://dx.doi.org/10.1016/j.jcis.2005.07.014 -
27Madejová J. FTIR techniques in clay mineral studies. Vib Spectrosc. 2003;31:1-10. http://dx.doi.org/10.1016/S0924-2031(02)00065-6
» http://dx.doi.org/10.1016/S0924-2031(02)00065-6 -
28Makó E, Senkár Z, Kristóf J, Vágvölgyi V. Surfacemodification of mechanochemically activated kaolinites by selective leaching. J Colloid Interface Sci. 2006;294:362-70. http://dx.doi.org/10.1016/j.jcis.2005.07.033
» http://dx.doi.org/10.1016/j.jcis.2005.07.033 -
29Oinuma K, Hayashi H. Infrared study of mixed-layer clay minerals. Am Mineral. 1965;50:1213-27.
-
30Worasith N, Goodman BA, Neampan J, Jeyachoke N, Thiravetyan P. Characterization of modified kaolin from the Ranong deposit Thailand by XRD, XRF, SEM, FTIR and EPR techniques. Clay Miner. 2011;46:539-59. http://dx.doi.org/10.1180/claymin.2011.046.4.539
» http://dx.doi.org/10.1180/claymin.2011.046.4.539 -
31Ritz M, Zdrálková J, Valásková M. Vibrational spectroscopy of acid treated vermiculites. Vib Spectrosc. 2014;70:63-9. http://dx.doi.org/10.1016/j.vibspec.2013.11.007
» http://dx.doi.org/10.1016/j.vibspec.2013.11.007 -
32Boulingui JE, Nkoumbou C, Njoya D, Thomas F, Yvon J. Characterization of clays from Mezafe and Mengono (Ne-Libreville, Gabon) for potential uses in fired products. Appl Clay Sci. 2015;115:132-44. http://dx.doi.org/10.1016/j.clay.2015.07.029
» http://dx.doi.org/10.1016/j.clay.2015.07.029 -
33Chmielarz L, Wojciechowska M, Rutkowska M, Adamski A, Węgrzyn A, Kowalczyk A, et al. Acid-activated vermiculites as catalysts of the DeNO(x) process. Catal Today. 2012;191:25-31. http://dx.doi.org/10.1016/j.cattod.2012.03.042
» http://dx.doi.org/10.1016/j.cattod.2012.03.042 -
34Akyuz S, Akyuz T, Basaran S, Bolcal C, Gulec A. Analysis of ancient potteries using FT-IR, micro-Raman and EDXRF spectrometry. Vib Spectrosc. 2008;48(2):276-80. http://dx.doi.org/10.1016/j.vibspec.2008.02.011
» http://dx.doi.org/10.1016/j.vibspec.2008.02.011 -
35Poensin D, Carpentier P, Féchoz C, Gasparini S. Effects of mud pack treatment on skin microcirculation. Joint Bone Spine. 2003;70:367-70. http://dx.doi.org/10.1016/S1297-319X(03)00064-2
» http://dx.doi.org/10.1016/S1297-319X(03)00064-2 -
36Brasil. Agência Nacional de Vigilância Sanitária – ANVISA. Resolução RDC n° 481, de 23 de setembro de 1999. Estabelece os parâmetros de controle microbiológico para os produtos de higiene pessoal, cosméticos e perfumes. Diário Oficial da União; Brasília; 27 setembro 1999.
-
37British Pharmacopeia. London: Her Majesty's Stationery Office; 2008.
-
38Hong E, Kang D. Effect of sequential dry heat and hydrogen peroxide treatment on inactivation of Salmonella typhimurium on alfalfa seeds and seeds germination. Food Microbiol. 2016;53:9-14. http://dx.doi.org/10.1016/j.fm.2015.08.002
» http://dx.doi.org/10.1016/j.fm.2015.08.002 -
39Baranova I, Kovalenko SM, Khokhlenkova NV, Martyniuk TV, Kutsenko SA. Prospects of using synthetic and semi- synthetic gelling substances in development of medicinal and cosmetic gels. Asian Journal of Pharmaceutic. 2017;11:2-9. http://dx.doi.org/10.22377/ajp.v11i02.1267
» http://dx.doi.org/10.22377/ajp.v11i02.1267 -
40Fairclough JP, Norman J. Structure and rheology of aqueous gels. Annu Rep Prog Chem. 2003;99:243-76.
-
41Modabberi S, Namayandeh A, López-Galindo A, Viseras C, Setti M, Ranjbaran M. Characterization of Iranian bentonites to be used as pharmaceutical materials. Appl Clay Sci. 2015;116-117:193-201. http://dx.doi.org/10.1016/j.clay.2015.03.013
» http://dx.doi.org/10.1016/j.clay.2015.03.013 -
42Weiss-Angeli V, Poletto FS, Zancan LR, Baldasso F, Pohlmann AR, Guterres SS. Nanocapsules of octyl methoxycinnamate containing quercetin delayed the photodegradation of both components under ultraviolet a radiation. J Biomed Nanotechnol. 2008;4(1):80-9. http://dx.doi.org/10.1166/jbn.2008.004
» http://dx.doi.org/10.1166/jbn.2008.004 -
43Viseras C, Cultrone G, Cerezo P, Aguzzi P, Baschini MT, Valles J, et al. Characterisation of northern Patagonian bentonite for pharmaceutical uses. Appl Clay Sci. 2006;31:272-81. http://dx.doi.org/10.1016/j.clay.2005.11.002
» http://dx.doi.org/10.1016/j.clay.2005.11.002 -
44Buhse L, Kolinski R, Westenberger B, Wokovich A, Spencer J, Chenb CW, et al. Topical drug classification. Int J Pharm. 2005;295:101-12. http://dx.doi.org/10.1016/j.ijpharm.2005.01.032
» http://dx.doi.org/10.1016/j.ijpharm.2005.01.032 -
45Estanqueiro M, Amaral MH, Lobo SJM. Comparison between sensory and instrumental characterization of topical formulations: impact of thickening agents. Int J Cosmet Sci. 2016;38:389-98. http://dx.doi.org/10.1111/ics.12302
» http://dx.doi.org/10.1111/ics.12302 -
46Bastos-Leal L, Sousa GD, Seixas CB, Souza PHN, Davi Pereira de Santana DP. Determination of the critical hydrophile-lipophile balance of licuri oil from Syagrus coronata: application for topical emulsions and evaluation of its hydrating function. Brazilian of Pharmaceutical Sciences. 2013;49:167-73. http://dx.doi.org/10.1590/S1984-82502013000100018
» http://dx.doi.org/10.1590/S1984-82502013000100018 -
47Silva PSC, Oliveira SMB, Farias L, Fávaro DIT, Mazzilli BP. Chemical and radiological characterization of clay minerals used in pharmaceutics and cosmetics. Appl Clay Sci. 2011;52:145-9. http://dx.doi.org/10.1016/j.clay.2011.02.013
» http://dx.doi.org/10.1016/j.clay.2011.02.013
Publication Dates
-
Publication in this collection
01 June 2020 -
Date of issue
2020
History
-
Received
16 Oct 2019 -
Reviewed
19 Feb 2020 -
Accepted
25 Apr 2020