Abstracts
Mahanarva fimbriolata (Stål) is an important pest in Latin America and causes significant reduction in sugarcane productivity. There is no information regarding the effect of this pest on the quality of cane juice used for sugar and alcohol production. This work aimed at evaluating the quality of sugarcane juice from plants attacked by spittlebugs. The experiment was arranged in a completely randomized design with 15 replications, and comprised two treatments: control and chemical treatment with thiamethoxam. An average of 9.2 ± 4.44 spittlebug nymphs m-1 were found in the plots prior to insecticide application. Nymphs were counted 18, 35, 55, and 82 days after the initial sampling (december/2003). During the mid growing season (July 2004), the juice was extracted from stalks and analyzed for Brix, Pol, RS, pH, fiber, purity, TRS, dextran, starch, and total phenolic compounds. Stalk yield was also measured. Chemical treatment was efficient in reducing spittlebug population, and elevated both stalk yield and juice pH. The accumulated infestation expressed as insect-days was significantly and negatively correlated to yield, Pol, pH, and purity. The concentration of phenolic compounds increased with pest infestation, while dextran and starch levels were not affected. The infestation of 2.4 and 7.3 nymphs m-1 day-1 caused reductions of 8.3% and 29.8% in yield; 1.9% and 5.8% in Pol; 0.4% and 1.1% in pH and 0.4% and 1.2% in purity, respectively, in comparison to areas where the pest population was extremely low (< 0.1 nymphs m-1).
Spittlebug; insect-day; chemical control
Mahanarva fimbriolata (Stål) é considerada praga importante na América Latina por reduzir a produtividade de cana-de-açúcar. Há pouca informação sobre o efeito do inseto na qualidade da cana que será utilizada para produção de açúcar e álcool. Assim, objetivou-se avaliar a qualidade do caldo da cana de plantas atacadas pela cigarrinha-das-raízes. Adotou-se o delineamento experimental inteiramente casualizado com 15 repetições e dois tratamentos: testemunha e controle químico com tiametoxam. Nas parcelas experimentais foram encontradas em média 9,2 ± 4,44 ninfas m-1 em monitoramento inicial (dezembro/2003). As ninfas foram contadas aos 18, 35, 55, e 82 dias após a primeira contagem, sendo a infestação expressa em insetos-dia acumulados. Em julho de 2004, procedeu-se à colheita de colmos e extração do caldo, analisando-se o Brix, Pol, açúcares redutores, pH, fibra, pureza, açúcares redutores totais, dextrana, amido, compostos fenólicos totais e produtividade. O controle químico reduziu a população do inseto e elevou a produtividade de colmos e do pH do caldo. A infestação acumulada foi correlacionada significativa e negativamente com a produtividade, Pol, pH, e pureza. O teor de compostos fenólicos aumentou com a elevação da infestação, enquanto que os valores de dextrana e amido não foram alterados. Infestações de 2,4 e 7,3 ninfas m-1 dia-1 causaram reduções da ordem de 8,3% e 29,8% na produtividade; 1,9 e 5,8% na Pol; 0,4% e 1,1% no pH e 0,4% e 1,2% na pureza, respectivamente, em comparação com áreas de população baixa (< 0,1 ninfa m-1).
Cigarrinha-das-raízes; inseto-dia; controle químico
CROP PROTECTION
Influence of Mahanarva fimbriolata (Stål) (Hemiptera: Cercopidae) injury on the quality of cane juice
Influência da injúria de Mahanarva fimbriolata (Stål) (Hemiptera: Cercopidae) na qualidade do caldo de cana
Leonardo L. MadalenoI; Gisele C. RavaneliI; Leandro E. PresottiI; Miguel A. MuttonII; Odair A. FernandesIII; Márcia J.R. MuttonI
IDepto. Tecnologia
IIDepto. Produção Vegetal
IIIDepto. Fitossanidade. FCAV, Universidade Estadual de São Paulo - UNESP, 14884-900, Jaboticabal SP
ABSTRACT
Mahanarva fimbriolata (Stål) is an important pest in Latin America and causes significant reduction in sugarcane productivity. There is no information regarding the effect of this pest on the quality of cane juice used for sugar and alcohol production. This work aimed at evaluating the quality of sugarcane juice from plants attacked by spittlebugs. The experiment was arranged in a completely randomized design with 15 replications, and comprised two treatments: control and chemical treatment with thiamethoxam. An average of 9.2 ± 4.44 spittlebug nymphs m-1 were found in the plots prior to insecticide application. Nymphs were counted 18, 35, 55, and 82 days after the initial sampling (december/2003). During the mid growing season (July 2004), the juice was extracted from stalks and analyzed for Brix, Pol, RS, pH, fiber, purity, TRS, dextran, starch, and total phenolic compounds. Stalk yield was also measured. Chemical treatment was efficient in reducing spittlebug population, and elevated both stalk yield and juice pH. The accumulated infestation expressed as insect-days was significantly and negatively correlated to yield, Pol, pH, and purity. The concentration of phenolic compounds increased with pest infestation, while dextran and starch levels were not affected. The infestation of 2.4 and 7.3 nymphs m-1 day-1 caused reductions of 8.3% and 29.8% in yield; 1.9% and 5.8% in Pol; 0.4% and 1.1% in pH and 0.4% and 1.2% in purity, respectively, in comparison to areas where the pest population was extremely low (< 0.1 nymphs m-1).
Key words: Spittlebug, insect-day, chemical control
RESUMO
Mahanarva fimbriolata (Stål) é considerada praga importante na América Latina por reduzir a produtividade de cana-de-açúcar. Há pouca informação sobre o efeito do inseto na qualidade da cana que será utilizada para produção de açúcar e álcool. Assim, objetivou-se avaliar a qualidade do caldo da cana de plantas atacadas pela cigarrinha-das-raízes. Adotou-se o delineamento experimental inteiramente casualizado com 15 repetições e dois tratamentos: testemunha e controle químico com tiametoxam. Nas parcelas experimentais foram encontradas em média 9,2 ± 4,44 ninfas m-1 em monitoramento inicial (dezembro/2003). As ninfas foram contadas aos 18, 35, 55, e 82 dias após a primeira contagem, sendo a infestação expressa em insetos-dia acumulados. Em julho de 2004, procedeu-se à colheita de colmos e extração do caldo, analisando-se o Brix, Pol, açúcares redutores, pH, fibra, pureza, açúcares redutores totais, dextrana, amido, compostos fenólicos totais e produtividade. O controle químico reduziu a população do inseto e elevou a produtividade de colmos e do pH do caldo. A infestação acumulada foi correlacionada significativa e negativamente com a produtividade, Pol, pH, e pureza. O teor de compostos fenólicos aumentou com a elevação da infestação, enquanto que os valores de dextrana e amido não foram alterados. Infestações de 2,4 e 7,3 ninfas m-1 dia-1 causaram reduções da ordem de 8,3% e 29,8% na produtividade; 1,9 e 5,8% na Pol; 0,4% e 1,1% no pH e 0,4% e 1,2% na pureza, respectivamente, em comparação com áreas de população baixa (< 0,1 ninfa m-1).
Palavras-chave: Cigarrinha-das-raízes, inseto-dia, controle químico
The spittlebug Mahanarva fimbriolata (Stål) was considered a secondary pest in sugarcane fields in Brazil. The adoption of green cane harvesting has contributed to a significant increase in the pest population (Dinardo-Miranda & Ferreira 2004). The layer of straw left on the field during cane harvest conserves soil moisture and helps to reduce temperature variation. These conditions are favorable to the development of spittlebug nymphs, which suck roots under the straw layer (Dinardo-Miranda et al. 2000a).
In addition to M. fimbriolata, several other species of spittlebugs, including Aeneolamia postica (Walker) in Mexico, Eoscarta carnifex (F.) in Australia, Mahanarva bipars (Walker) in Colombia, and Aeneolamia varia (F.) in Trinidad, are listed to as sugarcane pests. These insects cause significant yield losses in Trinidad, some areas in Mexico and South America. It is difficult to evaluate the damage caused by this pest, although Thompson (2004) estimated that 5% reduction in sugarcane production can be attributed to spittlebugs, which corresponds to 350 million dollars year-1 in losses.
The sugarcane harvest season in Brazil is from April through December (mid fall to late spring). The infestation by M. fimbriolata nymphs starts in early spring (September), when the raining season begins, lasting until late summer (March). During this period sugarcane grows at very high rates. Over most of the harvest season, which is characterized by dry weather, the spittlebug population decreases, undergoing diapause in the egg stage due to low moisture and temperature conditions (Dinardo-Miranda 2003).
The injury intensity caused by this pest vary depending on the plant development stage. If plants are attacked when the stalks are well developed (8-10 months), damage is much lower than if the plants are younger (less than eight months). In the latter situation, plants are at the vegetative stage and stalks still have to go through a long period of development. During this stage, according to Dinardo-Miranda et al. (1999), injury can lead to a yield loss of at least 41%.
Injuries caused by M. fimbriolata affect cane quality (Mendonça et al. 1996, Dinardo-Miranda et al. 2000b, Gonçalves et al. 2003). The studies conducted so far, however, have only analyzed conventional parameters (Brix, Pol, fiber and theoretically recoverable sugar -TRS), and have not considered the pest infestation period or the interaction which can lead to the synthesis of plant defense molecules during attack. These compounds may interfere in the production process of sugar and alcohol. In this study, we determine how spittlebug injury affects cane juice quality, also taking into account defense and stress-related molecules that are undesirable during sugar and ethanol production.
Material and Methods
A field trial was conducted during the crop season 2003/2004, in a second ratoon, green cane area of the variety SP80-1816, which is susceptible to spittlebugs (Dinardo-Miranda 2003), in Jaboticabal, Sao Paulo, Brazil (lat. 21º 15; long. 48º 18).
The experiment was arranged in a completely randomized design with 15 replications and comprised two treatments, control and chemical treatment. Plots corresponded to two 4 m long rows (side-by-syde), spaced 1.5 m. Side borders of 6 m were kept to avoid inteference between plots.
The first nymph count was performed on December 12th, 2003, at the rainy season when there are favorable environmental conditions for spittlebug development. Nymph counts were performed on both rows of each plot. The straw layer of both sides of a 2-m sugarcane row was removed by hand. Nymphs were counted and the straw layer was replaced. At the beginning of the experiment, nymphs were mostly at the 3rd instar. Infestation averaged 9.2 ± 4.44 nymphs m-1 during the first counting.
The pesticide thiamethoxam (Actara 250WG®), used as negative control, was applied at a rate of 0.2 kg a.i. ha-1, as recommended for spittlebug control in sugarcane (Dinardo-Miranda et al. 2003). A backpack sprayer at constant pressure (15 kg cm-2) was used for application on December 30th, 2003, when plants were five months old. The spray was directed towards stalk base. A total of 150 L ha-1 of spray mix was used. Spittlebug nymphs were again counted 18, 35, 55, and 82 days after the first count. There was a great reduction in spittlebug population due to low moisture and temperature, which induced eggs to undergo diapause. No infestation was detected after 82 days, so, nymph counting was discontinued. The cumulative insect-day parameter for each plot was calculated according to Ruppel (1983) to express the infestation.
Infestation of the stalk borer Diatraea saccharalis (F.) (Lepidoptera: Pyralidae), another important sugarcane pest, was monitored through the Infestation Index (II %), and by the time the crop was nine months old, an II as low as 0.5% was observed. The occurrence of diseases that could affect stalk yield or juice quality was negligible.
At harvest, in July 2004, ten stalks were collected at random from a 1-m row in the center of each plot. Leaves, sheath and dry leaves were removed. Stalks were topped at the apex bud line. Cane juice was extracted according to Tanimoto (1964).
The apparent sucrose content (Pol) and the level of soluble solids (Brix) of the juice were determined according to Scheneider (1979). The pH was measured with a digital pH-meter and reducing sugars (RS) were determined by Lane & Eynon's volumetric method (1934) and expressed in glucose. Fiber, purity, and theoretically recoverable sugar (TRS) were calculated according to Consecana (2004). Dextran was determined according to Copersucar (2001) and the starch content was measured as recommended by Chavan et al. (1991). The level of total phenolic compounds was determined by diluting the juice 10 times with HCl acidified methanol (1v/0.01v), adding 2.5 ml of Folin-Cicalteau reagent and 2 ml of Na2CO3 at 7.5%, heating the mix in water bath at 45ºC for 15 min, reading at 765 nm in a Uvmini 1240 spectrophotometer. Phenolic compounds were expressed in µl of catequin ml-1 of juice. To estimate stalk yield, the fresh mass of 10 stalks was determined and transformed to tons per hectare, considering a stand of 80,000 plants.
Data were subjected to analysis of variance, and correlations were performed between the variables used to evaluate infestation and cane quality. The significant correlations were subjected to linear regression analysis. Analyses were performed using PROC GLM, PROC CORR and PROC REG procedures (SAS Institute 1996).
Results and Discussion
A significant yield increase (33%) (F = 44.23; d.f. = 1, 28; P < 0.0001) and reductions in both juice pH (F = 5.44; d.f. = 1, 28; P < 0.0270) and pest infestation (F = 17.26; d.f. = 1, 28; P = 0.0003) were observed in treated plots (Fig. 1). The infestation was reduced on plots treated with thimethoxam (Table 1) as expected once this active ingredient is an effective insecticide used against sap-sucking insects (Dinardo-Miranda & Ferreira 2004, Tomizawa & Casida 2005). Although infestation on untreated plots (check) also reduced, the nymph population reached 7 and 8 insect m-1 at 55 and 82 days after initial sampling whereas treated plot reached 1 nymph m-1 at the last sampling date (Table 1).
The accumulated infestation expressed by insect-days correlated significantly and negatively to yield, Pol, pH, and purity (Table 2). This result confirms that spittlebug infestation reduces sugar yield (Fig. 2). Mendonça et al. (1996) observed reduction in sucrose content in a similar way to the reduction of Pol (Fig. 3) and purity (Fig. 4), and Gonçalves et al. (2003) reported that spittlebug infestation causes reduction in sucrose content. Because of the lower water absorption through roots due to xylem sap sucking nymphs, plants express similar symptoms to water deficit (Hagley & Blackman 1966). Leaves may also show burnt-like symptoms under high pest infestation and extreme stress (Mendonça et al. 1996). Consequently, photosynthesis is affected, leading to yield, Pol and purity reduction. The smaller amounts of carbon skeletons and energy resulted in lower growth rates and carbohydrate accumulation, especially sucrose (Buchanan et al. 2000).
In addition, the reduction in sucrose levels may be a consequence of the biosynthesis of plant defense compounds, such as phenols, which requires energy and carbon (Buchanan et al. 2000, Taiz & Zeiger 2004, Silva et al. 2005). There was a significant correlation between the levels of total phenolic compounds, RS (Reducing Sugars) and TRS (Theoretically Recoverable Sugar) (Table 1). There is an increase in sucrose hydrolysis forming glucose and fructose (RS). These hexoses enter the glycolytic pathway to provide energy for plant growth, synthesis of proteins and molecules related to secondary metabolism, such as phenolic compounds (Buchanan et al. 2000). As a result, TRS, which is an indicator of the amount of sugar that would be produced, is reduced.
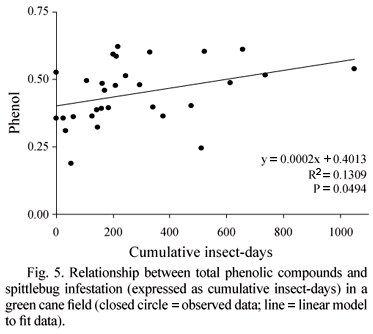
A significant correlation between the cumulative insect-day index and total phenolic compounds was observed (Table 1). The higher the accumulated infestation, the higher was the level of total phenolics in the juice (Fig. 5). Phenol may react with Iron (Fe) (Godshall 1999) or oxidizes itself through the action of the polyphenoloxidase (PPO) enzyme, forming quinones that connect to other cellular compounds such as proteins and starch (Vickers et al. 2005). These substances could be included during the sucrose crystallization, increasing the intensity of the dark color, which negatively affects sugar quality. Not even the clarification process is able to remove the excess of phenolic compounds. In addition, the increase of the phenolic compounds concentration also affects fermentation. The phenolic compounds decreased yeast cell and bud viability, which interfere in fermentation capacity, and consequently in ethanol production (Ravaneli et al. 2006).
The increase of nymph population led to a pH reduction (Fig. 6) probably due to cane deterioration. Egan (1971) reported that pH decrease indicates lower juice quality. However, Stupiello (1992) showed it is not recommended to adopt the pH solely to evaluate sugarcane juice quality, because compounds such as organic acids and gums are formed during cane deterioration.
Starch and dextran contents were not influenced by spittlebug infestation (Table 1). The increase of starch levels in the juice is associated to higher amounts of plant impurities (leaves, tops and sheaths) which are processed together with the stalk (Rein 2005). Dextran is synthesized by Leuconostoc mesenteroides NRRL B512 (F) especially when stalks are subjected to long storage times or tissues are exposed due to damage. Besides, diseases and pests facilitate the entrance of L. mesenteroides in stalks, and high mineral impurity in the cane juice is also related to high dextran levels (Singleton et al. 2001). The low levels of starch and dextran found in this study are probably due to the manual harvest, which eliminates most impurities. Also, stalks were quickly processed for chemical analysis. This reduced the probability of bacterial proliferation.
With the linear regression equations it is possible to estimate loss in cane quality due to spittlebug infestation. In low infestation (< 0.1 nymphs m-1 day-1), 100.29 t ha-1 of stalk yield is expected (Fig. 2), 17.6 of Pol (Fig. 3), pH 5.8 (Fig. 4) and 89.3% purity (Fig. 5). However, with populations of 2.4 and 7.3 nymphs m-1 day-1, which correspond to 200 and 600 cumulative insect days in 82 days, respectively, there would be 8.3% and 29.8% reductions in productivity, 1.9% and 5.8% in Pol, 0.4% and 1.1% in pH and 0.4% and 1.2% in purity. Further studies including a larger range of infestation levels are necessary to consolidate the results obtained in this work because correlations were significant, but the correlation indeces (r2) did show strong influence of CID on yield, pol, pH, purity and phenolic compounds.
Acknowledgments
The authors are grateful to Syngenta Crop Protection Ltda. for financial support; to Louis Dreyfus Commodities Bioenergia S/A São Carlos Sugarmill for technical suport; to CNPq for scholarships given to L. L. Madaleno and G. C. Ravaneli and to Capes for scholarship given to L. E. Presotti; to two anonymous reviewers for suggestions.
Received 26/IX/06. Accepted 09/X/07.
- Buchanan, B., W. Gruissem & R. Jones. 2000. Biochemistry and biology of plants. American Society of Plant Physiologists, Rockville, Maryland, 1367p.
- Chavan, S.M., A. Kumar & S.J. Jadhav. 1991. Rapid quantitative analysis of starch in sugarcane juice. Int. Sugar J. 93:56-59.
- Consecana. 2004. Normas de avaliação da qualidade da cana-de-açúcar. (http://www.unica.com.br/ files/consecana/normasepreços.pdf). Accessed in 21/dec/2004.
- Copersucar. 2001. Manual de controle químico da fabricação de açúcar. Piracicaba, SP, avaliable in CD Room.
- Dinardo-Miranda, L.L. 2003. Cigarrinha-das-raízes em cana-de-açúcar. Instituto Agronômico, Campinas, São Paulo, 70p.
- Dinardo-Miranda, L.L., G. Nakamura, L. Zotarelli, B.A. Braz & O. Euzébio. 2003. Viabilidade técnica e econômica de Actara 250WG, aplicado em diversas doses, no controle da cigarrinha-das-raízes. Stab 22: 39-43.
- Dinardo-Miranda, L.L. & J.M.G. Ferreira. 2004. Eficiência de inseticidas no controle da cigarrinha das raízes Mahanarva fimbriolata (Stal) (Hemiptera: Cercopidae), em cana-de-açúcar. Stab 22: 35-39.
- Dinardo-Miranda, L.L., J.M.G. Ferreira, A.M.P.R. Durigan & V. Barbosa. 2000a. Eficiência de inseticidas e medidas culturais no controle de Mahanarva fimbriolata em cana-de-açúcar. Stab 18: 34-6.
- Dinardo-Miranda, L.L., J.M.G. Ferreira & P.A.M. Carvalho. 2000b. Influência da cigarrinha-das-raízes, Mahanarva fimbriolata, sobre a qualidade tecnológica da cana-de-açúcar. Stab 19: 34-5.
- Dinardo-Miranda, L.L., P. Figueiredo, M.G.A. Landell, J.M.G. Ferreira & P.A.M. Carvalho. 1999. Danos causados pelas cigarrinhas-das-raízes (Mahanarva fimbriolata) a diversos genótipos de cana-de-açúcar. Stab 17: 48-52.
- Egan, B.T. 1971. Post harvest deleration losses in sugarcane. Sugar J. 33: 9-13.
- Godshall, M.A. 1999. Removal of colorants and polysaccharides and the quality of white sugar. In Association A.V. H. 6ş Symposium, 1999, Reims, France, p.28-35.
- Gonçalves, T.D., M.A. Mutton, D. Perecin, J.M. Campanhão & M.J.R. Mutton. 2003. Qualidade da matéria prima em função de diferentes níveis de danos promovidos pela cigarrinha-das-raízes. Stab 22: 29-33.
- Hagley, E.A.C. & J.A. Blackman. 1966. Site of feeding of the sugarcane froghopper, Aeneolamia varia saccharina (Homoptera: Cercopidae). Ann. Entomol. Soc. Am. 59: 1289-1291.
- Lane, J.H. & L. Eynon. 1934. Detemination of reducing sugars by Fehling solution with methylene blue indicator. Norman Rodger, London, 8p.
- Mendonça, A.F., G.V.S. Barbosa & E.J. Marques. 1996. As cigarrinhas da cana-de-açúcar (Hemiptera: Cercopidae) no Brasil, p.171-192. In A.F. Mendonça (ed.), Pragas da cana-de-açúcar. Insetos & Cia, Maceió, 239p.
- Ravaneli, G.C., L.L. Madaleno, L.E. Presotti, M.A. Mutton & M.J.R. Mutton. 2006. Spittlebug infestation in sugarcane affects ethanolic fermentation. Sci. Agri. 6: 534-539.
- Rein, P.W. 2005. The effect of green cane harvesting on a sugar mill. Proceedings of the XXV congress, Internacional society of sugar cane technologists, Guatemala City, 2: 513-520.
- Ruppel, R.F. 1983. Cumulative insect-days as an index of crop protection. J. Econ. Entomol. 76: 375-377.
- SAS Institute.1996. Proprietary software release 6.12. SAS Institute, Cary, NC, USA.
- Scheneider, F., 1979. (ed.), Sugar analysis ICUMSA methods, Peterborough, 256p.
- Silva, R.J.N., E.R. Guimarães, J.F. Garcia, P.S.M. Botelho, M.I.T. Ferro, M.A. Mutton & M.J.R. Mutton. 2005. Infestation of froghopper nymphs changes the amounts of total phenolics in sugarcane. Sci. Agric. 62: 543-546.
- Singleton, V., J. Horn, C. Bucke & M. Adlard. 2001. A new polarimetric method for the analysis of dextran and sucrose. Int. Sugar J. 103: 251-254.
- Stupiello, J.P. 1992. Produção de aguardente: Qualidade da matéria-prima, p.9-21. In M.J.R. Mutton & M.A. Mutton, Aguardente de cana produção e qualidade. Jaboticabal, Funep, 163p.
- Taiz, L. & E. Zeiger. 2004. Fisiologia vegetal. Artmed, Porto Alegre, 719p.
- Tanimoto, T. 1964. The press method of cane analysis. Hawaian Planters Rec. 57: 133-50.
- Thompson, V. 2004. Associative nitrogen fixation, C4 photossyntesis, and the evolution of spittlebugs (Hemiptera: Cercopidae) as major pests of neotropical sugarcane and forage grasses. Bull. Entomol. Res. 94: 189-200.
- Tomizawa, M. & J.E. Casida. 2005. Neonicotinoid insecticide toxicology: Mechanisms of selective action. Annu. Rev. Pharmacol. Toxicol. 45: 247-268.
- Vickers, J.E., C.P.L. Grof, G.D. Bonnett, P.A. Jackson, D.P. Knight, S.E. Roberts & S.P. Robinson. 2005. Overexpression of polyphenol oxidase in transgenic sugarcane results in darkes juice and raw sugar. Crop Sci. 45: 354-362.
Publication Dates
-
Publication in this collection
17 Mar 2008 -
Date of issue
Feb 2008
History
-
Received
26 Sept 2006 -
Accepted
09 Oct 2007