Abstracts
BmMNPV, a Nucleopolyhedrovirus isolated from infected Bombyx mori (L.) larvae in Paraná State - Brazil, was used to inoculate healthy 5th-instar B. mori larvae and examine the infection on central nervous system (CNS) cells. Samples of nervous tissue were removed from the infected insects, at different sampling times, and processed for cytopathology studies by light and transmission electron microscopy using routine techniques. The experiment included both inoculated and non-inoculated larvae (control). BmMNPV infection was detected on the 5th day after inoculation in CNS cells. Initially, infection was characterized by nuclear hypertrophy and the presence of virogenic stroma, in which the progeny virions were produced. Virions are enveloped and occluded into protein crystal, the polyhedra. Lyses of infected CNS cells were undetected; however, free mature polyhedra were seen in spaces inside the CNS. These polyhedra possibly came from trachea that penetrate the CNS and its cells, which are susceptible to BmMNPV and lyses after infection. We conclude that the tracheal system is responsible for disseminating BmMNPV infection in B. mori CNS and that the tracheal branches allow non-occluded virions to pass through the blood-brain barrier.
Insect; silkworm; Baculoviridae; neural lamella; perineurium
O Nucleopolyhedrovirus é um vírus que infecta uma série de insetos, como o bicho-da-seda, Bombyx mori (L.). Um isolado geográfico do vírus, o Bombyx mori Nucleopolyhedrovirus múltiplo (BmMNPV), foi utilizado para se analisar a citopatologia em células do sistema nervoso central (SNC) de lagartas de B. mori. O BmMNPV foi inoculado experimentalmente e segmentos do tecido nervoso foram processados para microscopia de luz e microscopia eletrônica de transmissão. Células do SNC (nervosas, gliais e do perineuro) revelaram indícios de infecção no 5° dia pós-inoculação com o BmMNPV, cujas características citopatológicas foram: hipertrofia nuclear, presença do estroma virogênico, onde são sintetizados os virions, e formação dos poliedros. Não foi observada lise das células do SNC infectadas, uma característica das infecções pelo NPV; contudo, poliedros maduros foram evidenciados em espaços nos gânglios e conectivos nervosos. Esses poliedros possivelmente são oriundos das traquéias que penetram no sistema nervoso, e suas células, susceptíveis ao BmMNPV, sofrem lise após infecção. Os resultados indicam, ainda, que o sistema traqueal é responsável pela dispersão da infecção causada pelo BmMNPV no SNC de lagartas de B. mori. Neste sentido, os ramos que formam o sistema traqueal possibilitam o rompimento da barreira hemolinfa/sistema nervoso, permitindo que os virions tenham acesso 'a matriz extracelular do SNC e, conseqüentemente, às suas células constituintes.
Insecta; bicho-da-seda; Baculoviridae; lamela neural; perineuro
SYSTEMATICS, MORPHOLOGY AND PHYSIOLOGY
Nucleopolyhedrovirus infected central nervous system cells of Bombyx mori (L.) (Lepidoptera: Bombycidae)
Citopatologia causada pelo Nucleopolyhedrovirus em células do sistema nervoso central de Bombyx mori (L.) (Lepidoptera: Bombycidae)
Ednéia F.B. TorquatoI; Marcilio H. de Miranda NetoII; Rose M.C. BrancalhãoI
ILaboratório de Biologia Celular. Universidade Estadual do Oeste do Paraná - Campus de Cascavel R. Universitária, 2069 - Jardim Universitário, 85814-110, Cascavel, PR
IILaboratorio de Anatomia Humana. Universidade Estadual de Maringá, Avenida Colombo, 5790 - Jardim Universitário, 87020-900, Maringá, PR
ABSTRACT
BmMNPV, a Nucleopolyhedrovirus isolated from infected Bombyx mori (L.) larvae in Paraná State Brazil, was used to inoculate healthy 5th-instar B. mori larvae and examine the infection on central nervous system (CNS) cells. Samples of nervous tissue were removed from the infected insects, at different sampling times, and processed for cytopathology studies by light and transmission electron microscopy using routine techniques. The experiment included both inoculated and non-inoculated larvae (control). BmMNPV infection was detected on the 5th day after inoculation in CNS cells. Initially, infection was characterized by nuclear hypertrophy and the presence of virogenic stroma, in which the progeny virions were produced. Virions are enveloped and occluded into protein crystal, the polyhedra. Lyses of infected CNS cells were undetected; however, free mature polyhedra were seen in spaces inside the CNS. These polyhedra possibly came from trachea that penetrate the CNS and its cells, which are susceptible to BmMNPV and lyses after infection. We conclude that the tracheal system is responsible for disseminating BmMNPV infection in B. mori CNS and that the tracheal branches allow non-occluded virions to pass through the blood-brain barrier.
Key words: Insect, silkworm, Baculoviridae, neural lamella, perineurium
RESUMO
O Nucleopolyhedrovirus é um vírus que infecta uma série de insetos, como o bicho-da-seda, Bombyx mori (L.). Um isolado geográfico do vírus, o Bombyx mori Nucleopolyhedrovirus múltiplo (BmMNPV), foi utilizado para se analisar a citopatologia em células do sistema nervoso central (SNC) de lagartas de B. mori. O BmMNPV foi inoculado experimentalmente e segmentos do tecido nervoso foram processados para microscopia de luz e microscopia eletrônica de transmissão. Células do SNC (nervosas, gliais e do perineuro) revelaram indícios de infecção no 5° dia pós-inoculação com o BmMNPV, cujas características citopatológicas foram: hipertrofia nuclear, presença do estroma virogênico, onde são sintetizados os virions, e formação dos poliedros. Não foi observada lise das células do SNC infectadas, uma característica das infecções pelo NPV; contudo, poliedros maduros foram evidenciados em espaços nos gânglios e conectivos nervosos. Esses poliedros possivelmente são oriundos das traquéias que penetram no sistema nervoso, e suas células, susceptíveis ao BmMNPV, sofrem lise após infecção. Os resultados indicam, ainda, que o sistema traqueal é responsável pela dispersão da infecção causada pelo BmMNPV no SNC de lagartas de B. mori. Neste sentido, os ramos que formam o sistema traqueal possibilitam o rompimento da barreira hemolinfa/sistema nervoso, permitindo que os virions tenham acesso 'a matriz extracelular do SNC e, conseqüentemente, às suas células constituintes.
Palavras-chave: Insecta, bicho-da-seda, Baculoviridae, lamela neural, perineuro
Nuclear polyhedrosis viruses (NPV) are entomopathogenic, belong to the family Baculoviridae (Murphy et al. 1995, Herniou et al. 2003), and infect mainly holometabolous lepidoptera, such as the silkworm, Bombyx mori (L.). B. mori infection by NPV results in insect developmental changes, leading to farmers' economic losses and to serious problems for the silk industry (Sengupta et al. 1990).
In Brazil, NPV infected B. mori caterpillars in the States of São Paulo and Paraná (Brancalhão 1998). Studies conducted with this pathogen in Paraná, the largest silk producer in the country, led to the identification of the multiple viral phenotype, BmMNPV (Brancalhão 2002). In these studies, analyses of viral symptoms, histology, and cytology showed infection of several silkworm caterpillar tissues. However, there is insufficient information about cell infection in the host's central nervous system (CNS).
We studied the susceptibility of CNS cells of B. mori caterpillars to the geographic isolate of BmMNPV. We also analyzed the system entrance route for virions, to understand BmMNPV infectious cycle.
Materials and Methods
Forth-instar, hybrid B. mori caterpillars were obtained from silk companies in the State of Paraná. At the time of ecdysis to 5th instar, the caterpillars were fed on mulberry foliar discs that were previously sprayed with a viral suspension of BmMNPV, 2 x 108 POBs/ml (POBs = polyhedral occlusion bodies); previously infected caterpillars were removed. Twenty µl of viral suspension were sprayed on each foliar disc (2 cm3 diameter); after drying at room temperature, the suspension was given to the unfed caterpillars.
We conducted four inoculation experiments with BmMNPV, each with 50 caterpillars: 25 were fed on foliar discs with viral suspension and 25 on control foliar discs. During feeding, caterpillars were individually placed in disposable cups until the foliar disc and the viral suspension were completely ingested.
Next, the caterpillars were identified and placed in cardboard boxes; then, they were transferred to a room under controlled temperature and humidity. Caterpillars fed on mulberry leaves without BmMNPV daily.
Caterpillars were dissected in different post-inoculation (p.i.) periods for light and electronic microscopy analyses. Dissection started on the 3rd day p.i. and finished on the 7th day p.i., at 24h intervals. Caterpillars were anesthetized with ethylic ester on dorsal position, and their tegument was cut in length, from the anal region to the head. The intestine was removed to show the nervous system. Cerebral, thoracic and abdominal ganglia, and nerve connectives were removed and fixed in DuBosq Brasil (Beçak & Paulete 1976) for 24h, at 4ºC. Some of the material was fixed in modified Karnovsky (Karnovsky 1965), for transmission electronic microscopy.
For light microscopy analysis, nervous system segments were washed in alcohol 70%, following routine histological techniques with adaptations (Brancalhão 1998). Sequencial, 3 µm to 7 µm-thick cuts were stained, following the hematoxylin and eosin technique (Junqueira & Junqueira 1983) for the study of nervous systems; the azan technique, modified for viral occlusion (Hamm 1966), was used for analyzing cell and tissue infection caused by BmMNPV.
After light microscopy analyses and the confirmation of nervous system cell infection by BmMNPV, the material was fixed for electronic microscopy, following Brancalhão (1998).
Control caterpillars were analyzed following the same procedures as for materials inoculated with BmMNPV.
Results and Discussion
Light microscopy showed that on the 5th day p.i., cerebral, thoracic and abdominal ganglia cells, and nerve connectives cells had their first symptoms of infection by BmMNPV. Fatty and tracheal tissues in histological cuts revealed first symptoms of infection after the third and 4th days p.i., respectively. These p.i. periods repeated those obtained by Moto et al. (2003), who used recombining BmNPV. Although p.i. periods in our study were different from periods shown by Brancalhão (2002), differences frequently occur in infections by NPV due to several intervening factors, such as caterpillar age, temperature, food availability, population density, incidence of other pathogens, quantity of ingested viruses, and virus virulence (Granados & Williams 1986, Adams & McClintock 1991).
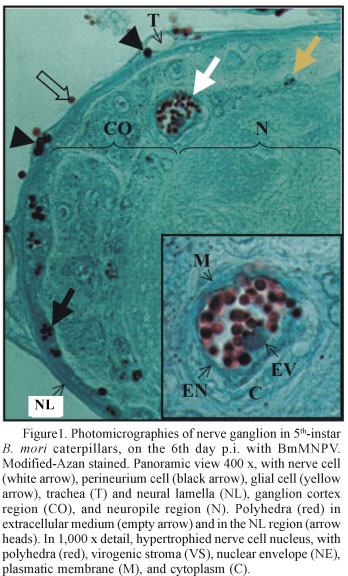
Polyhedra sizes were inversely proportional to their numbers and varied among and within cellular types; within each nucleus, all polyhedra were usually at the same development stage (Fig. 1). The quantity of polyhedra per infected nucleus varied in the same direction as polyhedra size; we observed nuclei with large and small quantities of polyhedra, and nuclei with one polyhedron.
Variations in size and quantity of polyhedra are common in NPVs, and can be related to number of occluded nucleocapsids, to polyhedra development stages, to infected-cell metabolism, and to virus genetic variation (Adams & McClintok 1991, Murphy et al. 1995). Polyhedra position in the nuclei, and ganglia and nerve connectives' angulation and depth during microscopic preparations can increase virus genetic variation still more (Brancalhão 1998).
B. mori CNS cells showed hypertrophic nuclei containing the virogenic stroma, virions, and polyhedric occlusion bodies (Figs.1 e 2 ), thus confirming infection by BmMNPV, which are similar to infections found in other tissues (Brancalhão 2002, Brancalhão & Ribeiro 2003). These authors also found that the cytolysis ends the NPV infectious cycle in host cells, thereby freeing the polyhedra into the extracellular medium. During the p.i. periods studied, lyses of nerve, glia and perineurium cells in ganglia and connectives were not observed, and the polyhedra remained in the infected nuclei (Fig. 1). In spite of the absence of cytolysis in the histopatologic analyses, polyhedra were found in spaces inside the ganglia (Fig. 3) and connectives, and between layers of the neural lamella (NL) (Fig. 1). The NL, a non-cellular structure that surrounds ganglia and nerve connectives, is formed by macromolecules organized in concentric layers. The NL maintains the nervous system format and helps to keep the appropriate ionic balance for neural metabolism (Chapman 1998).
The tracheal system is the NPV target. Previous studies (Brancalhão 2002) have shown that the tracheal cells develop lyses after viral infection, thus freeing a large quantity of polyhedra into the extracellular medium (Fig. 3). In the nervous system, the trachea enters the NL (Figs. 1 and 3) and ramifies within the ganglia and nerve connectives (Chapman 1998). The polyhedra freed by lyse of these cells are maintained between NL layers, disorganizing it (Fig. 1).
NL is a basal lamina (BL) that occurs in the nerve tissue (Schofield 1989, Carlson et al. 2000), being thick during the 5th-instar larval stage (Ashhurst & Richards 1964, Osinska 1981). In Lepidoptera, BL differs according to insect instar and development stage in each tissue (Sedlak & Gilbert 1979, Locke 1986). According to Etzel (1973), BL thinning can help to disperse entomopathogenic viruses. Tanada et al. (1984) argue that tracheal cell infection by NPV is caused by the lateral virus movement in BL. Based on these observations, BL organization and thickness seem to influence the passage of particles through its structure. Reddy & Locke (1990) studied BL behavior of 5th instar Calpodes ethlius (L.) (Lepidoptera: Hesperiidae), concerning the passage of gold particles. They found that particles smaller than 15 nm crossed the BS of fatty, epidermal, and pericardial tissues, whereas particles larger than 6 nm were retained in the silk gland BL, in the Malpighian tubule, in the muscle, and in the connective of the midgut connective tissue.
In 5th instar B. mori caterpillars, NL has a resistant structure organized in concentric layers, which is not completely removed when the insect leaves the pupa stage (Steopoe & Dornesco 1935). In addition, BmMNPV size (95 nm diameter and 315 nm length) (Brancalhão 2002) suggest that NL and perineurium cells can act as effective barriers against the passage of virion from insect hemolymph and other infected tissues (Engelhard et al. 1994, Brancalhão 2002). In perineurium cells, which are located below the NL, the simple squamous epithelium, maintained by mainly thin and septate intercellular junctions, is an efficient barrier against small molecules and ions (Carlson et al. 2000, Kniesel & Wolburg 2000). In the nervous system, dispersion of the infection caused by BmMNPV does not seem to occur by virions in the hemolymph because the virions can not reach the organ due to the nervous system/hemolymph barrier provided by NL and perineurium cells.
The tracheal system is important for dispersing viral infection in insects, particularly because infection can spread to all tissues, being intimately associated with its cells. Infection can also spread as tissues grow, by tracheoblasts. As tracheoblasts cross the NL, they let virions break the barrier, and the tracheal epithelium develops a continuous system that improves viral dispersion (Elazar et al. 2001). As mentioned before, the trachea in B. mori nervous system was infected by BmMNPV after the 4th day p.i; the nervous system was infected on the 5th day p.i. Therefore, during the NPV replication process in the nuclei of trachea cells, several virions sprout and reach the extracellular matrix in the nervous system. By endocytosis (Blissard 1996, Herniou et al. 2003), the virions penetrate the cytoplasm of glial, nerve, and perineurium cells. In the nuclei of these cells, the virions start a new reproductive cycle until polyhedra formation. Consequently, the trachea can in fact be a medium for infection dispersion in B. mori central nervous system.
However, not all nervous system cells of infected caterpillars had signs of infection by BmMNPV, and had a healthy appearance (Fig. 3). This can be a result of the differential expression of viral genes in the host cell's genome because NPVs usually replicate assynchronically (Adams & McClintock 1991, Brancalhão 2002). Nonetheless, the hypothesis that some cells are coded to bypass invasion and replication mechanisms, therefore showing resistance to some viruses (Hess & Falcon 1987), cannot be forgotten. Furthermore, the different kinds of nerve and glial cells in insect ganglia (Auld 1999, Oland & Tolbert 2003) can respond differently to the inoculated virus.
Acknowledgment
I thank the silk companies in the State of Paraná (BRATAC, COCAMAR e KANEBO), for providing the caterpillars; the Núcleo de Apoio a Pesquisa em Microscopia Eletrônica Aplicada à Pesquisa Agropecuária (NAP/MEPA), Escola Superior de Agricultura "Luiz de Queiroz"/Universidade de São Paulo (ESALQ/USP), Piracicaba, SP, for providing equipment and facilities during virus preparation; Professors Marcílio Hübner de Miranda Neto and Rose Meire Costa Brancalhão for the advice and dedication.
Received 31/I/05 - Accepted 24/VI/05.
- Adams, J.R. & J.T. Mcclintock. 1991. Baculoviridae Nuclear polyhedrosis viruses. Part 1. Nuclear polyhedrosis viruses of insects, p. 89-180. In J.R.Adams & J.R.Bonami (eds.), Atlas of invertebrate viruses. Florida, CRC Press, 684p.
- Ashhurst, D.E. & A.G. Richards. 1964. A study of the changes occurring in the connective tissue associated with the central nervous system during the pupal stage of the wax moth, Galleria mellonella L. J. Morph. 114: 225-236.
- Auld, V. 1999. Glia as mediators of growth cone guidance: Studies from insect nervous system. Cell Mol. Life Sci. 55: 1377-1385.
- Beçak, W. & Paulete J. 1976. (ed.) Técnicas de citologia e histologia.v. I. Rio de Janeiro, Livros Técnicos e Científicos, 305p.
- Blissard, G.W. 1996. Baculovirus-insect cell interactions. Cytotechnology 20: 73-93.
- Brancalhão, R.M.C. 1998. Nucleopolyhedrovirus em Bombyx mori L., 1758 (Lepidoptera: Bombycidae), no estado do Paraná. Tese de doutorado, Curitiba, UFPR, 99p.
- Brancalhão, R.M.C. 2002. Vírus entomopatogênico no bicho-da-seda: Taxonomia e citopatologia causada por Nucleopolyhedrovirus em células de Bombyx mori Biotec. 24: 54-58.
- Brancalhão, R.M.C. & L.F.C. Ribeiro. 2003. Citopatologia da infecção causada por BmNPV no tegumento de Bombyx mori L., 1758 (Lepidoptera: Bombycidae). Arq. Ciên. Vet. Zool. 6:15-20.
- Carlson, S.D., J.L. Juang, S.L. Hilgers & M.B. Garment. 2000. Blood Barriers of the insect. Ann Rev. Entomol. 45: 151-174.
- Chapman, R.F. 1998. (ed.) The insect structure and function. New York, American Elsevier Publishing Company, 770p.
- Elazar, M., L. Rafi & E. Zlotkin 2001. Targeting of an expressed neurotoxin by its recombinant baculovirus. J. Exp. Biol. 204: 2637-2645.
- Engelhard E.K., L.N.W. Kam-Morgan, J.O. Washburn & L.E. Volkman. 1994. The insect tracheal system: A conduit for the systemic spread of Autographa californica M nuclear polyhedrosis virus. Proc. Natl. Acad. Sci. USA. 91: 3224-3227.
- Etzel, L. 1973. Purification and partial characterization of the Granulosis virus of codling moth wit emphasis on trans-ovum and transstadial transmission. Ph. D. thesis, University of California, Berkeley, 57p.
- Granados, R.R. & K.A. Williams. 1986. In vivo infection and replication of baculovirus, p.89-108. In R.R. Granados & B.A. Federici. (eds.), The biology of baculoviruses. Florida, CRC Press, 259p.
- Hamm, J.J. 1966. A modified azan staining technique for inclusion body viruses. J. Invertebr. Pathol. 8: 125-126.
- Herniou, E.A., J.A. Olszewski, J.S. Cory & D.R.O. Reilly. 2003. The genome sequence and evolution of baculoviruses. Annu. Rev. Entomol. 48: 211-234.
- Hess, R.T. & L.A. Falcon. 1987. Temporal events in the invasion of the codling moth, Cydia pomonella, by a granulosis virus: an electron microscope study. J. Invert. Pathol. 50: 85-105.
- Junqueira, L.C. & L.M.M.S. Junqueira. 1983. (eds.) Técnicas básicas de citologia e histologia. São Paulo, Santos, 123p.
- Karnovsky, M.J. 1965. A formaldehyde-glutaraldehyde fixative of high osmolarity for use in electron microscopy. J. Cell. Biol. 27: 137A.
- Kniesel, U. & H. Wolburg. 2000. Tight junctions of the blood-brain barrier. Cell. Mol. Neurobiol. 20: 57-76.
- Locke, M. 1986. The development of the plasma membrane reticular system in the fat body of an insect. Tissue Cell 18: 853-867.
- Moto, K., H. kojima, M. Kurihara, M. Iwami & S. Matsumoto. 2003. Cell-specific expression of enhanced green fluorescence protein under the control of neuropeptide gene promoters in the brain of the silkworm, Bombyx mori, using Bombyx mori nucleopolyhedrovirus-derived vectors. Insec. Biochem. Mol. Biol. 33: 7-12.
- Murphy, F.A., C.M. Fauquet, D.H.L. Bishop, S.A. Ghabrial, A.W. Jarvis, G.P. Martelli, M.A. Mayo & M.D. Summers. 1995. (eds.) Virus taxonomy: Classification and nomenclature of viruses. Sixth Report of the International Committee on Taxonomy of Viruses. New York, Springer-Verlag, Wien, 586p.
- Oland, L.A. & L.P. Tolbert. 2003. Key interactions between neurons and glial cells during neural development in insects. Annu. Rev. Entomol. 48: 89-110.
- Osinska, H.E. 1981. Ultrastructural study of the postembryonic development of the neural lamella of Galleria melonella L. (Lepidoptera). Cell. Tissue Res. 217: 425-433.
- Reddy, J.T. & M. Locke. 1990. The size limited penetration of gold particles through insect basal lamina. J. Insect Physiol. 36: 397-407.
- Schofield, P.K. 1989. Electrical charge properties of connective tissue in the insect central nervous system, with regard to ionic homeostasis of the nerve cell environment. Symp. Soc. Exp. Biol. 43: 379- 388.
- Sedlak, B.J. & L.I. Gilbert. 1979. Correlation between epidermal cell structure and endogenous hormone titers during the fifth larval instar of the tobacco hornworm, Manduca sexta Tissue Cell. 11: 642-653.
- Sengupta, K., P. Kumar, M. Baig & M. Govindaiah. 1990. (eds.) Handbook on pest and disease control of mulberry and silkworm. Bangkok, UNESCAP - United Nations Economic and Social Commission for Asia and the Pacific, 88p.
- Steopoe, J. & G.T. Dornesco. 1935. Études sur le système nerveux des insects pendant la metamorphose. La gaine periganglionnaire. Arch. Zool. Exp. Gén. 78: 99-115.
- Tanada, Y., R.T. Hess & E.M. Omi. 1984. The movement and invasion of an insect baculovirus in tracheal cells of the armyworm, Pseudaletia unipuncta J. Invertbr. Pathol. 44: 198-208.
Publication Dates
-
Publication in this collection
03 Apr 2006 -
Date of issue
Feb 2006
History
-
Accepted
24 June 2005 -
Received
31 Jan 2005