Abstracts
Even though much improvement has been made in plant transformation methods, the screening of transgenic plants is often a laborious work. Most approaches for detecting the transgene in transformed plants are still timeconsuming, and can be quite expensive. The objective of this study was to search for a simpler method to screen for transgenic plants. The infiltration of kanamycin (100 mg/mL) into tobacco leaves resulted in conspicuous chlorotic spots on the non-transgenic plant leaves, while no spots were seen on the leaves of transformed plants. This reaction occurred regardless of age of the tested plants, and the method has proven to be simple, fast, non-destructive, relatively cheap, and reliable. These results were comparable to those obtained by the polymerase chain reaction (PCR) amplification of the transgene using specific primers.
kanamycin; genetically modified organisms; transgenic plants; testing
Apesar do grande desenvolvimento observado na área de transformação de plantas, a avaliação de plantas transgênicas ainda é difícil de ser realizada. Os métodos mais comuns de detecção do transgene em plantas transformadas ainda são demorados e de alto custo. O objetivo deste estudo foi testar um método simples de avaliação de plantas de fumo transgênicas. Para isso, 100 mg/mL de canamicina foi infiltrada em folhas de fumo, resultando no aparecimento de manchas cloróticas nas folhas de plantas não-transgênicas e nenhuma mancha nas folhas de plantas transformadas. Essa reação ocorreu independentemente da idade das plantas testadas e evidenciou a simplicidade, rapidez, confiabilidade e baixo custo do método. Os resultados foram comparáveis aos obtidos por amplificações do transgene, utilizando-se primers específicos, por meio da reação da polimerase em cadeia (PCR).
canamicina; organismo geneticamente modificado; planta transgênica; teste
SCIENTIFIC NOTES
A simple and reliable method for the screening of transgenic tobacco plants1 1 Extracted from Ph.D. dissertation presented by the first author to the University of Florida (UF), Florida, USA. Supported by the Florida Agricultural Experiment Station and the USDA-Tropical/Subtropical Agriculture Research Program
Método simples e confiável para avaliação de plantas de fumo transgênicas
Juliana Freitas-AstuaI; Gustavo Astua-MongeII; Jane Elisabeth PolstonIII; Ernest HiebertIV
IEmbrapa-Centro Nacional de Pesquisa de Milho e Sorgo, Caixa Postal 151, CEP 35701-970 Sete Lagoas, MG. Email: jfastua@cnpms.embrapa.br
IIInstituto Agronômico, Centro Avançado de Pesquisa Tecnológica do Agronegócio de Citros Sylvio Moreira, Caixa Postal 4, CEP 13490-970 Cordeiropólis, SP. E-mail: gamo@centrodecitricultura.br IIIUF, Gulf Coast Research & Education Center, 5007 60th St. East, Bradenton, FL, 34203, USA. Email: jep@mail.ifas.ufl.edu
IVUF, Plant Pathology Dept, 1453 Fifield Hall, Gainesville, FL, 32611, USA. Email: ehi@mail.ifas.ufl.edu
ABSTRACT
Even though much improvement has been made in plant transformation methods, the screening of transgenic plants is often a laborious work. Most approaches for detecting the transgene in transformed plants are still timeconsuming, and can be quite expensive. The objective of this study was to search for a simpler method to screen for transgenic plants. The infiltration of kanamycin (100 mg/mL) into tobacco leaves resulted in conspicuous chlorotic spots on the non-transgenic plant leaves, while no spots were seen on the leaves of transformed plants. This reaction occurred regardless of age of the tested plants, and the method has proven to be simple, fast, non-destructive, relatively cheap, and reliable. These results were comparable to those obtained by the polymerase chain reaction (PCR) amplification of the transgene using specific primers.
Index terms: kanamycin, genetically modified organisms, transgenic plants, testing.
Resumo
Apesar do grande desenvolvimento observado na área de transformação de plantas, a avaliação de plantas transgênicas ainda é difícil de ser realizada. Os métodos mais comuns de detecção do transgene em plantas transformadas ainda são demorados e de alto custo. O objetivo deste estudo foi testar um método simples de avaliação de plantas de fumo transgênicas. Para isso, 100 mg/mL de canamicina foi infiltrada em folhas de fumo, resultando no aparecimento de manchas cloróticas nas folhas de plantas não-transgênicas e nenhuma mancha nas folhas de plantas transformadas. Essa reação ocorreu independentemente da idade das plantas testadas e evidenciou a simplicidade, rapidez, confiabilidade e baixo custo do método. Os resultados foram comparáveis aos obtidos por amplificações do transgene, utilizando-se primers específicos, por meio da reação da polimerase em cadeia (PCR).
Termos para indexação: canamicina, organismo geneticamente modificado, planta transgênica, teste.
Plant transformation has become a very important and common tool in biological studies. A difficult aspect of this technology, however, is that it often requires screening of a large number of lines to identify the desired transformants. The neomycin phosphotransferase II (nptII) gene product confers resistance to kanamycin and some related aminoglycosides and has been one of the most widely used selectable markers for screening transformed cells and plants since the early 1980's (Bevans et al., 1983; Herrera-Estrella et al., 1983). However, according to Xiang et al. (1999), its use has not evolved along with the improvements in transformation technology, since most approaches for detecting the nptII gene or its product in transgenic plants are still laborious and time-consuming, especially for the screening of large populations of transformants (Weide et al., 1989). In addition, some of these tests can be quite expensive and, in many instances, sensitive transformants can be lost for further analysis (Weide et al., 1989).
A common screening method using the addition of filteredsterilized kanamycin to the sowing medium requires the use of seeds and thus, plants from R1 or more advanced generations. Furthermore, this method is sampledestructive for the non-transformed plants, since such plants are killed by the antibiotic. This characteristic might be undesirable for evaluating nontransformed plants that have come through the transformation protocol and can be used as controls.
An alternative to this method is the use of small leaf disks from the sample plants. However, not only the in vitro step is maintained but this method also involves an additional step of surface sterilization of the tissue, which is often difficult to obtain, particularly when the plant has large amounts of trichomes, as some tobacco varieties.
Other methods used for the screening of transformed plants include PCR and Southern blot to detect either the presence of the transgene itself or the nptII gene, and ELISA to test for the presence of the NPTII protein. However, these assays are more expensive and may not be feasible for the assessment of large amount of samples.
It should be noted that some less wellequipped laboratories, particularly in developing countries, have been heavily involved in the assessment of transgenic plants, even though many of them do not participate in the actual process of transformation. In order to facilitate the screening of transgenic plants, efforts have been made to develop simpler methods to test the presence of the nptII gene product. Weide et al. (1989) and Xiang et al. (1999) reported high efficiency in the detection of nptII gene product through the resistance to kanamycin when transformed tomato and Arabidopsis plants were sprayed with this antibiotic, respectively. However, such reported efficiency was not observed in the present study. When kanamycin was sprayed onto five non-transgenic Nicotiana tabacum cv. Xanthi (tobacco) plants, the results were not conclusive and only two of them exhibited the expected chlorotic spots.
The objective of this study was to assess the possibility of using a simpler and yet reliable method to screen for transgenic plants.
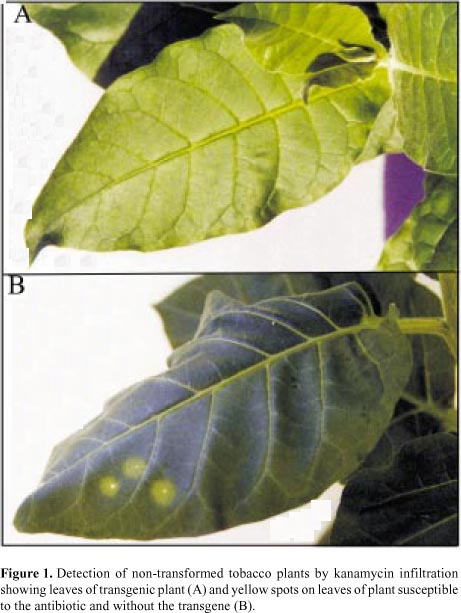
Consistent results were observed when intermediary leaves of tobacco were infiltrated with a 100 mg/mL kanamycin solution, using a syringe and a needle. The volume of the solution infiltrated varied according to the leaf size, but did not seem to interfere with the results. Conspicuous chlorotic spots were seen on the non-transgenic plants, whereas no spots were seen on the transformed plants (Figure 1). The first chlorotic symptoms appeared three to four days after the assay was performed, and the results were clearer 10 to 14 days post-infiltration, regardless of the age/size of the plants tested, which varied from the 3 to 4-leaf stage to adult plants.
A comparison of NPTII detection through kanamycin infiltration (K) and transgene detection by PCR amplification (PCR) using specific primers (FreitasAstua, 2001) is presented in Table 1. The infiltration of the kanamycin into tobacco leaves was effective in identifying transgenic from the non-transformed plants (Table 1). The kanamycin infiltration test and the transgene amplification by PCR exhibited an extremely high level of agreement when transgenic R1 and R2 plants were screened. A lower level of agreement was seen when R0 plants were tested. Most result disagreements between the two techniques occurred with plants that did not have the transgene. This observation has minimal negative implication, since no transgenic plants would be erroneously discarded. Moreover, higher correlation levels were identified in plants of R1 and R2 than R0 generations, indicating that the infiltration test tends to be more reliable as the selfing progresses and the transgene is integrated into the genome. The overall correlation coefficient between infiltration method and PCR analysis was 0.993.
The reliability of the test indicates that it can be used for the first screening of transgenic tobacco plants. Furthermore, the test is simple, fast to perform, non-destructive, relatively cheap, and can be done at any developmental stage of the plant.
Acknowledgements
To Eugene Crawford and Alba R. NavaFereira, for their help with the greenhouse work; to Conselho Nacional de Desenvolvimento Científico e Tecnológico, for the fellowship to the first author.
Accepted for publication on April 2, 2003
- BEVANS, M. W.; FLAVELL, R. B.; CHILTON, M. D. A chimaeric antibiotic resistance gene as a selectable marker for plant cell transformation. Nature, London, v. 304, p. 184-187, 1983.
- FREITAS-ASTUA, J. Characterization of resistance in transgenic tobacco plants expressing begomovirus genes 2001. 99 p. Dissertation (Ph.D. in Plant Pathology) - University of Florida, Gainesville, FL.
- HERRERA-ESTRELLA, L.; DE BLOCK, M.; MESSENS, E.; HERNALSTEENS, J. P.; MONTAGU, M. van; SCHELL, J. Chimeric genes as dominant selectable markers in plant cells. EMBO Journal, Oxford, v. 2, p. 987-995, 1983.
- WEIDE, R.; KOORNNEED, M.; ZABEL, P. A simple, nondestructive spraying assay for the detection of an active kanamycin resistance gene in transgenic tomato plants. Theoretical and Applied Genetics, Berlin, v. 78, p. 169-172, 1989.
- XIANG, C.; HAN, P.; OLIVER, D. J. In solium selection for Arabidopsis transformants resistant to kanamycin. Plant Molecular Biology Reporter, Dordrecht, v. 17, p. 59-65, 1999.
Publication Dates
-
Publication in this collection
03 Feb 2004 -
Date of issue
July 2003
History
-
Accepted
02 Apr 2003