Abstract
The accumulation of polymeric residues has been one of the most impacting environmental problems in recent human history, coming, above all, from disposable artefacts, such as plastic bags. Processing polyolefins with pro-oxidant additives is an alternative to favour the abiotic degradation process of macromolecules, including thermooxidation, so that the oxygenated fragments produced can be assimilated by microorganisms. The objective of this work was to evaluate the process of thermomechanical oxidative degradation of polyethylene (PE) during tubular extrusion of HDPE/LDPE films, without and with 1% of two different pro-oxidants, d2wTM and benzoin. The results of viscosimetric and MFI analyses indicated smaller chain sizes in the additivated films. The FTIR spectra and contact angles indicate a higher presence of polar functional groups in the samples with pro-oxidants. The surface morphological analysis by SEM indicated difference of PE homogeneity in the films. Benzoin, however, proved to be a better pro-oxidant than d2wTM.
Keywords:
benzoin; d2wTM; polyethylene; pro-oxidants; thermooxidation
1. Introduction
The use of polymeric materials has been growing worldwide since the 1940s, replacing the use of metals, ceramics and wood in many industrial branches[11 Thompson, R. C., Moore, C. J., vom Saal, F. S., & Swan, S. H. (2009). Plastics, the environment and human health: current consensus and future trends. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 364(1526), 2153-2166. http://dx.doi.org/10.1098/rstb.2009.0053. PMid:19528062.
http://dx.doi.org/10.1098/rstb.2009.0053...
2 Vázquez-Morillas, A., Beltrán-Villavicencio, M., Alvarez-Zeferino, J. C., Osada-Velázquez, M. H., Moreno, A., Martínez, L., & Yañez, J. M. (2016). Biodegradation and ecotoxicity of polyethylene films containing pro-oxidant additive. Journal of Polymers and the Environment, 24(3), 221-229. http://dx.doi.org/10.1007/s10924-016-0765-8.
http://dx.doi.org/10.1007/s10924-016-076...
-33 Silva, E. A., & Moita Neto, J. M. (2015). Environmental impacts the production of polyethylene bottles in a Teresina-PI factory. Polímeros: Ciência e Tecnologia, 25(No. esp.), 59-67. http://dx.doi.org/10.1590/0104-1428.1949.
http://dx.doi.org/10.1590/0104-1428.1949...
]. Parallel to this, a major environmental problem regarding the use of this class of materials arises: the accumulation of plastic waste in the environment (soil, rivers and oceans) due to incorrect and unconscious disposal, especially of disposable items, such as plastic bags, sacks, cups and bottles[33 Silva, E. A., & Moita Neto, J. M. (2015). Environmental impacts the production of polyethylene bottles in a Teresina-PI factory. Polímeros: Ciência e Tecnologia, 25(No. esp.), 59-67. http://dx.doi.org/10.1590/0104-1428.1949.
http://dx.doi.org/10.1590/0104-1428.1949...
4 Vasconcelos, Y. (2019). Planeta plástico. Pesquisa FAPESP, 281, 18-24. Retrieved in 2021, August 1, from https://revistapesquisa.fapesp.br/planeta-plastico/
https://revistapesquisa.fapesp.br/planet...
-55 Thompson, R. C., Swan, S. H., Moore, C. J., & vom Saal, F. S. (2009). Our plastic age. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 364(1526), 1973-1976. http://dx.doi.org/10.1098/rstb.2009.0054. PMid:19528049.
http://dx.doi.org/10.1098/rstb.2009.0054...
]. Due to their low specific masses, easier processing and low cost, petrochemical resins are the most widely used and are also the most difficult to degrade, including PE[66 Silva, E. A., & Moita, J. M., No. (2016). Possibilities for environmental improvements in the polyethylene recycling process. Polímeros: Ciência e Tecnologia, 26(No. esp.), 49-54. http://dx.doi.org/10.1590/0104-1428.1954.
http://dx.doi.org/10.1590/0104-1428.1954...
7 Koutny, M., Lemaire, J., & Delort, A.-M. (2006). Biodegradation of polyethylene films with prooxidant additives. Chemosphere, 64(8), 1243-1252. http://dx.doi.org/10.1016/j.chemosphere.2005.12.060. PMid:16487569.
http://dx.doi.org/10.1016/j.chemosphere....
8 Lucas, N., Bienaime, C., Belloy, C., Queneudec, M., Silvestre, F., & Nava-Saucedo, J.-E. (2008). Polymer biodegradation: mechanisms and estimation techniques. Chemosphere, 73(4), 429-442. http://dx.doi.org/10.1016/j.chemosphere.2008.06.064. PMid:18723204.
http://dx.doi.org/10.1016/j.chemosphere....
9 Ammala, A., Bateman, S., Dean, K., Petinakis, E., Sangwan, P., Wong, S., Yuan, Q., Yu, L., Patrick, C., & Leong, K. H. (2011). An overview of degradable and biodegradable polyolefins. Progress in Polymer Science, 36(8), 1015-1049. http://dx.doi.org/10.1016/j.progpolymsci.2010.12.002.
http://dx.doi.org/10.1016/j.progpolymsci...
-1010 Passos, T. M., Marconato, J. C., & Franchetti, S. M. M. (2015). Biodegradation of films of low density polyethylene (LDPE), poly(hidroxibutyrate-co-valerate) (PHBV), and LDPE/PHBV (70/30) blend with Paecilomyces variotii. Polímeros: Ciência e Tecnologia, 25(1), 29-34. http://dx.doi.org/10.1590/0104-1428.1432.
http://dx.doi.org/10.1590/0104-1428.1432...
], which plays an important role in the largest volume of plastic waste.
An alternative to solve the environmental problem would be the use of biodegradable polymers, which are macromolecules that can be cleaved by the action of biological enzymes of microorganisms (fungi, bacteria and algae) and subsequently used as nutrients for the growth of colonies, provided in the appropriate environmental conditions[1111 Rosa, D. S., & Pantano, R., Fo. (2003). Biodegradabilidade – um ensaio com polímeros. Brazil: Moara.,1212 Franchetti, S. M. M., & Marconato, J. C. (2006). Polímeros biodegradáveis - uma solução parcial para diminuir a quantidade dos resíduos plásticos. Quimica Nova, 29(4), 811-816. http://dx.doi.org/10.1590/S0100-40422006000400031.
http://dx.doi.org/10.1590/S0100-40422006...
]. Thus, biodegradable polymers are returned to the environment as gaseous compounds and salts, such as CO2, H2O, CH4, depending on the presence or absence of oxygen, in a process called mineralization[1313 Luckachan, G. E., & Pillai, C. K. S. (2011). Biodegradable polymers - A review on recent trends and emerging perspectives. Journal of Polymers and the Environment, 19(3), 637-676. http://dx.doi.org/10.1007/s10924-011-0317-1.
http://dx.doi.org/10.1007/s10924-011-031...
]. Biodegradable polymers, however, are more expensive and difficult to process, which hinders their use when compared to petrochemical resins. Besides this, when they are low cost, they are not applicable to the required purpose, due to the absence of some property, generally mechanical[1414 Siracusa, V., Rocculi, P., Romani, S., & Rosa, M. D. (2008). Biodegradable polymer for food: a review. Trends in Food Science & Technology, 19(12), 634-643. http://dx.doi.org/10.1016/j.tifs.2008.07.003.
http://dx.doi.org/10.1016/j.tifs.2008.07...
].
Another, more viable option would be the use of oxo-biodegradable polymers, which are petrochemical resins processed with a pro-oxidant additive[1515 Ojeda, T. F. M., Dalmolin, E., Forte, M. M. C., Jacques, R. J. S., Bento, F. M., & Camargo, F. A. O. (2009). Abiotic and biotic degradation of oxo-biodegradable polyethylenes. Polymer Degradation & Stability, 94(6), 965-970. http://dx.doi.org/10.1016/j.polymdegradstab.2009.03.011.
http://dx.doi.org/10.1016/j.polymdegrads...
16 Sen, S. K., & Raut, S. (2015). Microbial degradation of low density polyethylene (LDPE): a review. Journal of Environmental Chemical Engineering, 3(1), 462-473. http://dx.doi.org/10.1016/j.jece.2015.01.003.
http://dx.doi.org/10.1016/j.jece.2015.01...
-1717 Santos, A. S. F., Freire, F. H. O., Costa, B. L. N., & Manrich, S. (2012). Sacolas plásticas: destinações sustentáveis e alternativas de substituição.Polímeros: Ciência e Tecnologia, 22(3), 228-237. http:// dx.doi.org/10.1590/S0104-14282012005000036.
https://doi.org/http:// dx.doi.org/10.15...
]. Pro-oxidant additives are responsible for favouring the abiotic degradation of the polymer, mainly by thermooxidation and photodegradation, with production of oxygenated fragments of lower molar mass that can be assimilated by microorganisms, a biotic process[1818 Chiellini, E., Corti, A., D’Antone, S., & Baciu, R. (2006). Oxo-biodegradable carbon backbone polymers – Oxidative degradation of polyethylene under accelerated test conditions. Polymer Degradation & Stability, 91(11), 2739-2747. http://dx.doi.org/10.1016/j.polymdegradstab.2006.03.022.
http://dx.doi.org/10.1016/j.polymdegrads...
19 Liu, X., Gao, C., Sangwan, P., Yu, L., & Tong, Z. (2014). Accelerating the degradation of polyolefins through additives and blending. Journal of Applied Polymer Science, 131(18), 9001-9015. http://dx.doi.org/10.1002/app.40750.
http://dx.doi.org/10.1002/app.40750...
-2020 Reddy, M. M., Gupta, R. K., Gupta, R. K., Bhattacharya, S. N., & Parthasarathy, R. (2008). Abiotic oxidation studies of oxo-biodegradable polyethylene. Journal of Polymers and the Environment, 16(1), 27-34. http://dx.doi.org/10.1007/s10924-008-0081-z.
http://dx.doi.org/10.1007/s10924-008-008...
]. In general, they are organic salts of transition metals, mainly stearates of Fe, Co and Mn[2020 Reddy, M. M., Gupta, R. K., Gupta, R. K., Bhattacharya, S. N., & Parthasarathy, R. (2008). Abiotic oxidation studies of oxo-biodegradable polyethylene. Journal of Polymers and the Environment, 16(1), 27-34. http://dx.doi.org/10.1007/s10924-008-0081-z.
http://dx.doi.org/10.1007/s10924-008-008...
21 Roy, P. K., Surekha, P., Raman, R., & Rajagopal, C. (2009). Investigating the role of metal oxidation state on the degradation behavior of LDPE. Polymer Degradation & Stability, 94(7), 1033-1039. http://dx.doi.org/10.1016/j.polymdegradstab.2009.04.025.
http://dx.doi.org/10.1016/j.polymdegrads...
22 Jakubowicz, I. (2003). Evaluation of degradability of biodegradable polyethylene (PE). Polymer Degradation & Stability, 80(1), 39-43. http://dx.doi.org/10.1016/S0141-3910(02)00380-4.
http://dx.doi.org/10.1016/S0141-3910(02)...
23 Corti, A., Muniyasamy, S., Vitali, M., Imam, S. H., & Chiellini, E. (2010). Oxidation and biodegradation of polyethylene films containing pro-oxidant additives: synergistic effects of sunlight exposure, thermal aging and fungal biodegradation. Polymer Degradation & Stability, 95(6), 1106-1114. http://dx.doi.org/10.1016/j.polymdegradstab.2010.02.018.
http://dx.doi.org/10.1016/j.polymdegrads...
24 Vogt, N. B., & Kleppe, E. A. (2009). Oxo-biodegradable polyolefins show continued and increased thermal oxidative degradation after exposure to light. Polymer Degradation & Stability, 94(4), 659-663. http://dx.doi.org/10.1016/j.polymdegradstab.2009.01.002.
http://dx.doi.org/10.1016/j.polymdegrads...
-2525 Babetto, A. S., Agnelli, J. A. M., & Bettini, S. H. P. (2015). Evaluation of the pro-degradant systems in the thermooxidative degradation of HDPE. Polímeros: Ciência e Tecnologia, 25(No. esp.), 68-76. http://dx.doi.org/10.1590/0104-1428.2022.
http://dx.doi.org/10.1590/0104-1428.2022...
]. But organic pro-oxidants have been investigated, such as benzoin, which showed promising results in the abiotic degradation of polypropylene (PP)[2626 Montagna, L. S., Catto, A. L., Forte, M. M. C., Chiellini, E., Corti, A., Morelli, A., & Santana, R. M. C. (2015). Comparative assessment of degradation in aqueous medium of polypropylene films doped with transition metal free (experimental) and transition metal containing (commercial) pro-oxidant/pro-degradant additives after exposure to controlled UV radiation. Polymer Degradation & Stability, 120, 186-192. http://dx.doi.org/10.1016/j.polymdegradstab.2015.06.019.
http://dx.doi.org/10.1016/j.polymdegrads...
] and PE[2727 Rosa, T. P. S. (2019). Polietileno modificado com pró-degradante orgânico para aplicação em embalagens flexíveis (Master’s Thesis). Universidade Federal do Rio Grande do Sul, Porto Alegre.].
However, during polymer processing, as in the tubular extrusion process, the polymer is subjected to high shear rates and high temperatures when passing through the barrel, which can be initiators of the degradation process that, in the presence of O2 from air, can be called oxidative thermomechanical degradation. The present work is aimed at evaluating how the presence of pro-oxidant additives, one based on organic salts of transition metals (d2wTM) and another totally organic (benzoin), influence the thermomechanical oxidative degradation of PE during processing by tubular extrusion to obtain films. The films were evaluated by dilute solution viscosimetry (DSV), with determination of viscosity average molar mass; Fourier-transform infrared spectroscopy (FTIR) and carbonyl index determination; flow index (MFI); contact angle and scanning electron microscopy (SEM).
2. Materials and Methods
2.1 Materials
High density polyethylene (HDPE) grade HE-150, with MFI of 1.0 g/10 min (190 ºC/ 2.16 kg), and low density polyethylene (LDPE) grade EB-853/72, with MFI of 2.7 g/min (190 ºC/ 2.16 kg), both produced by Braskem (Brazil), were used in this work. d2wTM pro-oxidant additive in masterbatch form with low density polyethylene (LDPE) as a base polymer, produced by Symphony Environmental (United Kingdom). Benzoin with a purity grade above 99%, produced by Merck KGaG (Germany). Decahydronaphthalene (Decalin) produced by Neon.
2.2 Obtaining films by tubular extrusion
According to Table 1, the HDPE and LDPE blend, in mass proportion of 90 and 10%, respectively, was processed without and with 1 wt.% of pro-oxidants. The mixture was properly homogenized for processing.
The blown films, with an average thickness of 30 μm, were obtained by tubular extrusion in a Seibt (Brazil) single-screw extruder, model ES 35-FR, with 5 heating zones, using the following temperature profile: 120/150/175/185/210 ºC, from zones 1 (feed) to 5 (die).
2.3 Characterization
2.3.1 Dilute Solution Viscosimetry (DSV) and Viscosity Average Molar Mass (
In determining the viscosity average molar mass, the dilute solution viscosimetry technique was used. Five PE solutions were prepared at concentrations of 0.2, 0.4, 0.6, 0.8 and 1.0 g/dL for each film analysed, using decalin as solvent. The solutions were obtained, one by one, by determining the mass needed to prepare 25 ml of each of the concentrations mentioned. The dissolution of the polymer took place under stirring and at 140oC for one hour. The viscosities of the solutions were measured in a Cannon-Fenske viscometer (no 50) with an internal capillary diameter of 0.44 mm. The procedures were carried out according to ASTM D445 and ASTM D446. The analyses were performed with the viscometer immersed in a thermostatic silicone oil bath of SOLAB brand, Model SL 150, at 135.0 ± 0.1ºC[2828 Brandup, J., Immergut, E. H., & Grulke, E. A. (2009). Polymer Handbook. 2nd ed. New York: Wiley-Interscience Publication.].
First, the relative viscosities were calculated. Subsequently, the reduced specific (ηesp/C) and inherent viscosities (ln ηrel/C) of each one of the solutions were determined. Plotting the graphs of such viscosities versus concentration, the intrinsic viscosity of the PE used in the preparation of the solutions was determined, from the extrapolation of the straight lines obtained by linear regression when the concentration tends to zero. The values found for the two straight lines tend to the same value and, for this reason, their average was used as intrinsic viscosity ([η]). For the determination of of PE, the Mark-Houwink-Sakurada equation was used, which relates the intrinsic viscosity ([η]) and the viscosity average molar mass , as presented in Equation 1:
The α and K constants were 0.7 and 62 x 10-5 dL/g, respectively[2828 Brandup, J., Immergut, E. H., & Grulke, E. A. (2009). Polymer Handbook. 2nd ed. New York: Wiley-Interscience Publication.]. The films evaluated are made of a mixture of HDPE and LDPE, i.e., both PE. As per the literature reference, the viscosimetric constants used are for PE, without distinction among its variations (whether HDPE, LDPE or LLDPE, for example).
2.3.2 Melt Flow Index (MFI)
The tests for determining the PE flow index of HDPE/LDPE films with and without pro-oxidant additives were performed in the CEAST modular MeltFlow plastomer equipment, Model 7026.000, according to method A of ASTM D1238. The conditions used were 190 ºC/ 2.16 kg, with a residence time of 420 seconds and time between cuts of 60 seconds, with a total test time of 900 seconds for each sample.
2.3.3 Fourier Transform Infrared Spectroscopy (FTIR) and Carbonyl Index (CI)
To investigate the changes in the chemical structure of the films, especially the appearance of new functional groups due to oxidation, the FTIR analysis was performed in Perkin Elmer equipment, Frontier model, according to ASTM E1131. The films were evaluated in the ATR (Total Attenuated Reflectance) mode and the spectra obtained at a controlled ambient temperature of 25ºC, air humidity of 30% and in an absorption region ranging from 4000 to 650 cm-1, with 10 scans for each sample analysed. From the spectra, the carbonyl indices (CI) were calculated. The poorly variable absorption band peak was adopted as that at 1463 cm-1[2929 Gulmine, J. V., Janissek, P. R., Heise, H. M., & Akcelrud, L. (2002). Polyethylene characterization by FTIR. Polymer Testing, 21(5), 557-563. http://dx.doi.org/10.1016/S0142-9418(01)00124-6.
http://dx.doi.org/10.1016/S0142-9418(01)...
], and the limits between 1468 and 1450 cm-1 (A1468-1450) were integrated. The absorption peaks of carbonyls adopted to verify the degree of PE oxidation were of esters and carboxylic acids (1300-1050 cm-1) and lactones (1780-1770 cm-1), and the limits between the adopted bands were integrated, named A1300-1050 and A1780-1770[2525 Babetto, A. S., Agnelli, J. A. M., & Bettini, S. H. P. (2015). Evaluation of the pro-degradant systems in the thermooxidative degradation of HDPE. Polímeros: Ciência e Tecnologia, 25(No. esp.), 68-76. http://dx.doi.org/10.1590/0104-1428.2022.
http://dx.doi.org/10.1590/0104-1428.2022...
,3030 Sugimoto, M., Shimada, A., Kudoh, H., Tamura, K., & Seguchi, T. (2013). Product analysis for polyethylene degradation by radiation and thermal ageing. Radiation Physics and Chemistry, 82, 69-73. http://dx.doi.org/10.1016/j.radphyschem.2012.08.009.
http://dx.doi.org/10.1016/j.radphyschem....
], respectively. The CI was calculated from Equation 2:
2.3.4 Contact angle
The contact angle test was performed using deionized water as the liquid, according to ASTM D7334-08. The analysis allows the determination of the substrate (film) hydrophilicity or hydrophobia. For each film, 10 repetitions were made, in which drops of water were placed on the surface. The images were obtained from a Knup microscope, model Kp-8012, and the contact angles determined from the Surftens 4.3 software for 3 seconds and 3 minutes.
2.3.5 Scanning Electron Microscopy (SEM)
The surface morphologies of the samples were obtained by scanning electron microscopy soon after processing by tubular extrusion, in a Jeol instrument, model JSM6510LV. The films were metallized with gold in a standard procedure in the Denton Caccum metallizer, model Desk. Micrographs were obtained with electron beams at 10 kV energy and magnifications of 1500 and 5000x.
3. Results and Discussions
After processing, the samples were characterized to evaluate the influence of the pro-oxidant on the process of thermomechanical oxidative degradation of PE during extrusion of the blown films.
3.1 Evaluation of and MFI
The intrinsic viscosity ([η]) of the PE was determined for each of the samples and is shown in Table 2.
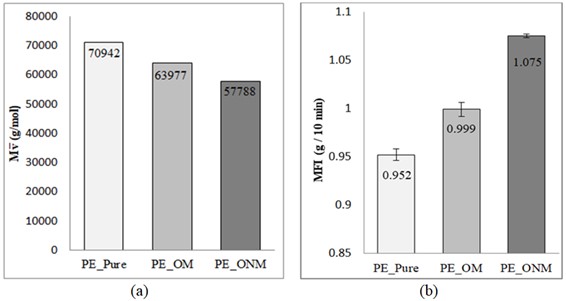
From the Mark-Howink-Sakurada equation (Equation 1), the of the PE in the evaluated films were determined, presented in Figure 1-a. In Figure 1-b, the MFI results of the evaluated films are presented.
In Figure 1-a is possible to observe a decrease of of the PE in the films extruded with the pro-oxidants if compared to the film without additives. The presence of the additives led to higher rates of chain scission and, consequently, a reduction in molar mass, indicating a greater degradation process of the polymer[3131 Albertsson, A.-C., Erlandsson, B., Hakkarainen, M., & Karlsson, S. (1998). Molecular weight changes and polymeric matrix changes correlated with the formation of degradation products in biodegradable polyethylene. Journal of Environmental Polymer Degradation, 6(4), 187-195. http://dx.doi.org/10.1023/A:1021873631162.
http://dx.doi.org/10.1023/A:102187363116...
,3232 Khajehpour-Tadavani, S., Nejabat, G.-R., & Mortazavi, S. M. M. (2018). Oxo-biodegradability of high-density polyethylene films containing limited amount of isotactic polypropylene. Journal of Applied Polymer Science, 135(6), 45843. http://dx.doi.org/10.1002/app.45843.
http://dx.doi.org/10.1002/app.45843...
]. The PE scission process was more intense in the presence of benzoin than for d2wTM, since the PE molar mass reduction in the PE_ONM film was higher than in the PE_OM film.
The MFI values, in Figure 1-b, indicate that there is a greater difficulty in the flow of the molten PE macromolecules in the PE_Pure film, possibly due to their larger size compared to the films containing pro-oxidants. The PE_ONM film containing benzoin, on the other hand, showed higher MFI, indicating higher fluidity and lower viscosity of the molten polymer, compatible with the lower of the sample and for which there is higher mobility, possibly due to the presence of smaller chains size and less likelihood of entanglement. The PE_OM film showed MFI with intermediate value, compatible with its .
It can be seen that there is a coherent correlation between the and the MFI, since the reduction of the is followed by an increase in MFI, indicating with the PE with smaller chains size has higher flow when melted, observed for the PE_ONM film. The opposite is also true, i.e., the PE in the PE_Pure sample, with higher , has the lowest flow when melted, hampered by the larger size of the polymer chains.
3.2 FTIR
Figure 2-a shows the FTIR spectra, between the 2000 and 1500 cm-1 bands, of the PE films just after processing by tubular extrusion. It is possible to observe that all samples present peaks between 1780 and 1700 cm-1, which indicates that the PE, in all samples, suffered oxidation during processing, with formation of oxidized products, such as esters, ketones, aldehydes, lactones and carboxylic acids(25, 34). Visually, the PE_Pure film presents lower peaks, mainly at 1780 and 1712 cm-1. The PE_OM film presents well defined peaks at 1712, 1723, 1730 and 1780 cm-1, indicating that the d2wTM additive favoured autooxidation. The PE_ONM film presents a clear peak at 1780 cm-1, suggesting that benzoin favoured PE autooxidation, however, the peak at 1723 cm-1 is overlap with the 1730 cm-1 peak, emphasizing that benzoin has a ketone group in its structure and, for this reason, the production of fragments containing esters and carboxylic acids groups was considered, observable in Figure 2-b, FTIR spectra, between the 1400 and 1000 cm-1, in which it is possible to observe that there is production of these oxygenated fragments in all samples. Visually, the PE_ONM film presents peak broadening between 1300 and 1250 cm-1, as well as between 1150 and 1100 cm-1, when compared to the other two films, which suggests a higher degree of oxidation. The PE_OM film, when compared to PE_Pure film, presents wider and deeper peak between 1100 and 1050 cm-1. However, it was decided to discuss the oxidation degree of the samples by the CI evaluation.
FTIR spectra of the evaluated samples: (a) between 2000 and 1500 cm-1, (b) between 1400 and 1000 cm-1.
Using Equation 2 and the Origin 8.5.1 software, the CI values for the samples were calculated and are shown in Figure 3. The different CI values indicate unequal degrees of oxidation of the PE in the samples during the extrusion process, favoured by exposure to high temperatures and high shear rates in the presence of O2 from the air[3333 Agnelli, J. A. M., & Chinelatto, M. A. (1992). Degradação de polipropileno: aspectos teóricos e recentes avanços em sua estabilização. Polímeros: Ciência e Tecnologia, 2(3), 27-31. Retrieved in 2021, August 1, from https://revistapolimeros.org.br/journal/polimeros/article/588371317f8c9d0a0c8b4792
https://revistapolimeros.org.br/journal/...
,3434 Gardette, M., Perthue, A., Gardette, J.-L., Janecska, T., Földes, E., Pukanszky, B., & Therias, S. (2013). Photo and thermal-oxidation of polyethylene: comparison of mechanisms and influence of unsaturation content. Polymer Degradation & Stability, 98(11), 2383-2390. http://dx.doi.org/10.1016/j.polymdegradstab.2013.07.017.
http://dx.doi.org/10.1016/j.polymdegrads...
].
In the case of the samples evaluated, the presence of carbonyl groups is evident in all, indicating that the PE suffered oxidation, producing, among others, carboxylic acids, esters, and lactones[1515 Ojeda, T. F. M., Dalmolin, E., Forte, M. M. C., Jacques, R. J. S., Bento, F. M., & Camargo, F. A. O. (2009). Abiotic and biotic degradation of oxo-biodegradable polyethylenes. Polymer Degradation & Stability, 94(6), 965-970. http://dx.doi.org/10.1016/j.polymdegradstab.2009.03.011.
http://dx.doi.org/10.1016/j.polymdegrads...
,1919 Liu, X., Gao, C., Sangwan, P., Yu, L., & Tong, Z. (2014). Accelerating the degradation of polyolefins through additives and blending. Journal of Applied Polymer Science, 131(18), 9001-9015. http://dx.doi.org/10.1002/app.40750.
http://dx.doi.org/10.1002/app.40750...
,2020 Reddy, M. M., Gupta, R. K., Gupta, R. K., Bhattacharya, S. N., & Parthasarathy, R. (2008). Abiotic oxidation studies of oxo-biodegradable polyethylene. Journal of Polymers and the Environment, 16(1), 27-34. http://dx.doi.org/10.1007/s10924-008-0081-z.
http://dx.doi.org/10.1007/s10924-008-008...
,3434 Gardette, M., Perthue, A., Gardette, J.-L., Janecska, T., Földes, E., Pukanszky, B., & Therias, S. (2013). Photo and thermal-oxidation of polyethylene: comparison of mechanisms and influence of unsaturation content. Polymer Degradation & Stability, 98(11), 2383-2390. http://dx.doi.org/10.1016/j.polymdegradstab.2013.07.017.
http://dx.doi.org/10.1016/j.polymdegrads...
]. However, the CI value is higher for PE_ONM and PE_OM. Comparing only the CI values, benzoin and d2wTM showed equal efficiency in accelerating PE autooxidation by thermooxidation, since the index values are similar.
3.3 Contact angle
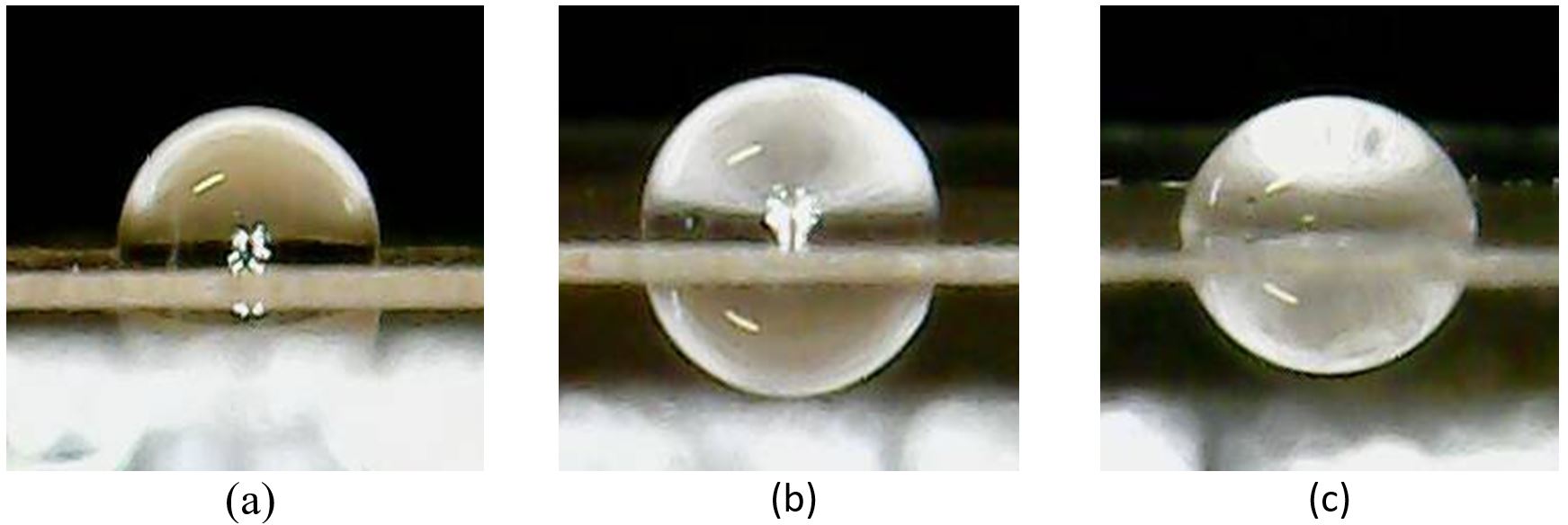
Another way to confirm the presence of functional groups is through the contact angle. Figure 4 shows the images of the water droplets on the surface of the films, in which it is possible to visualize the differences between the contours of the droplets and the contact angles for each of the films. It can be seen that the droplet on the PE_ONM sample (Figure 4-c) is more spread on the surface than the others, indicating that its surface is more hydrophilic.
Images of deionized water droplets arranged on the analysed films: (a) PE_Pure; (b) PE_OM; (c) PE_ONM.
The average surface contact values of the films of the evaluated samples are shown in Figure 5. The results found indicated that the PE_Pure film is the most apolar, with the largest contact angle, consistent with its low oxidation rate, if considered the CI value. The PE_OM film, is the second most apolar, with small reduction of the contact angle value, if compared to the PE_Pure film.
The reduction of the contact angle is possibly due to the greater insertion of carbonyls in the polymer chain, which changed its polarity. The PE_ONM film showed a marked reduction in the contact angle value compared to the other two films. Besides the insertion of carbonyl groups, the film also presented a higher concentration of hydroxyls (-OH), capable of establishing hydrogen bonds with water and reducing the contact angle presenting, therefore, the lowest value. The contact angle reduction indicates the PE degradation process in films additivated with pro-oxidants, with insertion of hydrophilic functional groups[3535 Muthukumar, T., Aravinthan, A., & Mukesh, D. (2010). Effect of environment on the degradation of starch and pro-oxidant blended polyolefins. Polymer Degradation & Stability, 95(10), 1988-1993. http://dx.doi.org/10.1016/j.polymdegradstab.2010.07.017.
http://dx.doi.org/10.1016/j.polymdegrads...
]. Benzoin, besides catalyzing the degradation of PE, altered the film surface by the insertion of hydroxyls in its structure.
3.4 SEM
The micrographs obtained to evaluate the surface morphology of the samples evaluated are presented in Figure 6 and in them it is possible to observe differences that indicate that the processing of PE, with and without pro-oxidant additives, leads to structural changes in the extruded films.
SEM images of the samples: (a) PE_Pure – 1500 x; (b) PE_Pure – 5000 x; (c) PE_OM – 1500 x; (d) PE_OM – 5000 x; (e) PE_ONM – 1500 x; (f) PE_ONM – 5000 x.
The film PE_Pure presents the highest surface homogeneity, while the film PE_ONM presents irregularities, possibly due to the presence of the polar chemical agent, benzoin, which hindered a better dispersion of the apolar polymer. The PE_OM film presents morphological variations with the appearance of agglomerations, which not indicate a total dissolution of the masterbatch containing the additive, whose processing took place in a single-screw extruder. Initially, these alterations only point out that the different organizations of the macromolecules may favour an increase in the fragility of the films for subsequent uses.
4. Conclusions
The tubular extrusion process is characterized by exposure of the polymer to high shear rates associated with high temperatures, and in this study, PE with pro-oxidants had its degradation accelerated, increasing the rate of scission and oxidation of the macromolecules. The benzoin pro-oxidant accelerates this degradation more markedly compared to the d2wTM additive, as it led to a greater reduction in the of the polymer evaluated. The structural changes in PE observed in this work need to be considered when using pro-oxidant additives to obtain oxybiodegradable PE, since they can alter important characteristics of a plastic artifact produced from it, such as disposable bags, reducing its shelf life.
5. Acknowledgements
Thanks to The National Council for Scientific and Technological (CNPq) for the financial support. Thanks to Federal Institute of Rio Grande do Sul (IF/RS) – Campus Farroupilha for carrying out FTIR analyses. Thanks to Feevale University for the SEM analyses.
6. References
-
1Thompson, R. C., Moore, C. J., vom Saal, F. S., & Swan, S. H. (2009). Plastics, the environment and human health: current consensus and future trends. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 364(1526), 2153-2166. http://dx.doi.org/10.1098/rstb.2009.0053 PMid:19528062.
» http://dx.doi.org/10.1098/rstb.2009.0053 -
2Vázquez-Morillas, A., Beltrán-Villavicencio, M., Alvarez-Zeferino, J. C., Osada-Velázquez, M. H., Moreno, A., Martínez, L., & Yañez, J. M. (2016). Biodegradation and ecotoxicity of polyethylene films containing pro-oxidant additive. Journal of Polymers and the Environment, 24(3), 221-229. http://dx.doi.org/10.1007/s10924-016-0765-8
» http://dx.doi.org/10.1007/s10924-016-0765-8 -
3Silva, E. A., & Moita Neto, J. M. (2015). Environmental impacts the production of polyethylene bottles in a Teresina-PI factory. Polímeros: Ciência e Tecnologia, 25(No. esp.), 59-67. http://dx.doi.org/10.1590/0104-1428.1949
» http://dx.doi.org/10.1590/0104-1428.1949 -
4Vasconcelos, Y. (2019). Planeta plástico. Pesquisa FAPESP, 281, 18-24. Retrieved in 2021, August 1, from https://revistapesquisa.fapesp.br/planeta-plastico/
» https://revistapesquisa.fapesp.br/planeta-plastico/ -
5Thompson, R. C., Swan, S. H., Moore, C. J., & vom Saal, F. S. (2009). Our plastic age. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 364(1526), 1973-1976. http://dx.doi.org/10.1098/rstb.2009.0054 PMid:19528049.
» http://dx.doi.org/10.1098/rstb.2009.0054 -
6Silva, E. A., & Moita, J. M., No. (2016). Possibilities for environmental improvements in the polyethylene recycling process. Polímeros: Ciência e Tecnologia, 26(No. esp.), 49-54. http://dx.doi.org/10.1590/0104-1428.1954
» http://dx.doi.org/10.1590/0104-1428.1954 -
7Koutny, M., Lemaire, J., & Delort, A.-M. (2006). Biodegradation of polyethylene films with prooxidant additives. Chemosphere, 64(8), 1243-1252. http://dx.doi.org/10.1016/j.chemosphere.2005.12.060 PMid:16487569.
» http://dx.doi.org/10.1016/j.chemosphere.2005.12.060 -
8Lucas, N., Bienaime, C., Belloy, C., Queneudec, M., Silvestre, F., & Nava-Saucedo, J.-E. (2008). Polymer biodegradation: mechanisms and estimation techniques. Chemosphere, 73(4), 429-442. http://dx.doi.org/10.1016/j.chemosphere.2008.06.064 PMid:18723204.
» http://dx.doi.org/10.1016/j.chemosphere.2008.06.064 -
9Ammala, A., Bateman, S., Dean, K., Petinakis, E., Sangwan, P., Wong, S., Yuan, Q., Yu, L., Patrick, C., & Leong, K. H. (2011). An overview of degradable and biodegradable polyolefins. Progress in Polymer Science, 36(8), 1015-1049. http://dx.doi.org/10.1016/j.progpolymsci.2010.12.002
» http://dx.doi.org/10.1016/j.progpolymsci.2010.12.002 -
10Passos, T. M., Marconato, J. C., & Franchetti, S. M. M. (2015). Biodegradation of films of low density polyethylene (LDPE), poly(hidroxibutyrate-co-valerate) (PHBV), and LDPE/PHBV (70/30) blend with Paecilomyces variotii. Polímeros: Ciência e Tecnologia, 25(1), 29-34. http://dx.doi.org/10.1590/0104-1428.1432
» http://dx.doi.org/10.1590/0104-1428.1432 -
11Rosa, D. S., & Pantano, R., Fo. (2003). Biodegradabilidade – um ensaio com polímeros Brazil: Moara.
-
12Franchetti, S. M. M., & Marconato, J. C. (2006). Polímeros biodegradáveis - uma solução parcial para diminuir a quantidade dos resíduos plásticos. Quimica Nova, 29(4), 811-816. http://dx.doi.org/10.1590/S0100-40422006000400031
» http://dx.doi.org/10.1590/S0100-40422006000400031 -
13Luckachan, G. E., & Pillai, C. K. S. (2011). Biodegradable polymers - A review on recent trends and emerging perspectives. Journal of Polymers and the Environment, 19(3), 637-676. http://dx.doi.org/10.1007/s10924-011-0317-1
» http://dx.doi.org/10.1007/s10924-011-0317-1 -
14Siracusa, V., Rocculi, P., Romani, S., & Rosa, M. D. (2008). Biodegradable polymer for food: a review. Trends in Food Science & Technology, 19(12), 634-643. http://dx.doi.org/10.1016/j.tifs.2008.07.003
» http://dx.doi.org/10.1016/j.tifs.2008.07.003 -
15Ojeda, T. F. M., Dalmolin, E., Forte, M. M. C., Jacques, R. J. S., Bento, F. M., & Camargo, F. A. O. (2009). Abiotic and biotic degradation of oxo-biodegradable polyethylenes. Polymer Degradation & Stability, 94(6), 965-970. http://dx.doi.org/10.1016/j.polymdegradstab.2009.03.011
» http://dx.doi.org/10.1016/j.polymdegradstab.2009.03.011 -
16Sen, S. K., & Raut, S. (2015). Microbial degradation of low density polyethylene (LDPE): a review. Journal of Environmental Chemical Engineering, 3(1), 462-473. http://dx.doi.org/10.1016/j.jece.2015.01.003
» http://dx.doi.org/10.1016/j.jece.2015.01.003 -
17Santos, A. S. F., Freire, F. H. O., Costa, B. L. N., & Manrich, S. (2012). Sacolas plásticas: destinações sustentáveis e alternativas de substituição.Polímeros: Ciência e Tecnologia, 22(3), 228-237. http:// dx.doi.org/10.1590/S0104-14282012005000036.
» https://doi.org/http:// dx.doi.org/10.1590/S0104-14282012005000036 -
18Chiellini, E., Corti, A., D’Antone, S., & Baciu, R. (2006). Oxo-biodegradable carbon backbone polymers – Oxidative degradation of polyethylene under accelerated test conditions. Polymer Degradation & Stability, 91(11), 2739-2747. http://dx.doi.org/10.1016/j.polymdegradstab.2006.03.022
» http://dx.doi.org/10.1016/j.polymdegradstab.2006.03.022 -
19Liu, X., Gao, C., Sangwan, P., Yu, L., & Tong, Z. (2014). Accelerating the degradation of polyolefins through additives and blending. Journal of Applied Polymer Science, 131(18), 9001-9015. http://dx.doi.org/10.1002/app.40750
» http://dx.doi.org/10.1002/app.40750 -
20Reddy, M. M., Gupta, R. K., Gupta, R. K., Bhattacharya, S. N., & Parthasarathy, R. (2008). Abiotic oxidation studies of oxo-biodegradable polyethylene. Journal of Polymers and the Environment, 16(1), 27-34. http://dx.doi.org/10.1007/s10924-008-0081-z
» http://dx.doi.org/10.1007/s10924-008-0081-z -
21Roy, P. K., Surekha, P., Raman, R., & Rajagopal, C. (2009). Investigating the role of metal oxidation state on the degradation behavior of LDPE. Polymer Degradation & Stability, 94(7), 1033-1039. http://dx.doi.org/10.1016/j.polymdegradstab.2009.04.025
» http://dx.doi.org/10.1016/j.polymdegradstab.2009.04.025 -
22Jakubowicz, I. (2003). Evaluation of degradability of biodegradable polyethylene (PE). Polymer Degradation & Stability, 80(1), 39-43. http://dx.doi.org/10.1016/S0141-3910(02)00380-4
» http://dx.doi.org/10.1016/S0141-3910(02)00380-4 -
23Corti, A., Muniyasamy, S., Vitali, M., Imam, S. H., & Chiellini, E. (2010). Oxidation and biodegradation of polyethylene films containing pro-oxidant additives: synergistic effects of sunlight exposure, thermal aging and fungal biodegradation. Polymer Degradation & Stability, 95(6), 1106-1114. http://dx.doi.org/10.1016/j.polymdegradstab.2010.02.018
» http://dx.doi.org/10.1016/j.polymdegradstab.2010.02.018 -
24Vogt, N. B., & Kleppe, E. A. (2009). Oxo-biodegradable polyolefins show continued and increased thermal oxidative degradation after exposure to light. Polymer Degradation & Stability, 94(4), 659-663. http://dx.doi.org/10.1016/j.polymdegradstab.2009.01.002
» http://dx.doi.org/10.1016/j.polymdegradstab.2009.01.002 -
25Babetto, A. S., Agnelli, J. A. M., & Bettini, S. H. P. (2015). Evaluation of the pro-degradant systems in the thermooxidative degradation of HDPE. Polímeros: Ciência e Tecnologia, 25(No. esp.), 68-76. http://dx.doi.org/10.1590/0104-1428.2022
» http://dx.doi.org/10.1590/0104-1428.2022 -
26Montagna, L. S., Catto, A. L., Forte, M. M. C., Chiellini, E., Corti, A., Morelli, A., & Santana, R. M. C. (2015). Comparative assessment of degradation in aqueous medium of polypropylene films doped with transition metal free (experimental) and transition metal containing (commercial) pro-oxidant/pro-degradant additives after exposure to controlled UV radiation. Polymer Degradation & Stability, 120, 186-192. http://dx.doi.org/10.1016/j.polymdegradstab.2015.06.019
» http://dx.doi.org/10.1016/j.polymdegradstab.2015.06.019 -
27Rosa, T. P. S. (2019). Polietileno modificado com pró-degradante orgânico para aplicação em embalagens flexíveis (Master’s Thesis). Universidade Federal do Rio Grande do Sul, Porto Alegre.
-
28Brandup, J., Immergut, E. H., & Grulke, E. A. (2009). Polymer Handbook 2nd ed. New York: Wiley-Interscience Publication.
-
29Gulmine, J. V., Janissek, P. R., Heise, H. M., & Akcelrud, L. (2002). Polyethylene characterization by FTIR. Polymer Testing, 21(5), 557-563. http://dx.doi.org/10.1016/S0142-9418(01)00124-6
» http://dx.doi.org/10.1016/S0142-9418(01)00124-6 -
30Sugimoto, M., Shimada, A., Kudoh, H., Tamura, K., & Seguchi, T. (2013). Product analysis for polyethylene degradation by radiation and thermal ageing. Radiation Physics and Chemistry, 82, 69-73. http://dx.doi.org/10.1016/j.radphyschem.2012.08.009
» http://dx.doi.org/10.1016/j.radphyschem.2012.08.009 -
31Albertsson, A.-C., Erlandsson, B., Hakkarainen, M., & Karlsson, S. (1998). Molecular weight changes and polymeric matrix changes correlated with the formation of degradation products in biodegradable polyethylene. Journal of Environmental Polymer Degradation, 6(4), 187-195. http://dx.doi.org/10.1023/A:1021873631162
» http://dx.doi.org/10.1023/A:1021873631162 -
32Khajehpour-Tadavani, S., Nejabat, G.-R., & Mortazavi, S. M. M. (2018). Oxo-biodegradability of high-density polyethylene films containing limited amount of isotactic polypropylene. Journal of Applied Polymer Science, 135(6), 45843. http://dx.doi.org/10.1002/app.45843
» http://dx.doi.org/10.1002/app.45843 -
33Agnelli, J. A. M., & Chinelatto, M. A. (1992). Degradação de polipropileno: aspectos teóricos e recentes avanços em sua estabilização. Polímeros: Ciência e Tecnologia, 2(3), 27-31. Retrieved in 2021, August 1, from https://revistapolimeros.org.br/journal/polimeros/article/588371317f8c9d0a0c8b4792
» https://revistapolimeros.org.br/journal/polimeros/article/588371317f8c9d0a0c8b4792 -
34Gardette, M., Perthue, A., Gardette, J.-L., Janecska, T., Földes, E., Pukanszky, B., & Therias, S. (2013). Photo and thermal-oxidation of polyethylene: comparison of mechanisms and influence of unsaturation content. Polymer Degradation & Stability, 98(11), 2383-2390. http://dx.doi.org/10.1016/j.polymdegradstab.2013.07.017
» http://dx.doi.org/10.1016/j.polymdegradstab.2013.07.017 -
35Muthukumar, T., Aravinthan, A., & Mukesh, D. (2010). Effect of environment on the degradation of starch and pro-oxidant blended polyolefins. Polymer Degradation & Stability, 95(10), 1988-1993. http://dx.doi.org/10.1016/j.polymdegradstab.2010.07.017
» http://dx.doi.org/10.1016/j.polymdegradstab.2010.07.017
Publication Dates
-
Publication in this collection
13 Dec 2021 -
Date of issue
2021
History
-
Received
05 Aug 2021 -
Reviewed
07 Oct 2021 -
Accepted
25 Oct 2021