ABSTRACT:
Porcine proliferative enteropathy (PPE) is one of the most common enteric diseases in growing and finishing pigs. PPE is characterized by reduced growth performance, accompanied or not by diarrhea. PPE is highly prevalent in several countries of the Americas, Europe and Asia, causing high economic losses in swine herds. The most common form of PPE control in pigs is antibiotic therapy. The objective of this study was to evaluate a new product based on tylosin injectable (Eurofarma Laboratórios S.A.) to control PPE in experimentally inoculated animals. Sixty 5-week-old pigs with mean weight of 9.5kg were divided into two experimental groups of 30 animals: medication and control. All pigs were challenged with Lawsonia intracellularis, the etiologic agent of PPE, on day zero. Fecal score, body condition score, and behavior were daily evaluated. Pigs were weighted on days -2, 13 and 21 of the experiment. Pigs in the Medication Group received tylosin injectable 13 days after inoculation, in three doses with a 12-hour interval between them. Pigs in the Control Group received injectable saline solution following the same protocol. In the Control Group, 23pigs presented with diarrhea before day 13. After day 13, the number of diarrheic animals in this group was reduced to 17. In the Medication Group, 26 pigs presented with diarrhea in the initial period, and in the period after medication, only 11 animals had diarrhea. The score of gross intestinal PPE lesions in the Medication Group was lower than that in the Control Group (p=0.031). The Medication Group also showed lower score for Lawsonia intracellularis antigen-labeling by immunohistochemistry compared with that of the Control Group (p=0.032), showing lower level of infection. These results demonstrate that tylosin injectable (Eurofarma Laboratórios S.A.), administrated in three doses (1mL/20kg) every 12 hours, was effective for the control of PPE in experimentally inoculated pigs.
INDEX TERMS:
Tylosin injectable; treatment; porcine proliferative enteropathy; pigs; macrolides; ileitis; antimicrobial; Lawsonia intracellularis; metaphylactic; diarrhea; practice; clinics
RESUMO:
Enteropatia proliferativa suína (EPS), causada pela bactéria Lawsonia intracellularis, é uma das doenças entéricas mais comuns em suínos de recria e terminação. A EPS caracteriza-se por redução no desempenho dos animais, acompanhada ou não por diarreia. É uma doença altamente prevalente em diversos países da América, Europa e Ásia, provocando elevados prejuízos econômicos nos rebanhos suínos. A forma de controle da EPS mais adotada em rebanhos suínos é a antibioticoterapia. O objetivo deste estudo foi avaliar um novo produto à base de tilosina (Eurofarma Laboratórios S.A.) na forma injetável para controlar a EPS em animais experimentalmente inoculados. Foram utilizados 60 leitões, de cinco semanas de idade, com peso médio de 9,5kg, divididos em dois grupos experimentais (n=30), medicados e não medicados. Todos os leitões foram desafiados com Lawsonia intracellularis no dia zero. Avaliações clínicas de escore fecal, escore corporal e comportamento foram realizadas diariamente além da pesagem individual dos animais realizada nos dias -2, 13 e 21 do experimento. Os leitões do grupo medicado receberam tilosina injetável 13 dias após a inoculação em três doses com intervalo de 12 horas cada. Já os leitões do grupo não medicado receberam solução salina injetável com o mesmo protocolo. O grupo não medicado apresentou 23 animais com diarreia antes do dia 13 e 17 após este período. No grupo medicado, 26 animais apresentaram diarreia previamente à medicação e apenas 11 após a medicação a partir do dia 13. Os leitões medicados apresentaram extensão de lesão macroscópica, caracterizada por espessamento de mucosa intestinal, menor em comparação com o grupo não medicado (p=0,031). A imunomarcação para Lawsonia intracellularis foi menor no grupo medicado (p<0,032), mostrando redução no grau de infecção por L. intracellularis nos animais medicados. Estes resultados demonstram que a tilosina injetável (Eurofarma Laboratórios S.A.) (1mL/20kg) em três doses, a cada 12 horas, foi eficaz no tratamento da enteropatia proliferativa suína em animais experimentalmente inoculados.
TERMOS DE INDEXAÇÃO:
Tilosina injetável; tratamento; enteropatia proliferativa; leitões; macrolídeos; antimicrobianos; Lawsonia intracellularis; metafilático; diarreia; suínos; clínica
Introduction
Porcine proliferative enteropathy (PPE) is an infectious disease caused by the obligate intracellular bacterium Lawsonia intracellularis. PPE is characterized by thickening of the intestinal mucosa, and it affects mainly growing -finishing pigs. Its main clinical manifestations include three forms: acute or hemorrhagic, chronic, and subclinical (Guedes 2012). In hemorrhagic PPE, animals present with bloody diarrhea, apathy, and death (McOrist & Gebhart 2012McOrist S. & Gebhart C.J. 2012. IV: Bacterial diseases, proliferative enteropathy, p.2976-3004. In: Zimmerman J.J., Karriker L.A., Ramirez A., Schwartz K.J. & Stevenson G.W. (Eds), Diseases of Swine. 10th ed. John Wiley and Sons, Iowa.). The chronic form affects growing pigs and is characterized by failure to gain weight and transient diarrhea, whereas in the subclinical form animals also show reduction in weight gain, but with no evident diarrhea (Guedes 2012).
PPE is of great importance in swine production, and it causes significant economic losses resulting from diarrhea, increased mortality, decreased growth performance of animals, as well as from expenses with medicine, reaching an annual cost of US$ 20 million in the USA (McOrist 2005McOrist S. 2005. Prevalence and impact of proliferative enteropathy (ileitis) in East Asia. Proceedings II Asian Pig Veterinary Society Congress, Pasig City, Philippines, p.24-37. (Resumo)). PPE can be controlled through administration of antimicrobial drugs, mainly macrolides, tetracyclines, lincosamides, and pleuromutilins (França & Guedes 2008França S.A. & Guedes R.M.C. 2008. Antimicrobianos para o controle da enteropatia proliferativa suína. Ciência Rural 38(1):288-296. <http://dx.doi.org/10.1590/S0103-84782008000100050>
https://doi.org/10.1590/S0103-8478200800...
). Among them, tylosin, chlortetracycline, and tiamulin are the most frequently used (Burch 2000Burch D.G.S. 2000. Controlling ileitis in the “colitis” complex. Pig J. 45:131-149.). Tylosin is a macrolide antibiotic that inhibits bacterial protein synthesis (Kim et al. 2008Kim M., Gebru E., Chang Z., Choi J., Hwang M., Kang E., Lim J., Yun H. & Park S. 2008. Comparative Pharmacokinetics of Tylosin of Florfenicol after a single intramuscular administration at two different doses of tylosin-florfenicol combination pigs. J. Vet. Med. Sci. 70(1):99-102. <http://dx.doi.org/10.1292/jvms.70.99> <PMid:18250580>
https://doi.org/10.1292/jvms.70.99...
), acting as a bacteriostatic agent, and may also act as a bactericide when used in high concentrations (Barcellos et al. 2012Barcellos D., Sobestiansky J., Linhares D. & Sobestiansky T.B. 2012. Uso de antimicrobianos, p.839-845. In: Sobestiansky J. & Barcellos D. (Eds), Doenças dos suínos. 2ª ed. Cânone Editorial, Goiânia.).
McOrist et al. (1997)McOrist S., Morgan J., Veenhuizen M.F., Lawrence K. & Kroger H.W. 1997. Oral administration of tilosin phosphate for treatment and prevention of porcine proliferative enteropathy. Am. J. Vet. Res. 58(2):136-139. <PMid:9028475> demonstrated that in-feed tylosin phosphate is effective in the prevention and treatment of PPE. In another study addressing experimental inoculation of L. intracellularis conducted with 114 swine, the authors showed the efficacy of tylosin injected twice daily for three consecutive days in improving clinical signs, reducing elimination of bacteria in feces, enhancing growth performance, and reducing macro- and microscopic lesions (Marsteller et al. 2001Marsteller T., Winkelman N., Gebhart C., Armbruster G., Weldon W., Muller R., Weatherford J. & Symanowski J. 2001. Efficacy of intramuscular tylosin for the treatment and control of porcine proliferative enteropathy caused by Lawsonia intracellularis. Vet. Therap. 2(1):51-60.).
Indiscriminate use of in-feed antimicrobial drugs at low doses is associated with bacterial resistance (Maron et al. 2013Maron D.F., Smith T.J.S. & Nachman K.E. 2013. Restrictions on antimicrobial use in food animal production: an international regulatory and economic survey. Globalization Health 9(48):1-11. <PMid:24131666>). Some studies conducted in the USA have shown that, in order to preserve the efficacy of antibiotics for human and animal treatment, it is necessary to limit the use of antimicrobial drugs (Levy & Marshall 2004Levy S.B. & Marshall B. 2004. Antibacterial resistance worldwide: causes, challenges and responses. Nature 10(Suppl.12):122-129. <PMid:15577930>, Silbergeld et al. 2008Silbergeld E.K., Graham J. & Price L.B. 2008. Industrial Food Animal Production, antimicrobial resistance and human health. Annual Rev. Publ. Health 29(1):151-169. <http://dx.doi.org/10.1146/annurev.publhealth.29.020907.090904> <PMid:18348709>
https://doi.org/10.1146/annurev.publheal...
). As a result, many countries have restricted the use of antibiotics as growth promoters (Maron et al. 2013Maron D.F., Smith T.J.S. & Nachman K.E. 2013. Restrictions on antimicrobial use in food animal production: an international regulatory and economic survey. Globalization Health 9(48):1-11. <PMid:24131666>). The European Union banned the use of antimicrobial growth promoters in pig feed in 2006 (Gaggia et al. 2010Gaggìa F., Mattarelli P. & Biavati B. 2010. Probiotics and prebiotics in animal feeding for safe food production. Int. J. Food Microbiol. 141(Suppl.1):1-14. <http://dx.doi.org/10.1016/j.ijfoodmicro.2010.02.031> <PMid:20382438>
https://doi.org/10.1016/j.ijfoodmicro.20...
); a new regulation on the use of antibiotics in animal feed was enacted in the USA in 2017 (Beek 2017Beek V.T. 2017. US: Vaccines and biosecurity to replace antibiotics. Pig Progress. Available at <Available at http://www.pigprogress.net/Health/Articles/2017/1/US-vaccines and biosecurity to replace antibiotics 81584E/
> Accessed on Sept. 23, 2017.
http://www.pigprogress.net/Health/Articl...
). Consequently, the use of metaphylactic or water-soluble and/or injectable therapeutic medication has become increasingly common to the detriment of additive or preventive use (Callens et al. 2012Callens B., Persoons D., Maes D., Laanen M., Postma M., Boyen F., Haesebrouck F., Butaye P., Catry B. & Dewulf J. 2012. Prophylactic and metaphylactic antimicrobial use in Belgian fattening pig herds. Prev. Vet. Med. 106(1):53-62.). In this context, this study aimed to assess the effect of tylosin injectable in the treatment of PPE in pigs experimentally inoculated with L. intracellularis.
Materials and Methods
Animals and facilities. This study was approved by the Research Ethics Committee of the Universidade Federal de Minas Gerais (UFMG) under protocol no. 250/2015. Sixty male pigs aged five weeks, weighing 9.5kg on average, were used. The animals were purchased from a swine farm free of toxigenic Mycoplasma hyopneumoniae, Actinobacillus pleuropneumoniae, Brachyspira hyodysenteriae, Brachyspira pilosicoli, Salmonella enterica sorovar Choleraesuis, Pasteurella multocida, and suid herpesvirus type I.
The pigs were identified with ear tags and housed in an experimental barn of the College of Veterinary Medicine of the UFMG in 10 nursery pens (1.4x14m, 0.33m2/animal density) with slated plastic floors, artificial heating system, a nipple drinker, and a two-hole deposit feeder equipped with a stainless steel pan in its lower part to collect feed waste. The animals received feed and water ad libitum throughout the experiment.
Study design. Two days before inoculation (day-2), all animals were weighed and had their feces collected to be used as samples for Polymerase Chain Reaction (PCR) testing for Lawsonia intracellularis (Jones et al. 1993Jones G.F., Ward G.E., Murtaugh M.P., Lin G. & Gebhart C.J. 1993. Enhanced detection of intracellular organism of swine proliferative enteritis, ileal symbiont intracellularis, in feces by polymerase chain reaction. J. Clin. Microbiol. 31(10):2611-2615. <PMid:8253956>) in order to confirm their negativity for this bacterium prior to inoculation.
The 60 pigs were divided into two experimental groups of 30 animals each, distributed in five pens with six pigs each, with all animals allocated in each pen of the same treatment. The groups were balanced by weight as follows: light (7.99 and 7.89kg of mean weight, in pens of the Control and Medication Groups), moderately light (8.83 and 8.91kg), medium (9.58 and 9.54kg), moderately heavy (10.16 and 10.22kg), and heavy (10.88 and 10.91kg). Pigs in the Control Group were inoculated but not medicated, whereas those in the Medication Group were inoculated and medicated.
Inoculum production. Fragments of the small intestine of naturally infected pigs with typical lesions of proliferative enteropathy were submitted to bacteriological evaluation to discard the presence of other pathogens. Presence of moderate-to-severe infection was confirmed in histological sections by hematoxylin and eosin (HE) staining and by immunohistochemistry (IHC) using specific antibodies against L. intracellularis (Guedes & Gebhart 2003aGuedes R.M.C. & Gebhart C.J. 2003a. Preparation and characterization of polyclonal and monoclonal antibodies against Lawsonia intracellularis. J. Vet. Diagn. Invest. 15(5):438-446. <http://dx.doi.org/10.1177/104063870301500506> <PMid:14535543>
https://doi.org/10.1177/1040638703015005...
). The selected intestinal samples were frozen at -80°C until inoculation.
On inoculation day, the scraped mucosa from the intestines was defrosted and blended with sucrose-potassium-glutamate (SPG) solution (1:1 w/v), as described in Guedes et al. (2009)Guedes R.M.C., França S.A., Machado G.S., Blumer M.A. & Costa Cruz Junior E.C. 2009. Use of tylvalosin-medicated feed to control porcine proliferative enteropathy. Vet. Rec. 165(12):342-345. <http://dx.doi.org/10.1136/vr.165.12.342> <PMid:19767637>
https://doi.org/10.1136/vr.165.12.342...
and Guedes et al. (2009)Guedes R.M.C., França S.A., Machado G.S., Blumer M.A. & Costa Cruz Junior E.C. 2009. Use of tylvalosin-medicated feed to control porcine proliferative enteropathy. Vet. Rec. 165(12):342-345. <http://dx.doi.org/10.1136/vr.165.12.342> <PMid:19767637>
https://doi.org/10.1136/vr.165.12.342...
. The final product was also examined bacteriologically to ensure absence of enterotoxigenic Salmonellasp. and Escherichia coli species through detection of pathogens by multiplex PCR panels (Macedo et al. 2007Macêdo N.R., Menezes C.P., Lage A.P., Ristow L.E., Reis A. & Guedes R.M.C. 2007. Detecção de cepas patogênicas pela PCR multiplex e avaliação da sensibilidade a antimicrobianos de Escherichia coli isoladas de leitões diarreicos. Arq. Bras. Med. Vet. Zootec. 59(5):1117-1123. <http://dx.doi.org/10.1590/S0102-09352007000500005>
https://doi.org/10.1590/S0102-0935200700...
).
Inoculation. On day 0, all pigs were individually inoculated intragastrically with 43mL of a homogenate of intestinal mucosa inoculum of swine known to be infected by L. intracellularis, as described in Guedes (2002)Guedes R.M.C. 2002. Porcine proliferative enteropathy: diagnosis, immune response and pathogenesis. Doctoral Dissertation, University of Minnesota, St Paul. 261p.. Each animal received 1.6x107 L. intracellularis organisms. This quantification was performed through serial dilution and immunoperoxidase staining using leporine polyclonal antibodies, as described in Guedes & Gebhart (2003a)Guedes R.M.C. & Gebhart C.J. 2003a. Preparation and characterization of polyclonal and monoclonal antibodies against Lawsonia intracellularis. J. Vet. Diagn. Invest. 15(5):438-446. <http://dx.doi.org/10.1177/104063870301500506> <PMid:14535543>
https://doi.org/10.1177/1040638703015005...
.
Clinical assessment and growth performance. Individual clinical evaluations of all pigs were performed daily, from day-2 to the end of the experiment. The following parameters were observed: behavior, body score, and grade of diarrhea (grade 0 = without diarrhea, grade 1 =pasty feces, grade 2 = liquid feces, grade 3 = bloody diarrhea). Also, feed waste was collected and actual dietary intake per pen was evaluated daily. These data were divided into two periods: pre- and post-treatment. All animals were weighed individually on days -2, 13, and 21.
Therapy. On day 13 after inoculation, when at least 25% of the pigs showed diarrhea caused by L. intracellularis, the Medication Group was treated with tylosin (Tilosina 20%, Eurofarma Laboratórios S.A.), 1mL/20kg p.v., injected intramuscularly in the region of the neck, in three doses every 12 hours. All animals were previously weighed on day 13 to calculate the individual dose of the drug. Pigs in the Control Group received volume of sterile saline solution (0.9% NaCl) proportional to their body weight following the same protocol of the medication.
Euthanasia and post-mortem evaluation. All animals were weighed and euthanized by electrocution followed by bleeding on day 21 after inoculation, when a higher index of gross PPE lesions is expected (Guedes et al. 2017Guedes R.M.C., Machuca M.A., Quiroga M.A., Pereira C.E.R., Resende T.P. & Gebhart C.J. 2017. Lawsonia intracellularis in pigs: progression of lesions and involvement of apoptosis. Vet. Pathol. 54(4):620-628. <http://dx.doi.org/10.1177/0300985817698206> <PMid:28622490>
https://doi.org/10.1177/0300985817698206...
). In the post-mortem assessment, the macroscopic lesions compatible with PPE were graded and measured individually according to the following score: grade 0 = normal mucosa; grade 1 = hyperemia and thickened mucosa; grade 2 = thickened and necrotic mucosa; grade 3 = thickened mucosa with blood clots in the intestinal lumen (Guedes 2002Guedes R.M.C. 2002. Porcine proliferative enteropathy: diagnosis, immune response and pathogenesis. Doctoral Dissertation, University of Minnesota, St Paul. 261p.). For histopathology and immunohistochemistry, samples of the ileum, cecum, proximal colon, and mesenteric lymph node were fixed in 10% formalin (Guedes & Gebhart 2003bGuedes R.M.C. & Gebhart C.J. 2003b. Comparison of intestinal mucosa homogenate and pure culture of the homologous Lawsonia intracellularis isolate in reproducing proliferative enteropathy in swine. Vet. Microbiol. 93(2):159-166. <http://dx.doi.org/10.1016/S0378-1135(03)00013-0> <PMid:12637004>
https://doi.org/10.1016/S0378-1135(03)00...
).
Immunohistochemistry (IHC). The formalin-fixed intestine samples were routinely processed for histology, embedded in paraffin, and sectioned 3μ thick. The sections of ileum were stained immunohistochemically by the labeled Streptavidin method (Dako - Vila Real Carpinteria, EUA, K675) with leporine polyclonal antibodies to L. intracellularis (Guedes & Gebhart 2003aGuedes R.M.C. & Gebhart C.J. 2003a. Preparation and characterization of polyclonal and monoclonal antibodies against Lawsonia intracellularis. J. Vet. Diagn. Invest. 15(5):438-446. <http://dx.doi.org/10.1177/104063870301500506> <PMid:14535543>
https://doi.org/10.1177/1040638703015005...
) and Harris hematoxylin. Immunostaining was quantified as follows: grade 0 = no positive antigen for L. intracellularis labeled, grade 1 = positive antigen for L. intracellularis labeled in up to 25% of intestinal crypts, grade 2 = positive antigen labeled in up to 50% of the crypts, grade 3 = positive antigen labeled in up to 75% of the crypts; grade 4 = positive antigen labeled in 100% of the mucosa (Guedes et al. 2009Guedes R.M.C., França S.A., Machado G.S., Blumer M.A. & Costa Cruz Junior E.C. 2009. Use of tylvalosin-medicated feed to control porcine proliferative enteropathy. Vet. Rec. 165(12):342-345. <http://dx.doi.org/10.1136/vr.165.12.342> <PMid:19767637>
https://doi.org/10.1136/vr.165.12.342...
).
Statistical analysis. In the present study, statistical analysis of the data was processed using the SPSS Statistics 25 software with confidence interval of 95% (p<0.05). The Chi-squared test was applied to compare the frequency of animals with diarrhea between the Control and Medication Groups in the post-treatment period and the frequency of animals with intestinal lesions according to macroscopic features, histology, and grade of infection based on IHC. The Student’s t-test was used to compare the mean weight of the groups on days -2, 13, and 21 of the experiment, as well as the daily weight gain between the groups on days -2 to 13 and 14 to 21. Poisson regression was used to compare the number of days with diarrhea between the groups in the post-treatment period. The Mann-Whitney test was applied to compare the mean daily dietary intake between the groups in the pre- (days 0-13) and post-treatment (days 14-20) periods. Binomial regression was employed to compare data on the length of gross intestinal lesion between the groups.
Results
Clinical findings
All fecal samples collected before the pigs were inoculated (day 2) tested negative for the presence of Lawsonia intracellularis by the Polymerase Chain Reaction (PCR) technique. Results of the bacteriological examinations of the inoculum were negative for enterotoxigenic Salmonella sp. and Escherichia coli. However, a total of 27 pigs (45%), 13 from the Control Group and 14 from the Medication Group, presented with liquid and yellowish diarrhea in the first four days after inoculation. As the period after inoculation was too short for occurrence of diarrhea as a result of infection by L. intracellularis, infection by enterotoxigenic E. coli was suspected. Fecal samples were collected for bacteriological examination and beta-hemolytic E. coli was isolated and tested positive for the Sta and Stb genes. Based on these results, zinc oxide (3.000ppm) was added to the feed of all animals for three days.
As of day 6, the number of pigs showing diarrhea associated with E. coli began to decline, and on day-9 the animals began to present pasty diarrhea compatible with that caused by L. intracellularis. On day12 of the experiment, 19 of the 60 pigs showed diarrhea: 10 (33.3%) in the Control Group and nine (30%) in the Medication Group, reaching the expected minimum of 25% of animals with diarrhea to begin treatment (Fig.1).
Number of pigs with diarrhea in each experimental group (Control and Medication) on the days after inoculation (days 1 to 21).
After treatment with tylosin injectable, which occurred on days 13 and 14, clinical evaluations continued to be performed in the same manner, and a gradual reduction of diarrhea was observed in both groups, more numerically accentuated in the Medication Group. In the Control Group, 23 and 17 pigs showed diarrhea before and after day 13, respectively; whereas, in the Medication Group, 26 and 11 animals showed diarrhea in the pre- and post-treatment periods, respectively.
Poisson regression analysis showed statistical difference (p=0.001) between the groups relative to the sum of the number of animals with and without diarrhea in the post-treatment period (Table 1). Pigs in the Medication Group presented, on average, one day (0.85 days) less without diarrhea than those in the Control Group (Table 1).
Total number of days with and without diarrhea between the animals in the Control and Medication Groups in the post-treatment period (days- 14 to 21 after inoculation)
On day 18 of the experiment, one of the pigs in the Medication Group was found dead. The animal had not been presented with any clinical signs before death. Necropsy identified that the death was caused by septicemia due to mitral valve endocarditis and the animal did not have gross lesions of proliferative enteropathy. The weight gain data of this animal were considered until day 18, and the final calculation was adjusted until the end of the experiment. At the end of the experiment, five pigs in the Control Group were thin, showing lack of uniformity of the group.
Growth performance
Although the pigs in the Medication Group were, on average, 730g heavier than those in Control Group 21 days after inoculation, no significant difference was found between the groups regarding the variables mean weight and mean daily weight gain (Table 2). Mean daily dietary intake was 39g higher in the Medication Group compared with that in the Control Group, but with no significant difference (Table 2).
Comparison between mean weight of animals in the Control and Medication Groups on days -2, 13, and 21. Comparison between mean daily weight gain (MDWG) in the Control and Medication Groups in the periods between days -2 and 13 and days 13 and 21. Comparison between mean daily dietary intake (MDDI) per animal, calculated by the mean of the pen, in the Control and Medication Groups in the periods: total (days 0 to 20), pre-treatment (days 0 to 13), and post-treatment (days 14 to 20)
Gross lesions
At necropsy, typical PPE grade 1 lesions were observed in the ileum of 16 animals, with the Control Group showing a larger number (10 pigs) compared with that (6 pigs) of the Medication Group (p>0.05). The lesions comprised discrete thickening of the intestinal mucosa in the ileum with mild hyperemia, and their length for each animal ranged from 6 to 65cm. The total lesion length observed in pigs in the Control Group (366cm of intestinal lesion) was statistically larger compared with that in pigs in the Medication Group (97cm of intestinal lesion) (p=0.031). The mean lesion lengths per affected pig were 16.16 and 36.6cm in the Medication and Control Groups, respectively (p=0.093). The mean lesion lengths by the total number of animals were 3.2 and 12.2cm in the Medication and Control Groups, respectively (p=0.151) (Table 3).
IHC
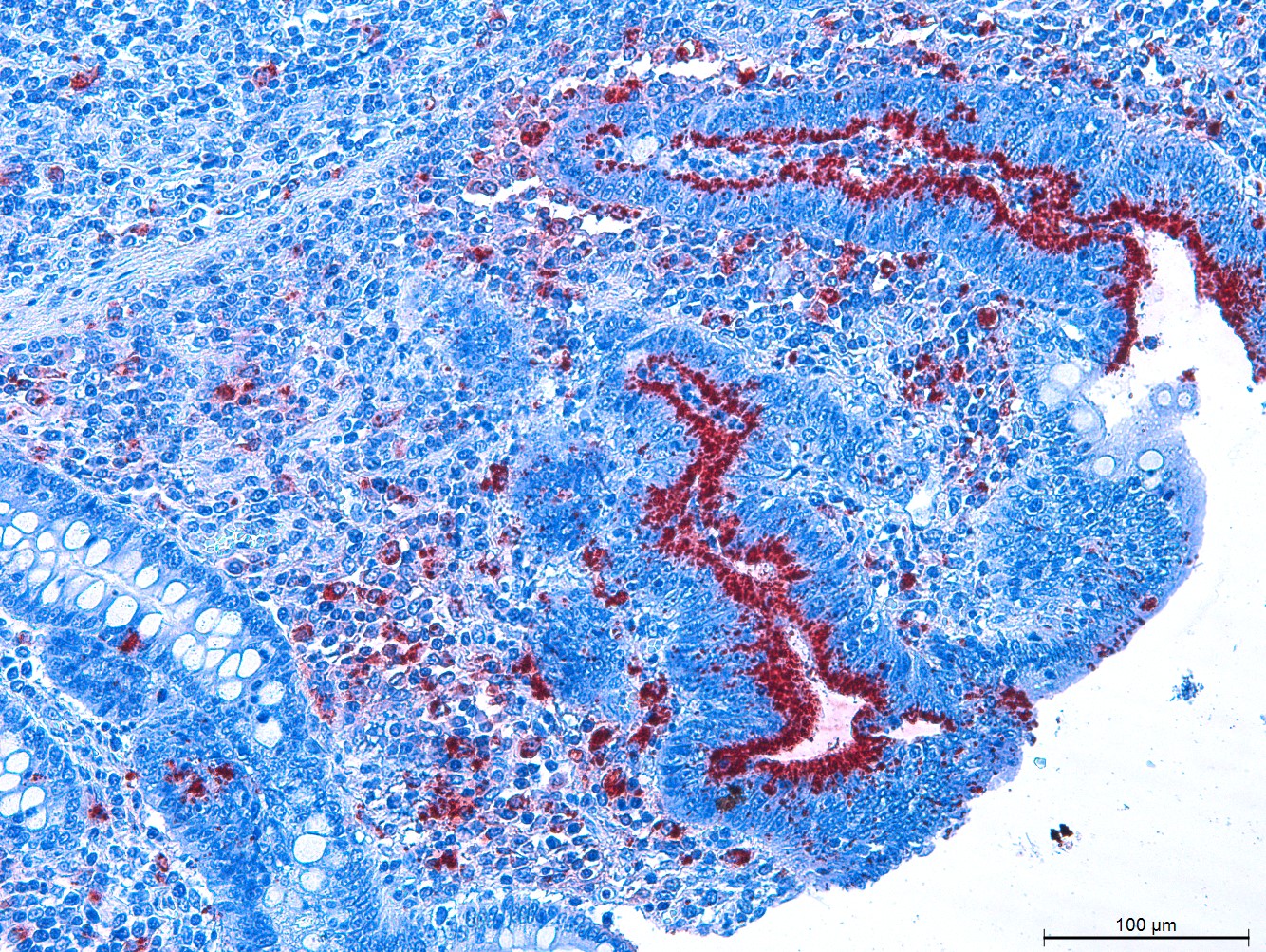
Statistically significant difference (p<0.032) in immunostaining was observed between the groups, present in 16 pigs in the Control Group (53.3%) and in eight pigs in the Medication Group (26.6%). All labels were observed in the ileum, and were classified as grade 1 (positive antigen labeled in up to 25% of the intestinal crypts) (Figs.2 and 3).
Histology section of ileum of Pig 37, Control Group (PAb 1999, Guedes & Gebhart 2003aGuedes R.M.C. & Gebhart C.J. 2003a. Preparation and characterization of polyclonal and monoclonal antibodies against Lawsonia intracellularis. J. Vet. Diagn. Invest. 15(5):438-446. <http://dx.doi.org/10.1177/104063870301500506> <PMid:14535543>
https://doi.org/10.1177/1040638703015005... ). Grade 1, positive antigen labeled in up to 25% of the crypts. IHC anti-Lawsonia intracellularis, obj.10x.
Histology section of ileum of Pig 8, Control Group (PAb 1999, Guedes & Gebhart 2003aGuedes R.M.C. & Gebhart C.J. 2003a. Preparation and characterization of polyclonal and monoclonal antibodies against Lawsonia intracellularis. J. Vet. Diagn. Invest. 15(5):438-446. <http://dx.doi.org/10.1177/104063870301500506> <PMid:14535543>
https://doi.org/10.1177/1040638703015005... ). IHC anti-Lawsonia intracellularis, obj.20x.
Discussion
Based on clinical signs, gross lesions, and immunostaining for Lawsonia intracellularis observed in the pigs in the Control Group, it can be stated that the experimental inoculation model was effective to reproduce the disease. The fecal sample collection from all animals in the study prior to inoculation and the negative result for L. intracellularis in all of them, characterize the absence of infection in these animals at the beginning of the study and show that a non-inoculated restricted Control Group is not necessary.
Considering that it takes at least 7 to 8 days for the onset of a clinical condition caused by infection with L. intracellularis (Guedes et al. 2017Guedes R.M.C., Machuca M.A., Quiroga M.A., Pereira C.E.R., Resende T.P. & Gebhart C.J. 2017. Lawsonia intracellularis in pigs: progression of lesions and involvement of apoptosis. Vet. Pathol. 54(4):620-628. <http://dx.doi.org/10.1177/0300985817698206> <PMid:28622490>
https://doi.org/10.1177/0300985817698206...
), despite the presence of diarrhea caused by enterotoxigenic Escherichia coli on the first days after inoculation, the higher incidence of diarrhea from day 9 to day 12 after inoculation is in agreement with findings of other studies that used a similar experimental infection model (Paradis et al. 2004Paradis M.A., Pauling G.E., Brennan J., Winkelmand N.L., Bagg R.N., Dick C.P. & Wilson J. 2004. Evaluation of tylosin tartrate in drinking water for treatment of porcine proliferative enteropathy (ileitis). J. Swine Health Prod. 12(4):177-181.).
For zinc oxide, used in the first days after inoculation to contain the diarrhea caused by enterotoxigenic E. coli, there are no studies that clinically assessed animals infected with L. intracellularis. However, it is known that zinc oxide, at the doses applied in the present study, can be used during the administration of the live attenuated vaccine of L. intracellularis (Enterisol Ileitis, Boehringer Ingelheim VetMedica) without compromising its effectiveness, according to the manufacturer’s information. Thus, we strongly believe that the use of zinc oxide did not affect infection by L. intracellularis, but it was effective in controlling the initial diarrhea induced by E. coli.
Although the growth performance results were numerically different between the experimental groups of this study, no significant statistical difference was observed, which can be justified by the high coefficient of variation of the analyzed variables (body weight, mean daily weight gain, and mean daily dietary intake) (Veenhuizen et al. 1998Veenhuizen M.F., Mowrey D.H., Moore G.M. & Watkins L.E. 1998. Evaluating a natural outbreak of porcine proliferative enteropathy and treatment with tylosin in the grow-finish phase. J. Swine Health Prod. 6(2):67-72., Paradis et al. 2005Paradis M.A., Mcya R.I., Wilson J.B., Vessie G.H., Winkelman N.L., Gebhart C.J. & Dick C.P. 2005. Subclinical ileitis produced by sequential dilutions of Lawsonia intracellularis in a mucosal homogenate challenge model. Am. Assoc. Swine Vet. 2005:189-191.).
Gross lesions were more frequently observed in pigs in the Control Group, which showed greater total length than that of pigs in the Medication Group (p<0.05). The lesions found on the day of euthanasia (day 21 post-inoculation) are consistent with those described in another study, in which most gross lesions were found between days 15 and 24 (Guedes et al. 2017Guedes R.M.C., Machuca M.A., Quiroga M.A., Pereira C.E.R., Resende T.P. & Gebhart C.J. 2017. Lawsonia intracellularis in pigs: progression of lesions and involvement of apoptosis. Vet. Pathol. 54(4):620-628. <http://dx.doi.org/10.1177/0300985817698206> <PMid:28622490>
https://doi.org/10.1177/0300985817698206...
).
Pigs in the Medication Group had less ileum immunostaining (p<0.032) compared with those in the Control Group, showing that the presence of the L. intracellularis antigen was more frequent in non-medicated animals. Similar findings were reported by Marsteller et al. (2001)Marsteller T., Winkelman N., Gebhart C., Armbruster G., Weldon W., Muller R., Weatherford J. & Symanowski J. 2001. Efficacy of intramuscular tylosin for the treatment and control of porcine proliferative enteropathy caused by Lawsonia intracellularis. Vet. Therap. 2(1):51-60. using tylosin injectable, in a different presentation and formulation, in experimentally inoculated pigs. It is worth noting that the number of applications was half that used by Marsteller et al. (2001)Marsteller T., Winkelman N., Gebhart C., Armbruster G., Weldon W., Muller R., Weatherford J. & Symanowski J. 2001. Efficacy of intramuscular tylosin for the treatment and control of porcine proliferative enteropathy caused by Lawsonia intracellularis. Vet. Therap. 2(1):51-60., which demonstrates clear practical advantage based on the time spent with handling animal medication. With respect to the active principle used for the treatment of PPE in the present study, tylosin is a macrolide, bacteriostatic agent that can act as a bactericide when in high concentrations (Kim et al. 2008Kim M., Gebru E., Chang Z., Choi J., Hwang M., Kang E., Lim J., Yun H. & Park S. 2008. Comparative Pharmacokinetics of Tylosin of Florfenicol after a single intramuscular administration at two different doses of tylosin-florfenicol combination pigs. J. Vet. Med. Sci. 70(1):99-102. <http://dx.doi.org/10.1292/jvms.70.99> <PMid:18250580>
https://doi.org/10.1292/jvms.70.99...
). Macrolides bind to the subunit (50s) of the bacterial ribosome by inhibiting bacterial protein synthesis (Barcellos et al. 2012Barcellos D., Sobestiansky J., Linhares D. & Sobestiansky T.B. 2012. Uso de antimicrobianos, p.839-845. In: Sobestiansky J. & Barcellos D. (Eds), Doenças dos suínos. 2ª ed. Cânone Editorial, Goiânia.). Particularly important for the case of intracellular microorganisms, as L. intracellularis, in this class of antimicrobial drugs, it is the liposolubility that enables crossing of cell barriers and reaching the target agent more easily (Spinosa et al. 2002Spinosa H.S., Gorniak S.L. & Bernardi M.M. 2002. Farmacologia Aplicada à Medicina Veterinária. 3ª ed. Guanabara Koogan, Rio de Janeiro. 646p.).
Regarding previous studies addressing tylosin and L. intracellularis, despite showing values of minimum inhibitory concentration (MIC) in vitro, that is, little effective action against PPE in an in vitro study (McOrist et al. 1995McOrist S., Mackie R.A. & Lawson G.H.K. 1995. Antimicrobial susceptibility of ileal symbiont intracellularis isolated from pigs with proliferative enteropathy. J. Clin. Microb. 33(5):1314-1317. <PMid:7615747>), in-feed tylosin was effective to treat the disease in pure culture experimental inoculation when administered for 14 days (100ppm) (McOrist et al. 1997McOrist S., Morgan J., Veenhuizen M.F., Lawrence K. & Kroger H.W. 1997. Oral administration of tilosin phosphate for treatment and prevention of porcine proliferative enteropathy. Am. J. Vet. Res. 58(2):136-139. <PMid:9028475>). As previously mentioned, tylosin also showed satisfactory results in the treatment of PPE in another study (Marsteller et al. 2001Marsteller T., Winkelman N., Gebhart C., Armbruster G., Weldon W., Muller R., Weatherford J. & Symanowski J. 2001. Efficacy of intramuscular tylosin for the treatment and control of porcine proliferative enteropathy caused by Lawsonia intracellularis. Vet. Therap. 2(1):51-60.), in which it was injected twice daily for three consecutive days, twice as much as in the present study. The difference between in vivo and in vitro results may be justified by the fact that L. intracellularis is an obligate intracellular bacterium (McOrist et al. 2000McOrist S., Muller Wager A., Kratzer D. & Sjösten C.G. 2000. Therapeutic efficacy of water-soluble lincomycin-spectinomycin powder against porcine proliferative enteropathy in a European field study. Vet. Rec. 146(3):61-65. <http://dx.doi.org/10.1136/vr.146.3.61> <PMid:10674691>
https://doi.org/10.1136/vr.146.3.61...
).
In addition to management measures, medication is the most used form for the treatment and control of PPE (França & Guedes 2008França S.A. & Guedes R.M.C. 2008. Antimicrobianos para o controle da enteropatia proliferativa suína. Ciência Rural 38(1):288-296. <http://dx.doi.org/10.1590/S0103-84782008000100050>
https://doi.org/10.1590/S0103-8478200800...
). Metaphylactic use corresponds to the application of medication at therapeutic doses in the whole batch of animals, indicated when diseases begin to manifest in a small percentage of animals (Barcellos et al. 2012Barcellos D., Sobestiansky J., Linhares D. & Sobestiansky T.B. 2012. Uso de antimicrobianos, p.839-845. In: Sobestiansky J. & Barcellos D. (Eds), Doenças dos suínos. 2ª ed. Cânone Editorial, Goiânia.). Metaphylactic antibiotic therapy and treatment are the most indicated, because the use of antimicrobial drugs in inadequate doses and times, as growth promoters, may increase the chance of outbreaks of enteric diseases (Bane et al. 2001Bane D.P., Neumann E., Gebhart C.J., Gardner I.A. & Norby B. 2001. Porcine proliferative enteropathy: a case-control study in swine herds in the United States. J. Swine Health Prod. 9(4):155-158.), in addition to favoring the risk of bacterial resistance (Silbergeld et al. 2008Silbergeld E.K., Graham J. & Price L.B. 2008. Industrial Food Animal Production, antimicrobial resistance and human health. Annual Rev. Publ. Health 29(1):151-169. <http://dx.doi.org/10.1146/annurev.publhealth.29.020907.090904> <PMid:18348709>
https://doi.org/10.1146/annurev.publheal...
, Dosen et al. 2014Dosen R., Prodanov-radulovic J., Pusic I., Ratajac R., Stojanov I. & Grubac S. 2014. The uncontrolled use of antibiotics in pig production, a threat to public health. XVI International Symposium “Feed Technology”, p.20-24. (Resumo)). Administration of in-feed medication has been more associated with increased risk of bacterial resistance when compared with individual treatment (Dunlop et al. 1998Dunlop R.H., McEwen S.A., Meek A.H., Clarke R.C., Black W.D. & Friendship R.M. 1998. Associations among antimicrobial drug treatments and antimicrobial resistance of fecal Escherichia coli of swine on 34 farrow-to-finish farms in Ontario, Canada. Prev. Vet. Med. 34(4):283-305. <http://dx.doi.org/10.1016/S0167-5877(97)00095-0> <PMid:9618742>
https://doi.org/10.1016/S0167-5877(97)00...
, Haese & Silva 2004Haese D. & Silva B.A.N. 2004. Antibióticos como promotores de crescimento em monogástricos. Revta Eletrôn. Nutritime 1(1):7-19.).
Indiscriminate use of antimicrobial drugs in low dose diet is associated with bacterial resistance (Maron et al. 2013Maron D.F., Smith T.J.S. & Nachman K.E. 2013. Restrictions on antimicrobial use in food animal production: an international regulatory and economic survey. Globalization Health 9(48):1-11. <PMid:24131666>). Some studies conducted in the USA have shown that in order to preserve the efficacy of antibiotics for human and animal treatments, it is necessary to limit the use of antimicrobial drugs (Levy & Marshall 2004Levy S.B. & Marshall B. 2004. Antibacterial resistance worldwide: causes, challenges and responses. Nature 10(Suppl.12):122-129. <PMid:15577930>, Silbergeld et al. 2008Silbergeld E.K., Graham J. & Price L.B. 2008. Industrial Food Animal Production, antimicrobial resistance and human health. Annual Rev. Publ. Health 29(1):151-169. <http://dx.doi.org/10.1146/annurev.publhealth.29.020907.090904> <PMid:18348709>
https://doi.org/10.1146/annurev.publheal...
). As a result, many countries have restricted the use of antibiotics as growth promoters (Maron et al. 2013Maron D.F., Smith T.J.S. & Nachman K.E. 2013. Restrictions on antimicrobial use in food animal production: an international regulatory and economic survey. Globalization Health 9(48):1-11. <PMid:24131666>). The European Union banned the use of antimicrobial growth promoters in pig feed in 2006 (Gaggia et al. 2010Gaggìa F., Mattarelli P. & Biavati B. 2010. Probiotics and prebiotics in animal feeding for safe food production. Int. J. Food Microbiol. 141(Suppl.1):1-14. <http://dx.doi.org/10.1016/j.ijfoodmicro.2010.02.031> <PMid:20382438>
https://doi.org/10.1016/j.ijfoodmicro.20...
) and a new regulation proposed by the FDA (Food and Drugs Administration) on the use of human antibiotics in domestic animal feed was enacted in the USA in 2017 (FDA 2017FDA 2017. Guidance for Industry 203: New animal drugs and new animal drug combiation products administered in or on medicated feed or drinking water of food-producing animals, recommendations for drugs sponsors for voluntarily aligning product use conditions with GFI #209. Center for Veterinary Medicine, Food and Drug Administration, U.S. Department of Health and Human Services, USA. 18p., Beek 2017Beek V.T. 2017. US: Vaccines and biosecurity to replace antibiotics. Pig Progress. Available at <Available at http://www.pigprogress.net/Health/Articles/2017/1/US-vaccines and biosecurity to replace antibiotics 81584E/
> Accessed on Sept. 23, 2017.
http://www.pigprogress.net/Health/Articl...
). Therefore, the individual use of injectable antimicrobial drugs, as in this study, can assist with reversing the frequency of high bacterial resistance, as well as preventing the emergence of new resistant bacteria (Levy & Marshall 2004Levy S.B. & Marshall B. 2004. Antibacterial resistance worldwide: causes, challenges and responses. Nature 10(Suppl.12):122-129. <PMid:15577930>).
Most in-feed antibiotics provide low plasma levels of the drug compared with those of injectable drugs, especially macrolides and pleuromutilins, which also reduces bioavailability. In order to achieve treatment efficacy, the drug should be at the site of infection for sufficient time and concentration, otherwise it might favor development of bacterial resistance (Burch 2012Burch D.G.S. 2012. Examination of the pharmacokinetic/pharmacodynamic (PK/PD) relationships of orally administered antimicrobials and their correlation with the therapy of various bacterial and mycoplasmal infections in pigs. Royal Colege of Veterinary Surgeons, London, p.1-130.).
Presentation of medication in injectable form, as used in the present study, is advantageous, because it enables its complete absorption, ensuring that the animal receives the entire necessary dose (Karriker et al. 2012Karriker L.A., Coetzee J., Friendship R.M. & Prescott J.F. 2012. Drug pharmacology, therapy and prophylaxis, p.438-443. In: Zimmerman J.J., Karriker L.A., Ramirez A., Schwartz K.J. & Stevenson G.W. (Eds), Diseases of Swine. 10th ed. John Wiley and Sons, Iowa.). Animals infected with L. intracellularis present with atrophy and fusion of the villi, with reduction of digestive enzymes, and inhibition of membrane transporters, mechanisms that lead to malabsorption diarrhea (Argenzio 1980Argenzio R.A. 1980. Glucose-stimulated fluid absorption in the pig small intestine during the early stage of swine dysentery. Am. J. Vet. Res. 41(12):2000-2006. <PMid:7212433>, Vannucci & Guedes 2009Vannucci F.A. & Guedes R.M.C. 2009. Fisiopatologia das diarreias em suínos. Ciência Rural 39(7):2233-2242. <http://dx.doi.org/10.1590/S0103-84782009005000163>
https://doi.org/10.1590/S0103-8478200900...
), suggesting that it may result in low antibiotic uptake when this is administered orally. Intramuscular medication has another advantage compared with in-feed medication, because ill animals show lower feed intake (Apley et al. 2012Apley M.D., Bush E.J., Morrison R.B., Singer R.S. & Snelson H. 2012. Use estimates of In-feed antimicrobials in swine production in the United States. Foodborne Pathog. Dis. 9(3):1-8. <http://dx.doi.org/10.1089/fpd.2011.0983> <PMid:22324295>
https://doi.org/10.1089/fpd.2011.0983...
). It is true that intramuscular application is more laborious in larger animals, but long-acting formulations that do not need to be applied more than once have been increasingly growing (Burch 2012Burch D.G.S. 2012. Examination of the pharmacokinetic/pharmacodynamic (PK/PD) relationships of orally administered antimicrobials and their correlation with the therapy of various bacterial and mycoplasmal infections in pigs. Royal Colege of Veterinary Surgeons, London, p.1-130.).
Conclusion
Tylosin injectable (Eurofarma Laboratórios S.A), in the conditions of the present study, was effective in treating porcine proliferative enteropathy (PPE) in experimentally inoculated pigs, because it significantly reduced lesion length and grade of infection by Lawsonia intracellularis.
Acknowledgements
The authors are grateful to the research funding agencies Fapemig, Capes, and CNPq for the support provided to this study. RMCG holds a grant from CNPq for productivity in research.
References
- Apley M.D., Bush E.J., Morrison R.B., Singer R.S. & Snelson H. 2012. Use estimates of In-feed antimicrobials in swine production in the United States. Foodborne Pathog. Dis. 9(3):1-8. <http://dx.doi.org/10.1089/fpd.2011.0983> <PMid:22324295>
» https://doi.org/10.1089/fpd.2011.0983 - Argenzio R.A. 1980. Glucose-stimulated fluid absorption in the pig small intestine during the early stage of swine dysentery. Am. J. Vet. Res. 41(12):2000-2006. <PMid:7212433>
- Bane D.P., Neumann E., Gebhart C.J., Gardner I.A. & Norby B. 2001. Porcine proliferative enteropathy: a case-control study in swine herds in the United States. J. Swine Health Prod. 9(4):155-158.
- Barcellos D., Sobestiansky J., Linhares D. & Sobestiansky T.B. 2012. Uso de antimicrobianos, p.839-845. In: Sobestiansky J. & Barcellos D. (Eds), Doenças dos suínos. 2ª ed. Cânone Editorial, Goiânia.
- Beek V.T. 2017. US: Vaccines and biosecurity to replace antibiotics. Pig Progress. Available at <Available at http://www.pigprogress.net/Health/Articles/2017/1/US-vaccines and biosecurity to replace antibiotics 81584E/ > Accessed on Sept. 23, 2017.
» http://www.pigprogress.net/Health/Articles/2017/1/US-vaccines and biosecurity to replace antibiotics 81584E/ - Burch D.G.S. 2000. Controlling ileitis in the “colitis” complex. Pig J. 45:131-149.
- Burch D.G.S. 2012. Examination of the pharmacokinetic/pharmacodynamic (PK/PD) relationships of orally administered antimicrobials and their correlation with the therapy of various bacterial and mycoplasmal infections in pigs. Royal Colege of Veterinary Surgeons, London, p.1-130.
- Callens B., Persoons D., Maes D., Laanen M., Postma M., Boyen F., Haesebrouck F., Butaye P., Catry B. & Dewulf J. 2012. Prophylactic and metaphylactic antimicrobial use in Belgian fattening pig herds. Prev. Vet. Med. 106(1):53-62.
- Dosen R., Prodanov-radulovic J., Pusic I., Ratajac R., Stojanov I. & Grubac S. 2014. The uncontrolled use of antibiotics in pig production, a threat to public health. XVI International Symposium “Feed Technology”, p.20-24. (Resumo)
- Dunlop R.H., McEwen S.A., Meek A.H., Clarke R.C., Black W.D. & Friendship R.M. 1998. Associations among antimicrobial drug treatments and antimicrobial resistance of fecal Escherichia coli of swine on 34 farrow-to-finish farms in Ontario, Canada. Prev. Vet. Med. 34(4):283-305. <http://dx.doi.org/10.1016/S0167-5877(97)00095-0> <PMid:9618742>
» https://doi.org/10.1016/S0167-5877(97)00095-0 - FDA 2017. Guidance for Industry 203: New animal drugs and new animal drug combiation products administered in or on medicated feed or drinking water of food-producing animals, recommendations for drugs sponsors for voluntarily aligning product use conditions with GFI #209. Center for Veterinary Medicine, Food and Drug Administration, U.S. Department of Health and Human Services, USA. 18p.
- França S.A. & Guedes R.M.C. 2008. Antimicrobianos para o controle da enteropatia proliferativa suína. Ciência Rural 38(1):288-296. <http://dx.doi.org/10.1590/S0103-84782008000100050>
» https://doi.org/10.1590/S0103-84782008000100050 - Gaggìa F., Mattarelli P. & Biavati B. 2010. Probiotics and prebiotics in animal feeding for safe food production. Int. J. Food Microbiol. 141(Suppl.1):1-14. <http://dx.doi.org/10.1016/j.ijfoodmicro.2010.02.031> <PMid:20382438>
» https://doi.org/10.1016/j.ijfoodmicro.2010.02.031 - Guedes R.M.C. 2002. Porcine proliferative enteropathy: diagnosis, immune response and pathogenesis. Doctoral Dissertation, University of Minnesota, St Paul. 261p.
- Guedes R.M.C. & Gebhart C.J. 2003a. Preparation and characterization of polyclonal and monoclonal antibodies against Lawsonia intracellularis J. Vet. Diagn. Invest. 15(5):438-446. <http://dx.doi.org/10.1177/104063870301500506> <PMid:14535543>
» https://doi.org/10.1177/104063870301500506 - Guedes R.M.C. & Gebhart C.J. 2003b. Comparison of intestinal mucosa homogenate and pure culture of the homologous Lawsonia intracellularis isolate in reproducing proliferative enteropathy in swine. Vet. Microbiol. 93(2):159-166. <http://dx.doi.org/10.1016/S0378-1135(03)00013-0> <PMid:12637004>
» https://doi.org/10.1016/S0378-1135(03)00013-0 - Guedes R.M.C., França S.A., Machado G.S., Blumer M.A. & Costa Cruz Junior E.C. 2009. Use of tylvalosin-medicated feed to control porcine proliferative enteropathy. Vet. Rec. 165(12):342-345. <http://dx.doi.org/10.1136/vr.165.12.342> <PMid:19767637>
» https://doi.org/10.1136/vr.165.12.342 - Guedes R.M.C., Machuca M.A., Quiroga M.A., Pereira C.E.R., Resende T.P. & Gebhart C.J. 2017. Lawsonia intracellularis in pigs: progression of lesions and involvement of apoptosis. Vet. Pathol. 54(4):620-628. <http://dx.doi.org/10.1177/0300985817698206> <PMid:28622490>
» https://doi.org/10.1177/0300985817698206 - Haese D. & Silva B.A.N. 2004. Antibióticos como promotores de crescimento em monogástricos. Revta Eletrôn. Nutritime 1(1):7-19.
- Jones G.F., Ward G.E., Murtaugh M.P., Lin G. & Gebhart C.J. 1993. Enhanced detection of intracellular organism of swine proliferative enteritis, ileal symbiont intracellularis, in feces by polymerase chain reaction. J. Clin. Microbiol. 31(10):2611-2615. <PMid:8253956>
- Karriker L.A., Coetzee J., Friendship R.M. & Prescott J.F. 2012. Drug pharmacology, therapy and prophylaxis, p.438-443. In: Zimmerman J.J., Karriker L.A., Ramirez A., Schwartz K.J. & Stevenson G.W. (Eds), Diseases of Swine. 10th ed. John Wiley and Sons, Iowa.
- Kim M., Gebru E., Chang Z., Choi J., Hwang M., Kang E., Lim J., Yun H. & Park S. 2008. Comparative Pharmacokinetics of Tylosin of Florfenicol after a single intramuscular administration at two different doses of tylosin-florfenicol combination pigs. J. Vet. Med. Sci. 70(1):99-102. <http://dx.doi.org/10.1292/jvms.70.99> <PMid:18250580>
» https://doi.org/10.1292/jvms.70.99 - Levy S.B. & Marshall B. 2004. Antibacterial resistance worldwide: causes, challenges and responses. Nature 10(Suppl.12):122-129. <PMid:15577930>
- Macêdo N.R., Menezes C.P., Lage A.P., Ristow L.E., Reis A. & Guedes R.M.C. 2007. Detecção de cepas patogênicas pela PCR multiplex e avaliação da sensibilidade a antimicrobianos de Escherichia coli isoladas de leitões diarreicos. Arq. Bras. Med. Vet. Zootec. 59(5):1117-1123. <http://dx.doi.org/10.1590/S0102-09352007000500005>
» https://doi.org/10.1590/S0102-09352007000500005 - Maron D.F., Smith T.J.S. & Nachman K.E. 2013. Restrictions on antimicrobial use in food animal production: an international regulatory and economic survey. Globalization Health 9(48):1-11. <PMid:24131666>
- Marsteller T., Winkelman N., Gebhart C., Armbruster G., Weldon W., Muller R., Weatherford J. & Symanowski J. 2001. Efficacy of intramuscular tylosin for the treatment and control of porcine proliferative enteropathy caused by Lawsonia intracellularis Vet. Therap. 2(1):51-60.
- McOrist S. 2005. Prevalence and impact of proliferative enteropathy (ileitis) in East Asia. Proceedings II Asian Pig Veterinary Society Congress, Pasig City, Philippines, p.24-37. (Resumo)
- McOrist S. & Gebhart C.J. 2012. IV: Bacterial diseases, proliferative enteropathy, p.2976-3004. In: Zimmerman J.J., Karriker L.A., Ramirez A., Schwartz K.J. & Stevenson G.W. (Eds), Diseases of Swine. 10th ed. John Wiley and Sons, Iowa.
- McOrist S., Mackie R.A. & Lawson G.H.K. 1995. Antimicrobial susceptibility of ileal symbiont intracellularis isolated from pigs with proliferative enteropathy. J. Clin. Microb. 33(5):1314-1317. <PMid:7615747>
- McOrist S., Morgan J., Veenhuizen M.F., Lawrence K. & Kroger H.W. 1997. Oral administration of tilosin phosphate for treatment and prevention of porcine proliferative enteropathy. Am. J. Vet. Res. 58(2):136-139. <PMid:9028475>
- McOrist S., Muller Wager A., Kratzer D. & Sjösten C.G. 2000. Therapeutic efficacy of water-soluble lincomycin-spectinomycin powder against porcine proliferative enteropathy in a European field study. Vet. Rec. 146(3):61-65. <http://dx.doi.org/10.1136/vr.146.3.61> <PMid:10674691>
» https://doi.org/10.1136/vr.146.3.61 - Paradis M.A., Pauling G.E., Brennan J., Winkelmand N.L., Bagg R.N., Dick C.P. & Wilson J. 2004. Evaluation of tylosin tartrate in drinking water for treatment of porcine proliferative enteropathy (ileitis). J. Swine Health Prod. 12(4):177-181.
- Paradis M.A., Mcya R.I., Wilson J.B., Vessie G.H., Winkelman N.L., Gebhart C.J. & Dick C.P. 2005. Subclinical ileitis produced by sequential dilutions of Lawsonia intracellularis in a mucosal homogenate challenge model. Am. Assoc. Swine Vet. 2005:189-191.
- Silbergeld E.K., Graham J. & Price L.B. 2008. Industrial Food Animal Production, antimicrobial resistance and human health. Annual Rev. Publ. Health 29(1):151-169. <http://dx.doi.org/10.1146/annurev.publhealth.29.020907.090904> <PMid:18348709>
» https://doi.org/10.1146/annurev.publhealth.29.020907.090904 - Spinosa H.S., Gorniak S.L. & Bernardi M.M. 2002. Farmacologia Aplicada à Medicina Veterinária. 3ª ed. Guanabara Koogan, Rio de Janeiro. 646p.
- Vannucci F.A. & Guedes R.M.C. 2009. Fisiopatologia das diarreias em suínos. Ciência Rural 39(7):2233-2242. <http://dx.doi.org/10.1590/S0103-84782009005000163>
» https://doi.org/10.1590/S0103-84782009005000163 - Veenhuizen M.F., Mowrey D.H., Moore G.M. & Watkins L.E. 1998. Evaluating a natural outbreak of porcine proliferative enteropathy and treatment with tylosin in the grow-finish phase. J. Swine Health Prod. 6(2):67-72.
Publication Dates
-
Publication in this collection
Mar 2019
History
-
Received
30 Sept 2018 -
Accepted
02 Oct 2018