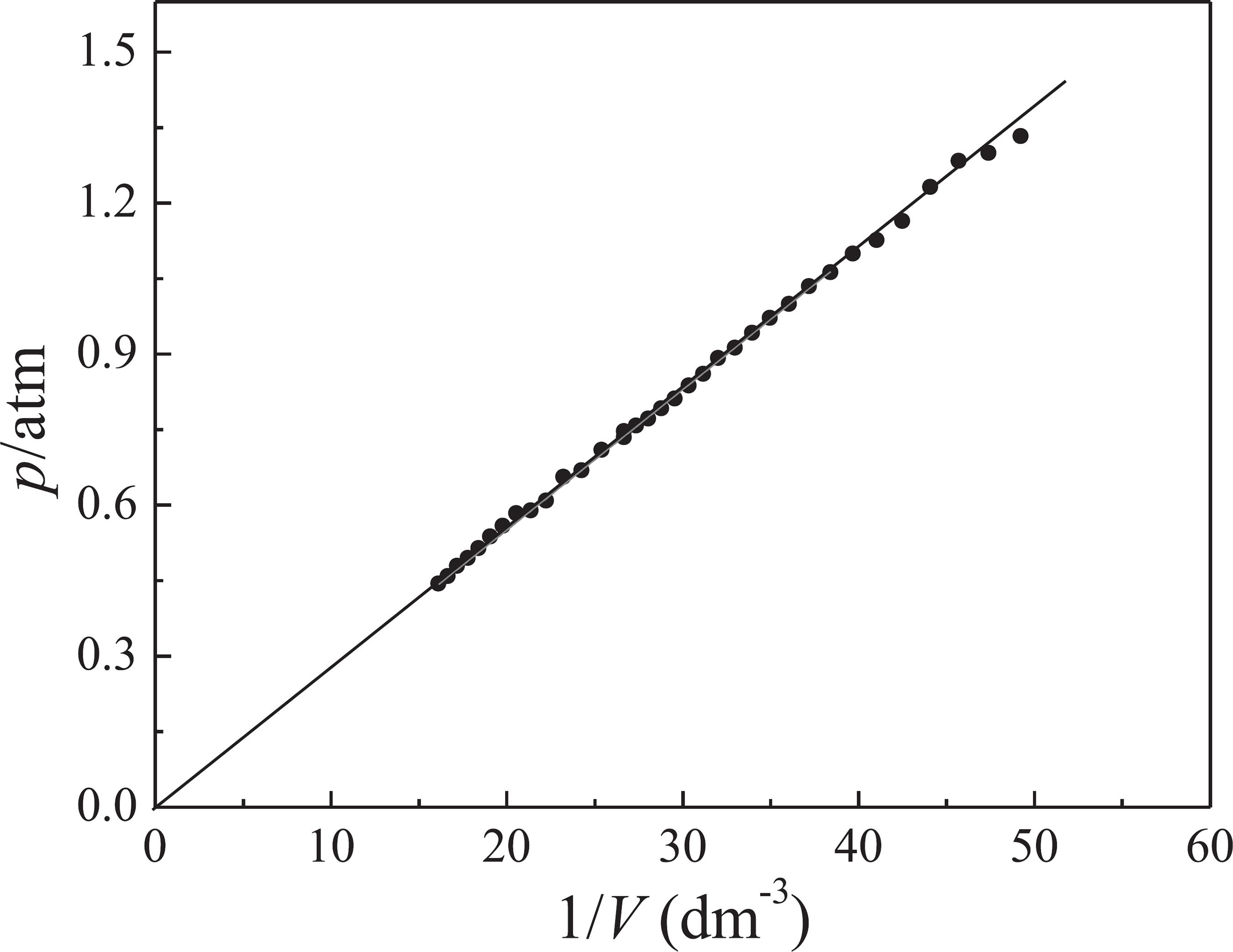
Relevant historical aspects concerning ideal gases were briefly reviewed to provide a condensed resource for the undergraduate student of chemistry. The importance of the barometer and the concept of atmospheric pressure were reviewed and discussed. A combination of Boyle's law with the well-known equation for determining the pressure produced by a column of stationary fluid was used to obtain a graphical method for determining atmospheric pressure under isothermal conditions in the laboratory. Important aspects related to the study of ideal gases were reviewed in light of the pressure-volume data that can be obtained by physical chemistry students using a simple apparatus composed of a commercial hypodermic syringe connected to a mercury manometer. Relevant concepts associated with the measurement of a physical quantity, the importance of linear regression, as well as the use of a graphical method to test the validity of a theory were reviewed from a pedagogical perspective through the experimental study of Boyle's law during regular experimental physical chemistry classes.
Keywords:
ideal gases; Boyle's law; atmospheric pressure; hypodermic syringe; experiments in physical chemistry