Abstract
Polychlorinated dibenzo-p-dioxins (PCDD) and dibenzofuranes (PCDF) and polychlorinated biphenyls (PCB) are types of persistent and bioaccumulating organic pollutants with enhanced chronic toxicity and carcinogenic properties and can be considered as environmental indicators of anthropogenic activities since their occurrence in the environment can always be linked to anthropogenic activities. The present paper reviews the main sources and behaviour of these compounds in the environment as well as the risks they represent to man and biota.
PCDD/PCDF; PCB; risk to environment and health
REVISÃO
Polychlorinated dibenzo-p-dioxins (PCDD), dibenzofurans (PCDF) and polychlorinated biphenyls (PCB): main sources, environmental behaviour and risk to man and biota
Márcia de Souza Pereira
Departamento de Geoquímica, Instituto de Química, Universidade Federal Fluminense, Outeiro de São João Baptista, s/n, 24020-007 Niterói - RJ
ABSTRACT
Polychlorinated dibenzo-p-dioxins (PCDD) and dibenzofuranes (PCDF) and polychlorinated biphenyls (PCB) are types of persistent and bioaccumulating organic pollutants with enhanced chronic toxicity and carcinogenic properties and can be considered as environmental indicators of anthropogenic activities since their occurrence in the environment can always be linked to anthropogenic activities. The present paper reviews the main sources and behaviour of these compounds in the environment as well as the risks they represent to man and biota.
Keywords: PCDD/PCDF; PCB; risk to environment and health.
INTRODUCTION
Since the Intergovernmental Forum on Chemical Safety promoted by UNEP (Decision 19/13 C from 1997) and later the Stockholm Convention (2001), international efforts have been made to eliminate and/or reduce the emissions and discharges of a set of 12 toxic organic chemicals, also called the "12 Dirties". These chemicals are classified as persistent organic pollutants (POP) and include different groups of molecules that are very resistant to (bio) degradation and thus prone to biomagnification, exerting their toxic effects at different trophic levels. Among them, polychlorinated dibenzo-p-dioxins (PCDD) and dibenzofuranes (PCDF) and polychlorinated biphenyls (PCB) constitute three groups of relevant persistent organic pollutants with enhanced chronic toxicity.
PCDD/F can be emitted by different human activities and industrial processes where they can be present as unwanted by-products. PCB are ubiquitous contaminants of the environment due to their large scale production until the end of the 1980s and their usage up to now. PCDD/F and PCB can also be emitted from biomass and fossil fuel burning and stationary sources like waste incineration. Taking into account this information, PCDD/F and PCB can be considered as environmental indicators of anthropogenic activities since their occurrence can always be linked to the human activities. The present paper reviews the physical-chemical properties, main sources and behaviour of these compounds in the environment as well as the risks they represent to man and biota.
POLYCHLORINATED DIBENZO-P-DIOXINS AND DIBENZOFURANES (PCDD/F)
Physical-chemical properties and nomenclature
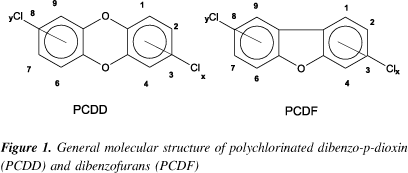
The polychlorinated dibenzo-p-dioxins (PCDD) and dibenzofuranes (PCDF), commonly called "dioxins", are two classes of "quasi-planar" tricycles aromatic ethers with 210 different compounds (congeners) in total. The PCDD/F have similar physical-chemical properties but different biological potencies1. Figure 1 shows the general structure of these classes of compounds.
According to the US-EPA, some rules should be observed in order to name the PCDD/F.
-
Congener: Each compound belonging to the same determined class of substances. In the case of PCDD/F there are 75 congeners of PCDD and 135 congeners of PCDF.
-
Homologue: compounds that have the same degree of chlorination.
-
Isomer: compounds that belong to the same homologue group and of which chlorine atoms are bonded at different positions at the body of the molecule.
-
A homologue group is named according to the number of chlorine atoms bound to the molecule: TetraCDD, TCDD, T4CDD, T4CDD or Cl4CDD designate the group of tetrachlorodibenzo-p-dioxins.
-
One specific congener is named when the following information is given: number of chlorine atoms and their position along the body molecule, name of the homologue group and class of compound to which this congener belongs.
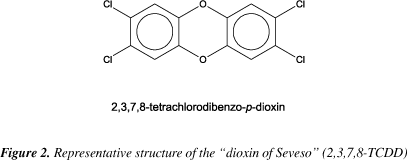
The most recognized example is the so-called "dioxin of Seveso", of which official nomenclature is 2,3,7,8-tetrachlorodibenzo-p-dioxin. Figure 2 shows the structure of this compound.
Table 1 shows the main physical-chemical properties of PCDD/F. Most of these data is calculated, since these compounds are only found as trace amounts in the environment2. PCDD/PCDF show high boiling points and low vapour pressures. Since their water solubility is extremely low, the air is their preferential transport medium and thereby these substances are contaminants of the fine and ultra-fine particulate matter3. Their distribution between particulate and gaseous forms depends on their physical and chemical characteristics.
In general, it is observed that the low molecular weight congeners can either be adsorbed to particulate material or appear in free gaseous form, whereas the heavier congeners are strongly adsorbed to particulate material2. The very low water solubility of PCDD/F decreases as the molecular weight and the number of chlorine substituents increase, which leads that in the aquatic medium and in soil, these substances are found preferentially bound to particulate material and to organic matter4.
Toxicokinetic metabolism and elimination of PCDD/PCDF
PCDD/F show, according to their pattern of chlorine substitution, the ability to bioaccumulate within trophic chains. Among the 210 different congeners, 17, the 2,3,7,8-chlorosubstituted, are by far the most bioaccumulating and the most toxic congeners5. Even within these 17 congeners the bioconcentration factors as well as the toxic potencies are not similar, but can vary up to three orders of magnitude due to steric effects. According to Körner (1995)6, the mechanism of biological action of these substances in the organism begins with the binding to and activation of the cytoplasmatical aryl hydrocarbon receptors (AhR) present in the liver and other organs and tissues. This binding and activation is greatly favoured by the 2,3,7,8-chlorine substitution, which enables that the PCDD/F molecules encompass a rectangle of about of 3 x 10 Å, which fits to the ligand binding domain of the AhR. Once formed, the dioxin-Ah receptor complex can move to the cellular nucleus and binds to specific base sequences, the so-called xenobiotic responsive elements (XRE). This leads to an increased transcription of genes which are under control of the XRE into the corresponding m-RNAs and finally to an increased biosynthesis of the corresponding proteins modifying the cell function. One important and well-defined interaction is the induction of the monooxygenase isozymes cytochrom P450 1A1 (CYP1A1) and P450 1A2 (CYP1A2). The induction of CYP1A1 and 1A2 is one of the most common parameters of AhR activation used in cellular bioassays and in in vivo studies7. The toxicokinetic behaviour of PCDD/F in humans (and other primates) depends on three major properties: lipophilicity, metabolism and binding to the CYP1A2 in the liver8. Lipophilicity controls the absorption and partitioning of PCDD/F. Metabolism acts as the rate-limiting step of elimination, thereby leading to bioaccumulation. The induction of CYP1A2, which as that of CYP1A1 is controlled by the AhR, promotes the hepatic sequestration of these substances by the liver.
2,3,7,8TCDD is the most toxic congener and is an unavoidable by-product of different organochlorine chemicals. This compound was the main contaminant found in 2,4,5-Trichloracetic acid (2,4,5-T), the so-called "Agent Orange", largely disseminated in the Vietnam War9 and was also the main contaminant in the "Seveso Episode"10. 2,3,7,8-TCDD has a high acute toxicity that is only exceeded by natural toxins like Botulin A and Diphtherietoxin and is a strong tumour promoter2. The toxic effects of PCDD/F are proven to animals but there are only a few epidemiological studies, mainly from the Seveso region, on carcinogenic and other (sub)chronic effects of PCDD/F in man8. These studies are mainly based on the effect of 2,3,7,8-TCDD. Nevertheless, the basic determinants of pharmacokinetic behaviour are similar in animals and in man8. Table 2 describes the routes of magnification, lethal doses (LD50), recommended values of tolerable daily intake (TDI) and levels of concentration where no effects are observed (No Observed Effect Levels - NOEL) as well as the main symptoms to man/biota related to PCDD/F exposure11. The oral bioavailability of PCDD/F depend on the doses, media and is specific for each congener. PCDD/F can be absorbed from animal dairy products (Milk, butter, meat and fats) or reabsorbed from soil, ash or smooth particles. Once absorbed, PCDD/F will accumulate mainly in lipoproteins in blood, liver, and fat tissues6.
The high toxicity and the ability that PCDD/F seems to have to add their effects in organism, lead the development of the international toxicity equivalency factors (I-TEF) for this substances. The I-TEF developed by the North Atlantic Treaty Organisation in joint with Committee on the Challenges of Modern Society (NATO/CCMS)5 and latter revised by the World Health Organisation (WHO), are supposed to foresee the toxic effects of the PCDD/F from an additive model. The I-TEF of the 2,3,7,8-substituted PCDD/F related to 2,3,7,8-TCDD according to NATO/CCMS (1988)5 and WHO (1998)8 are listed in Table 3.
The metabolism and elimination of PCDD/F is only possible through the transformation of these substances into polar metabolites. The biotransformation in organism involves the epoxidation of these molecules with the formation of hydroxyl-derivatives and glucuronidration of the PCDD/F12.
The hydroxylation phase occurred through the activation of cytochrom P-450 isozymes (CYP1A1, CYP1A2, CYP3A3 and CYP3A4) 13. The resulting metabolites are less toxic than the original molecules, so that the metabolisation can be viewed as a decontamination pathway. Other PCDD/F congeners seem to follow the same hydroxylation pathway. Studies with rats and dogs shown that the metabolisation of 2,3,7,8-TCDD occurred mainly in the liver. For humans there is no information about PCDD/F metabolisation until now13.
Unmetabolised PCDD/F are partially eliminated through the excrements. When the metabolisation occurred, the polar metabolites can be eliminated partially through the gall, excrements and in little extent through the urine. The elimination half-live of the high-chlorinated congeners (HxCDD/F-OCDD/F) in rat varies between third five days and seven years13. Studies from third six veterans of the Vietnam had shown a mean halve-live of 7, 1 years (confidence interval of 5,8-9,6 years)6,12.
Sources of PCDD/F to the environment: formation and degradation
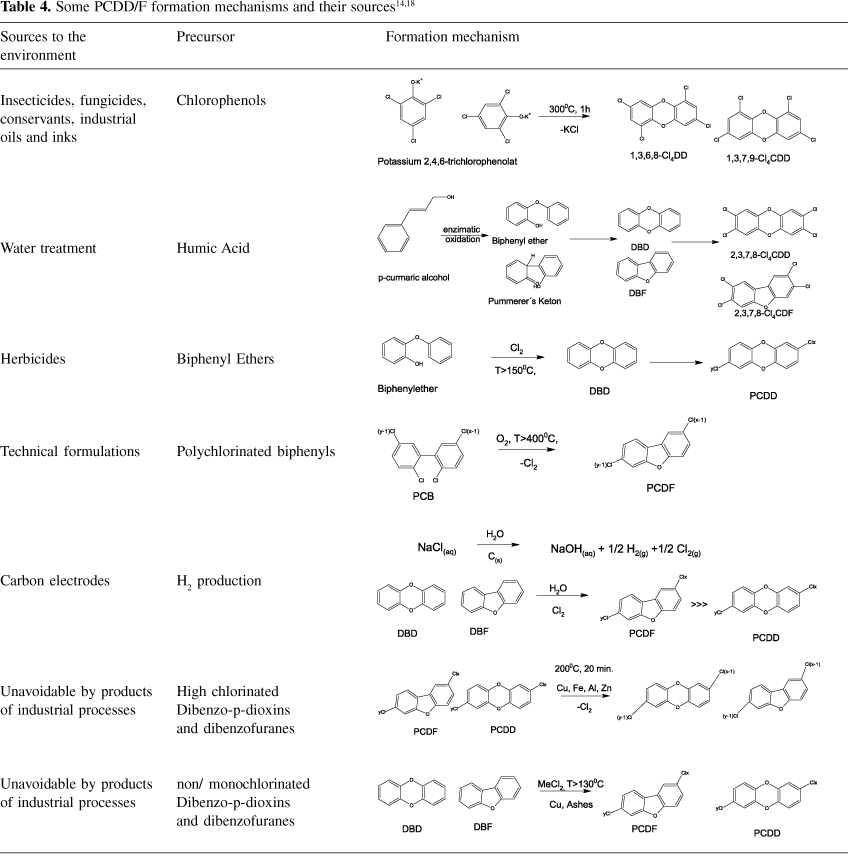
The main sources of PCDD/F to the environment are divided into three categories: stationary (thermal processes, chemical industries), diffuse (fuel burning, fires) and secondary sources or reservatories (biocompost, sewage sludge)3. Table 4 summarizes some mechanisms of PCDD/F formation from precursors during industrial processes. PCDD and PCDF are unwanted by-products during the combustion of organic materials containing (trace amounts of) chlorine and of stationary thermal sources like controlled burning of domestic, hospital or hazardous wastes14. Fuel burning in order to generate heat and energy (coal, wood or fossil fuels, Otto- and Diesel-engines)15,16 as well as cigarette smoke17 can be considered as diffuse sources of these contaminants to the environment. As main mechanisms to PCDD/F formation during combustion processes, different studies14-19 have pointed out reactions that involves the substitution of radicals, cyclisation and aromatization of molecules up to 600 ºC, condensation of precursors of PCDD/F, and free radical reactions mediated on the fly ash surface (de novo synthesis) over 300 ºC.
Chemical processes such as paper production19, petrochemistry20, production of herbicides/insecticides1, metallurgical processes and metal recycling are also stationary sources of PCDD/F2. As remote sources of PCDD/F some authors listen catastrophic events like volcanic eruptions2 and forest fires21. The mechanisms of formation occurring during industrial processes such as paper production, are also called "cold-formation" mechanisms due to the smooth conditions under which they occurred14. These reactions are always mediated by precursors18.
Typical natural process to PCDD/F destruction is the u.v. mediated dechlorinated mechanism occurred in the upper part of the atmosphere2,6. Intentional destruction methods already described in the literature are the catalytic dechlorination through metal and metalchloride1 and incineration above 1200 °C18,23. Anyway these methods require straight controlled conditions in order to avoid the formation of the toxic congeners through reaction such as dimerization and recondesation in the cooling zone of the incinerator23.
POLYCHLORINATED BIPHENYLS (PCB)
Physical-chemical properties and nomenclature
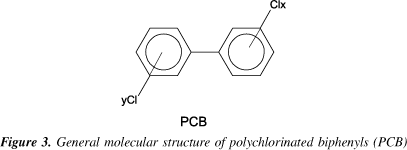
The polychlorinated biphenyls (PCB) are a class of organic compounds characterized by two benzene rings linked by a C-C bond, with up to 209 congeners with the general formula C12H(10-n)Cln (1=n=10) and a molecular weight between 189 and 499 g.mol-1 12. Figure 3 shows the general structural formula of PCB. The nomenclature of PCB follows the same basic rules of that for PCDD/F but, according to the Union for Pure and Applied Chemistry (IUPAC, 1988), some attention should be paid to the order of the chlorine atoms in the molecule in order to name the PCB:
-
The numbering of the two aromatic rings begins taking the C-C bond as reference point and using the 2-6 e 2'-6'numerals, respectively.
-
The numbering of the chlorine atoms in the molecule is made taking into account the arrangement that ables the lowest settlement possible between the two rings and in an increased sequence, where: 2<2'; 2'<3.
-
Not apostrophised numbers are used to the ring that has more chlorine atoms. In the case of equal number of substitutes, the main ring will be that with the first chlorine atom at the lower number. When both rings show the first chlorine atom at the same relative position, the main ring will be that with the second lowest chlorine-substitution and so on.
-
One congener is named when the following information is given: number of chlorine atoms and their position along the body molecule; name of the homologue group and; class of compounds to which this congener belongs.
However, the system proposed by the IUPAC was too complicated to be applied. In 1980 Ballschmiter and Zell proposed a new system in which PCB congeners were numerated from 1 to 209. This nomenclature system is used until now. For example, PCB nº 126 is named according to IUPAC rules as 3, 3',4,4',5-pentachlorobiphenyl. Table 5 shows the physical-chemical properties of the PCB at 25 ºC.
Because of their physical and chemical properties, the degradation of most PCB congeners is extremely difficult. In addition, the strong lipophilic character of PCB increases the risk of bioaccumulation in man and biota2. The difference in the chemical and physical properties as well as in the biological effects between the congeners is directly correlated to the degree of chlorination and the substitution pattern of the molecule.
Toxicokinetic, metabolism and elimination of PCB
The toxic effects of PCB depend on the positions of chlorine atoms and consequently on the steric structure of the molecule12. Congeners with chlorine atoms only in meta- and para-positions (at least tetra-substituted PCB in the lateral positions), the coplanar or "dioxin-like" PCB, will have similar effects in vivo as PCDD/F, whereas PCB substituted in one or more ortho-positions would have none, or only after application of high doses, similar effects as those observed for PCDD/F in organisms6. As one main biological effect, PCB have the ability to induce certain isozymes of the cytochrom P450 monooxygenase superfamily, where three main groups of PCB can be differentiated according to the enzymatic systems they activate23, as summarized in Table 6.
-
Methylcholanthren-type (MC-type). PCB in this group have the same toxicological properties in vivo like 2,3,7,8-TCDD and thus are named the "Dioxin-Like" PCB. As the PCDD/F, the MC-TYP-PCB can induce the cytochrom P450 1A1 (CYP1A1) and P450 1A2 (CYP1A2).
-
Phenobarbital-type (PB-type): PCB congeners, which have at least 2 ortho-substituted positions. They have the ability to induce the Cytochrom P-450 2B1 (CYP2B1) like phenobarbital and promote neoplasia and hepatomegalien.
-
Methylcholanthren and Phenobarbital-type (MC+PB-type): in this group are the PCB with asymmetrical chlorosubstitution on both phenyl rings. Moreover, these PCB have at least one substitution in the ortho-position and the meta and para-positions are chlorosubstituted in both rings.
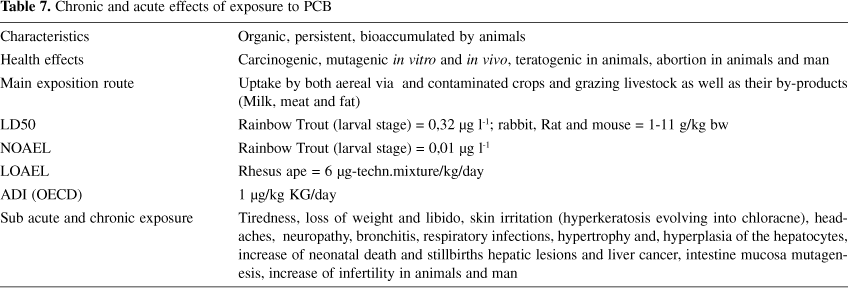
Major acute toxic effects of PCBs reported in humans are the eruption of chloracne, and pigmentation of the skin and nails. Due to two incidents with contaminated rice oil in Japan (Yusho 1968) and Taiwan (Yu-Cheng 1979), the epidemiological data base of PCB toxicity in man are better developed as that for PCDD/F 24. However, these data must to be taken into reserve since not only PCB but also PCDF were present in an appreciable concentration in both episodes.The main acute and chronic effects of PCB are summarized in Table 7.
The metabolism and elimination of PCB occurred through the biotransformation into polar metabolites as already described for PCDD/F6,12. Main reaction involved are the hydroxylation and epoxidation of PCB. The elimination half-live for PCB varies according to the congener. In rat fat tissues, the observed elimination half-live were of 453 days and 6 months for PCB-153 and PCB-180, respectively. From the Yusho human exposition, the elimination half-live of 10,4 years for PCB-169 was observed. Serum analysis from Workers exposed to Arochlor mixtures 1242 and 1254 had shown elimination half-lives form 2,6 until 4,8 years12.
Sources of PCB to the environment: production and destruction
PCB are considered as ubiquitous contaminants of the environment stemming from their large scale production and use all over the world between the 1930's and 1980's as technical mixtures with different degree of chlorination. From the 209 possible congeners of PCB, 130 were produced in industrial scale. Among others, the main productors in the whole world were Mosanto, USA (Arochlor trade mark) Bayer S. A., Germany (Clophen trade mark) and Kanegafuchi Chemical (Kanechlor trade mark)2.
The technical mixtures are very resistant to degradation, are thermally stable, and resistant to oxidation, acids, bases, and other chemical agents and with a consistency from mobile oils to viscous liquids or sticky resins. They are soluble in most of the common organic solvents and lipids, but only slightly soluble in water, glycerol, and glycols2,12.
Mixtures with a high degree of chlorination are used as cooling/isolation fluids in closed systems like transformators, hydraulic systems, gas turbines, and vacuum pumps, and also as fire retardants for electric/electronic equipments and as plasticizers in adhesives, textiles, surface coatings and sealants. Low chlorination degree mixtures are normally used as basis for resinous products and inks2,6. Such mixtures have different production names according to the manufacturer and the country of origin. The code name utilised in these mixtures gives the following information: manufacturer, country of origin and main composition. The Arochlors are identified by a four-digit numbering code, in which the two first digit mean that the parent molecule is biphenyl (12 carbons) and the last two digits, give the chlorine content in weight percent. e.g. Arochlor1260 is a mixture where the hexachlorobiphenyls predominate with 60% (wt) in chlorine and was produced by Monsanto, USA2.
The technical properties of these mixtures led to their massive use as cooling/isolation fluids as well as basis for resinous products. In 1966 Jensen discovered that the wanted physical-chemical properties had turned the PCB into a serious environmental problem, once they could be found in all compartments in a quite appreciable concentration (ppb-ppm)2.
The worldwide production of these compounds between 1930 and 1971 is assumed to be 1.5 million metric tons. Solely the US production yielded 500,000 tons, from which around 340,000 ton were produced between 1960 and 197123. Main components of these PCB mixtures are congeners in which one or more ortho-positions (respectively carbons 2,2' and 6,6') are chloro-substituted12,25. Behnisch12,28 proved that dioxin-like PCB are present in some extension in Arochlor mixtures in a variable concentration (1.4 to 17.6 µg TEQ/g of Arochlor formulation). For high-chlorinated mixtures the high-chlorinated PCB predominate whereas for low chlorinated formulations like Arochlor 1221 and 1232 the PCB-105, -118 and 77 predominate.
As main routes of PCB contamination of the environment diffuse sources like transformators, waste and sewage sludge can be cited. Due to the high stability of these molecules under environmental conditions, PCB can be transported over long distances and bioaccumulate through trophic chains. One of the consequences of this persistence in the environment and bioaccumulation observed nowadays is the strong decrease of the marine biota, especially of seabirds and some mammalian populations like seals and otters8. In order to foresee possible impacts coming from the contamination of the biota with these compounds, analysis and research was focused on those PCB congeners, also called indicator-PCB, that are present in great concentrations in the technical mixtures, in environmental matrices and food.
The production and use of polychlorinated biphenyls (PCB) has been forbidden or severely restricted now in the majority of the developed countries2,12,21-23. However, the release of materials containing PCB and of PCB-containing wastes spreads the contamination of the environment by this pollutant. Moreover, the discussion about the formation of dioxin-like PCB in thermal processes, especially by solid residue burning in incinerators, was stressed by the work of Tiernan et al.26 and Ballschmiter et al.27, where the formation of dioxin-like PCB during combustion processes was proven. Among the probable mechanisms of formation of PCB in combustion processes is the dimerization of 2 chlorobenzene molecules from free radical mediated processes12. The main evidence of this hypothesis is the difference between the profiles of congener concentrations in technical mixtures to those found in exhaustion gases and ashes of incinerators and thermal process plants. Dioxin-like PCB can also be produced from photolysis of high chlorinated PCB in the upper part of the atmosphere and released during wood burning and from cement kilns28. Table 8 lists the indicator-PCB according to the German guideline DIN 51527 and the WHO-TEF29 for coplanar PCB, as well as their IUPAC nomenclature.
As for PCDD/F, natural destruction of PCB can be mediated through photolysis in the upper atmosphere. The incineration above 1200 °C is the most used method for PCB destruction, but efforces should be made to avoid redimerization of coplanar PCB in the cold zone of the incinerator and PCDF formation due to inefficient burn-up28.
Behaviour, distribution and environmental fate of PCDD/F and PCB
The behaviour and distribution of PCDD, PCDF and PCB in the environment is similar to each other. Given their physical and chemical characteristics, the main transport route from their sources is atmospheric1-4. Once emitted, from air or directly from residual waters, these substances show a strong tendency to bind to particulate material (increasing with the number of chlorine atoms) and can be transported to other environmental compartments2,6,12. Eventually, they can enter trophic chains, and due to their lipophilic character they tend to bioaccumulate along trophic chains.
After being transported from their sources, PCDD/F and PCB may deposit on soil and plant surfaces where they show a very low mobility associated with high persistence. They preferentially stay in the upper soil surface, bound to the organic matter in soil. Regarding plants, the main route of contamination is by wet and dry deposition, affecting mostly the upper parts of the plants30. The transfer of PCDD/F and PCB to animals, and consequently biomagnification along the trophic chains, apparently occurs by ingestion. This led on one hand to the development of indices and limits regarding gaseous emissions (mainly from stationary as well as from diffuse sources) in order to control surface deposition and crop contamination and on the other hand to limits in the use of biocompost and sewage sludge on pastures and in agriculture30-32. Based on these recomendations, maximum levels of PCDD/F (including "dioxin-like" PCB) in soil and sewage sludge/compost in USA and Europe lie between 1,0 and 100,0 ng I-TEQ/kg (d.w.) respectively. Concerning stationary sources like incinerators,the emission levels in Europe should be under 1,0 ng I-TEQ/ Nm3. In Brazil there's no specific legislation regarding PCDD/F or PCB levels in soil and emissions until now.
Transfer of PCDD/F and PCB to man: WHO guidelines
The transport of persistent pollutants through the atmosphere often gives rise to uncontrolled exposure of larger populations to toxic (and bioaccumulating) substances. Concerning PCDD/F, a tolerable daily intake (TDI) of 1-4 pg TEQ/kg body weight (b.w.)/day is recommended, according to the WHO/EURO standard guidelines8. In 2001 the EU commission has recommended a weekly tolerable intake of 14 pg TEQ/kg b.w., where not only the 17 2,3,7,8-chlorosubstituted PCDD/F but also the 12 coplanar PCB must be taken into account7. For PCDD/F it was stated that around 95% of contamination occurred via food of animal origin, when people have been exposed to neither occupational nor acute events such as transformator fires33. Maximum PCDD/F and PCB level in foodstuffs varies from foodstuff (Meat, butter, fats, fish etc.) and from country to country. Anyway, studies from the World Health Organisation (WHO) in USA and Europe indicate that a daily intake of PCDD/F of 50-200 pg I-TEQ/person/day or 1-3 pg I-TEQ/kg b.w./day already occurred. These studies also showed that the food contamination by coplanar PCB is in the same degree or greater than that observed for PCDD/F, which leads in some cases, to an exceed of a factor 2-3 of the observed TDI values for these countries8. To PCDD/F the human exposure is primarily attributed to background contamination caused by atmospheric deposition of these pollutants coming from different sources and subsequently to biomagnification through the trophic chains. The control of stationary and diffuse sources of PCDD/F and revision of the main legislation in respect to land use are the main strategies to control exposure of the human population. For coplanar PCB, on the other hand, it has not yet entirely been answered which are the main stationary thermal sources and how much they contribute to the contamination of the environment25. Furthermore, it must be stated if the contamination coming from the industrial production and use of PCB yet plays an important role for the occurrence of these contaminants in the environment despite of the strict legislation concerning the use of these chemicals all over the world. In this way, besides the importance of determination of the actual concentrations and main sources of these substances in the environment, it is necessary to understand the distribution of these pollutants between different compartments, the role of the longe range transport of these substances in the bulk concentration observed in both developed countries and the third world block, and which are the significant steps to promote biomagnification along the trophic chains aiming to control the exposure levels of PCDD/F and PCB to man/biota.
Analytical methods for PCDD/F and PCB
There are different analytical methods to determination of PCDD/F and PCB according to the sample matrix, most of them already approved by federal agencies and organisations34-37. Current regulations concerning human exposure through food and environmental matrices, recommends the extrictly control of this contaminants. Therefore, PCDD/F and PCB analysis are usually time-consuming and costly. High controlled laboratory conditions and validation of the selected analytical method using standard reference compounds are needed. The standardisation of the reagents and solvents used are essential prior to analysis and to avoid possible contamination sources1,2,6,14,33-37. Four general steps in the PCDD/F and PCB analysis can be described: sampling/storage, extraction from the sample matrix, fractionation/cleanup and determination.
Sampling and storage methods are depending on sample matrix. PCDD/F and PCB are very stable compounds but the collecting methods must be so, that assure sample containers and storage environments free of contamination. Soil, sludge and sediment, as well as biological samples, should be sampled by means glass, aluminium or Teflon (PTFE) containers33-37. In the case of air samples (emissions, deposition), both sorbents resins (Florisil38, XAD-239) and deposition surfaces (Glass wool, Polyurethane foams)34-37 can be used.
The extraction of PCDD/F and PCB from sample matrix is generally achieved by partitioning of these contaminants into suitable organic solvents (e.g. Toluene/hexane/dichloromethane). For liquid samples, this can be done through liquid-liquid extraction. For solid samples the extraction using soxhlet apparatus, ultra-sound and centrifugation are the most cited methods1,2,6,34-36 . Recently, accelerated solvent extraction (ASE)40, supercritical fluid extraction (SFE), high performance liquid chromatography (HPLC) with porous graphite carbon (PGC) column41 and ultra-rapid automated solid phase extraction42 methods were described in the literature.
The Fractionation/cleanup step will minimise possible interferences between the analytes and other co-extracted materials prior to instrumental analysis. Different fractionation procedures are described in the literature, most of them are developed not only to PCDD/F and/or PCB fractionation but also PAH and pesticides in a multi-residue analysis. Adsorbents normally used are deactivated silica gel, Florisil and Alumina1,2,6,43. The use of mono-layer or multi-layer columns is described, as well. The clean-up procedures involve different steps and can be either acid1,2,6 or basic34-37 treatments and use different adsorbent materials and/or gel permeation chromatography (GPC)43.
Due to the high number of isomers involved, the identification and determination of PCDD/F and PCB requires sophisticated techniques and instrumental. Almost all literature data describe that capillary gas chromatography coupled to mass spectrometry (HRGC/MS) is the most useful instrumental method prior to PCDD/F analysis. Other methods already described to PCDD/F analysis are fast gas chromatography, two-dimensional gas chromatography44,45, GC coupled to high-resolution mass spectrometry (HRGC/HRMS)1,2,6,33-35 and time-of-flight mass spectrometry (TOFMS)46,47. Such methods are required in order to achieve lower detection limits normally required for food and environmental analysis. The GC/MS technique able the simultaneous detection, quantification and ordination of the peaks. This is made according to the level of chlorination of the PCDD/F. The identification is made through the electron ionisation method (EI) where the selected monitoring (SIM Modus) of the peaks of fragmented ions can be observed1,6,12. The quantification is made through the isotope dilution method. This method consist of the addition of a known quantity of standard target with carbon 13 (13C12)1. Because of the toxicological aspect involved, for the PCDD/F analysis, the determination of the 2,3,7,8- chlorosubstituted isomers is essential. Therefore almost all techniques described, recommend the addition of target standards containing at least all 2,3,7,8-chlorosubstituted congeners prior to PCDD/F analysis1,2,6,33-37.
The use of capillary columns is required to promote the separation of all congeners of PCDD/F. In the past the most used columns are no-polar and semi-polar columns6. The Non-polar columns (DB1-DB-5 HP-5, Ultra-2, etc) have a stationary phase constituted by methyl-polysiloxilan or methyl-phenyl-polysiloxilan groups6. They are able to promote a good separation between homologous groups, but have low performance for peak separation between isomers (isomeric-specific analysis). In order to promote the isomeric-specific analysis, polar columns like the CP-Sil 88 and similar, were used in a two-step analysis. The CP-Sil 88 columns and similar are high polar columns which have a stationary phase constituted by polysiloxilan highly substituted with cyanopropyl groups. The extremely high polarity ables maximum resolution in separations between isomers. Main disadvantage is the low thermal stability (~270 ºC), that interferes in the analysis of the high chlorinated isomers. Upon 1991 a 60 m length phase-modified column called DB-Dioxin, total designed for PCDD/F determination, is brought to the market. This column has a stationary phase constituted by 44% methyl-polysiloxilan, 28% phenyl-polysiloxilan, 20% cyanopropyl-polysiloxilan and 8% polyoxiethylen (Carbowax 20M). The DB-Dioxin column pursues higher thermal stability when compared with the CP-Sil 88 and efficiency to separate the 2,3,7,8-chlorsubstituted isomers in each homologous group and all homologous groups as well.
Until the 80's the gas chromatography coupled to an Electron capture Detector (GC/ECD) using packed columns was fast the unique instrumental technique for the determination of PCB and, in special Arochlors mixtures. Other detector already cited in the literature is the atomic emission detector (AED) for PCB determinations48,49. The ECD detector has a high sensibility and selectivity to determinate halogenated compounds. The sensibility to phthalates and non-linearity to high concentrations are the main disvantages this detector presents48.
In the 80's the main PCB determination method using GC/ECD, was done through the Webb-McCall method. The Webb-McCall method gaves only knowledge about the contamination level through PCB Mixtures and only the most abundant peaks of PCB could be surely identified43. For the identification and quantification, both an external calibration with Arochlor mixtures and the use of an internal standard could be made. In most of the cases the quantification was done through the total area under the Arochlor region.
Upon 1988 it was stated the quantification through the six Indicator-PCB congeners and the use of capillary columns coupled to ECD was developed. Recently, Due to the toxicological implications that Dioxin-like PCB have23, the analysis of PCB through high resolution chromatography coupled to mass spectrometry was developed. HRGC/MS-Technique ables to achieve lower detection limits and better separation of PCB congeners to quantification. The GC/MS method will require the same identification and quantification methods as for PCDD/F as well as the use of target standards (13C12-PCB). Most useful capillary columns for PCB analysis are DB-1 -DB-5 family, DBXLB, HT 8, Optima 5 and CP-Sil 88. As for PCDD/F, whatever column is used, there is poor or no resolution to separate some of the PCB congeners12,23,43:
INCIDENTS INVOLVING PCDD/F AND PCB AND HUMAN EXPOSURE
The Seveso incident
The most serious incident involving PCDD/F exposure occurred in 1976 at the Industrie Chimiche Meda Societa Azionaria chemical plant (ICMESA) in Meda, near Seveso, northern Italy. An explosion of one reactor during the production of 2,4,5- trichlorophenol released a toxic vapour cloud contained circa 3000 kg of various chemicals and about 100 g to 20 kg of 2,3,7,8-TCDD. The explosion was the result of an exothermic reaction between ethylene glycol and sodium hydroxide50. The release occurred during about 20 min before it was noticed and stopped. Anyway there was time enough to spread the cloud over a large area, contaminating humans, animals, crops and land in the vicinity of the plant51.
First health effects observed occurred a few hours later when children showing burn-like skin lesions entered the Hospitals and clinics in the region. Five days latter, the massive dead of little animal such as birds and rabbits, alerted the authorities about the gravity of the situation at hand. Two weeks later scientists stated that dioxin was the main contaminant10. Within three weeks, the population living closest to the plant were evacuated. Several cubic meters of topsoil were removed and incinerated. Three months later, a chloracne outbreak occurred among the people exposed to the cloud. Some pregnant women had abortions due to the potential danger to their unborn children. About 37,000 people are believed to have been exposed to the chemicals51,52. Concerning the farm animals, approximately 4 percent died and the survivors (circa 80,000 animals) were killed to prevent contamination from biomagnification through the food chain50.
The affected area was later, subdivided into three zones (A, B, and R) according to their decreasing mean levels of TCDD soil contamination. The Zone A, comprising 110 hectares, was the most contaminated area and was completely evacuated. Nowadays this zone is turned into the Seveso Oak Forest Park. The Zones B and R are the next-most contaminated areas and agricultural using as well as the consumption of local agricultural goods and meats, were strictly prohibited 10,50. Hormonal disruption seems to be one of the strongest effects of dioxin poisoning10. In 1983, a dramatical change in the sex ratio (Rfemale: male=1,64) among just-born children, whose parent were exposed to the accident, was observed51. Another Symptom observed among the exposed population, where immune system and neurological disorders as well as spontaneous abortions. Despite of this, the symptoms could not be related to the dioxin exposition until now10,52.
Agent Orange and TCDD exposure in Vietnam
Upon 1960 the United States Military forces had promoted a massive defoliation Program in the Republic of Vietnam. As main purpose, the defoliation Program aimed to destroy the natural coverage promoted by the native rain forest and thus to disable the enemy any chance to hide himself. Several different chemical mixtures were used. The code name of these formulations reminds the identification stripe that appeared on the container vessels53. From them, the Agent Orange was the most widely used, corresponding to 60% of the total of herbicides used in South Vietnam54.
The Agent orange was a 50:50 technical formulation containing esthers of 2,4-dichlorophenoxyacetic acid (2,4-D) and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T)55. The exposure to Agent Orange and consequent development of chronically diseases seems to be related to the contamination of 2,4,5-T with 2,3,7,8-TCDD, which concentration ranged from 0,05 to 50 ppm. As consequence, the toxicokinetic and long-therm health effect studies are the same as those described to the exposure to 2,3,7,8-TCDD53-55.
The Belgian PCB/dioxin incident
At the end of January of 1999, animal feed heavily contaminated with PCB and PCDD/F was introduced into the Belgian food supply, including exports, to the European Union. The contamination occurred when around 100 l of PCB oil (Technical formulations Arochlor 1260:1254, 75:25), was accidentally added to a storage tank containing animal fat. The concentrations of PCDD/F and "Dioxin-Like " PCB in the PCB oil was estimated to be around 1,0 g-TEQ and 2,0 g-TEQ, respectively56. The contaminated fat was then sold to different animal feed producers in Belgium, France, and the Netherlands and reached the European market a few weeks latter. In May 1999, the contamination was discovered, when around 2500 farmers have been supplied with contaminated feed. Main farm animals affected were hens, chicks, pigs and cattle.
As consequence of the contamination The EU- member together with countries like China, Hong Kong, Malaysia, Poland, South Korea and Brazil57 promoted a boycott against the Belgian animals and dairy products until a strictly dioxin monitoring program was adopted by belgian government and the danger of poisoning was banned.
Yusho and Yusheng and PCB exposure
Two large-scale food poisoning episodes involving PCB occurred in 1968 in the Kyushu islands, southern Japan and in 1979 in Taichung, central Taiwan. The incident occurred in Japan and the syndrome decurrent from the PCB contamination, were latter reported as Yusho, in reference to the name of the rice oil responsible for this incident. The name latter adopted to the incident in Taiwan, is actually the same writed Ideogram characters as used for Yusho but the different pronounce in both countries had turned the occidental name into Yusheng58. In both incidents, rice bran oil contaminated with technical formulations of PCB (Kanechlor 400 in Kyushu, Kanechlor 400-500 in Taichung) occurred. Some studies pointed out that rather than technical PCB present in the Kanechlor formulations, the main causal agents for the contamination were polychlorinated dibenzofuranes (PCDF) and coplanar PCB present in appreciable concentration in these formulations59. In both events, the contamination occurred during the rice oil production, when a leakage in the heat exchanger ables the contamination of edible oil with the technical formulation of PCB used as cooler fluid.
In Japan 1866 patients were reported, in Yusheng, 2061. Main acute symptoms in both episodes were: numbness in the extremities, loss of appetite and nausea, skin itching developing to pigmentation and chloroachne, pigmentation of skin, conjutiva, hyperaemia of the conjutiva and eye swelling. Terathogenic effects observed was the unusual increase of still birth and live births of abnormal colouring babies also called "black" or "cola colouring" babies58,59. Another Terathogenic effect found in Yusho were nail deformity among living babies. Time trend symptoms among Yusho patients, shown that symptoms like numbness of extremities, fatigue and headache occurred until now. Another significative observation is the statistical increase of development of malignant neoplasms and mortality decurrent from chronic liver disease and cirrhosis58,59.
Cidade dos Meninos Duque de Caxias, RJ
The efforces to remediate a hexachlorocyclohexane contaminated site in Cidade dos Meninos, Duque de Caxias RJ, had turned into a serious dioxin incident. The hexachlorocyclohexane factory was opened in 1949 by the Ministry of Health and closed ten years latter due to increased production costs60. The contamination of the site was stated in 1989, after denunciation of an illegal commerce of Hexachlorocyclohexane (HCH) in a free market in Rio de Janeiro. It was stated that an area of 13 Km2 around the factory was contaminated61. The remediation method used was the mixture of the soil with lime (CAO) in order to provide HCH degradation. The remediation method used had promoted PCDD/F formation60. PCDD/F concentrations in the remediated area reached values of 13900 ng I-TEQ/kg soil. Moreover, dioxin levels in cow's milk produced in this region is the highest concentration found in comparison to other regions in Brazil60. 1500 families were contaminated and 18 Cancer falls were reported as the contamination was discovered. Unfortunately the process against the government was archived a few years latter and the problem persists until now61.
Citrus pulp from Brazil and dairy products poisoning in Europe
An increase of the concentrations of PCDD/F in dairy products (Milk, butter) and food animal (Meat), first detected in Germany was the start point of an international food crisis involving countries of Europe and Brazil. The increased concentration of PCDD/F was first detected in the State of Baden-Württemberg south Germany, in September 1997. In February 1998 the PCDD/F concentration increased from 06 pg-TEQ/g fat to 1,41 pg-TEQ/g fat (mean values)62. Furthermore, the increased trend was not limited to south Germany but also different states in Germany and countries in Europe had reported similar increase. All food and dairy products samples had shown the same congeners pattern. This pattern was unknown in Europe and was latter found in the citrus pulp pellets (CPP) imported from Brazil63,64. These Pellets were treated with lime64 to promote neutralisation and drying prior to production. The pellets were exported to Europe and used in the feed animal production. Latter it was stated that the lime was heavier contaminated with PCDD/F (2,5 million pg I-TEQ/Kg)63, PCB and Chlorobenzenes (650000 ng/kg lime)64.
Main consequences were the withdraw of the dairy products and meat; withdraw of the CPP and related compound feed containing contaminated CP; The collapse of the Citrus Pulp (CP) market in some countries of Europe and the destruction of 92000 t of CP. Furthermore, a temporary tolerance for PCDD/F in citrus pulp of 500 pg I-TEQ/Kg, including upper bound detection limits was regulated by the European Community (EC) in 199864.
Recebido em 2/7/03; aceito em 17/3/04; publicado na web em 9/8/04
- 1. Hagenmaier, H.; Abschlussbericht zum Forschungs- und Untersuchungsvorhaben "Belastung der Umwelt mit Dioxinen", Umweltministerium Baden-Württemberg ed.: Tübingen, 1987.
- 2. Mahnke, K.; PhD Thesis, Organic Chemistry Institute, Eberhard-Karls-Universität Tübingen, Germany, 1997.
- 3. Hutzinger, O.; Fiedler. H.; Chemosphere 1993, 27, 121.
- 4. Fiedler, H.; Organohalogen Compnds 1994, 20, 229.
- 5. NATO/CCMS; Report nş 176, 1988.
- 6. Körner, W.; PhD Thesis, Eberhard-Karls-Universität Tübingen, Germany, 1995.
- 7. Schrenk, D.; Abstracts of the LfU-Kolloquium "Dioxinähnliche PCB in der Umwelt Quellen, Verbleib, Exposition und gesundheitliche Bewertung", Augsburg, Germany, 2003.
-
8WHO-ECEH/IPCS; Consultation on assessment of health risk of dioxins: re-evaluation of daily tolerable intake (TDI), Geneva: WHO, 1998.
- 9. Schecter, A. J.; Ryan, J. J.; Constable, J. D.; Chemosphere 1986, 15, 1613.
- 10. Mocarelli, P.; Chemosphere 2001, 43, 391.
- 11. Wacker, R.; Poiger, H.; Schlatter, C.; Chemosphere 1986, 15, 1473.
- 12. Behnisch, P. A. In Isomerenspezifische Untersuchungen über Eintrag, Verbleib und Risikoabschätzung der nicht-, mono- und di-ortho-chlorierten Biphenyle in der Umwelt; UFO Atelier für Gestaltung & Verlag GbR: Allensbach, 1997.
- 13. Berg Van den, M.; De Jongh, J.; Poiger, H.; Olson, J. R.; Crit. Rev. Toxicol. 1994, 24, 1.
- 14. Calaminus, B.; Fiedler, H.; Stahlberg, R.; Organohalogen Compounds 1997, 31, 354.
- 15. Ballschmiter, K.; Bacher, R. In Dioxine - Chemie, Analytik, Vorkommen, Umweltverhalten und Toxikologie der halogenierten Dibenzo-p-dioxine und Dibenzofurane; Ballschmitter, K.; Bacher, R., eds.; VCH Verlag: Weinheim, 1996.
- 16. Hagenmaier, H.; Organohalogen Compnds 1994, 20, 267.
- 17. Hagenmaier, H.; Dawidowsky, N.; Weberruss,U.; Hutzinger, O.; Schwind, K. H.; Thoma, H.; Essers, U.; Bühler, U.; Greiner, R. In Proceedings of the 11th International Symposium. on Chlorinated Dioxins, PCB and related Compounds; Bayreuth: Germany, 1990.
- 18. Matsueda, T.; Kurokawa, Y.; Nakamura, M.; Takada, S.; Fukamachi, K.; Organohalogen Compnds 1994, 20 , 331.
- 19. Schetter, G. In PCDD- und PCDF-Emissionen aus modernen Müllverbrenungsanlagen und ihre Bedeutung für die Umwelt; Thomè-Kozmiensky, K., ed.; EF-Verlag, Berlin, 1987, vol. 2, p. 629.
- 20. Paasivirta, J.; Koistinen, J.; Kuokkanen, T.; Maatela, P.; Mäntykoski, K.; Paukku, R.; Rantalainen, A-L.; Rantio, T.; Sinkkoen, S.; Welling, L.; Chemosphere 1993, 27, 447.
- 21. Fedorov, A. L.; Chemosphere 1993, 27, 477.
- 22. Buckland, S. J.; Dye, E. A.; Leathem, S. V.; Taucher, J. A.; Organohalogen Compnds 1994, 20, 85.
- 23. Ballschmitter, K.; Abstracts of the LfU-Kolloquium "Dioxinähnliche PCB in der Umwelt Quellen, Verbleib, Exposition und gesundheitliche Bewertung", Augsburg: Germany, 2003.
- 24. Ahlborg, U. G.; Younes, M.; Yränheikki, E.; Organohalogen Compnds 1994, 20, 563.
- 25. Körner, W. In ref. 23.
- 26. Tiernan, T. O.; Taylor, M. L.; Garret, J. H.; Van Ness, G. F.; Solchy, G.; Dais, D. A.; Wagel, D. J.; Chemosphere 1983, 12, 595.
- 27. Ballschmiter, K.; Niemcyk, R.; Schäfer, W.; Zoller, W.; Frezenius J. Anal. Chem. 1987, 328, 583.
- 28. Alcock, R. E.; Behnisch, P. A.; Jones, K. C.; Hagenmaier, H.; Chemosphere 1998, 37, 1457.
- 29. Berg Van den, M.; Birnbaum, L.; Bosveld, A. T.; Brunstrom, B.; Cook, P.; Feeley, M.; Giesy, J. P.; Hanberg, A.; Hasegawa, R.; Kennedy, S. W.; Kubiak, T.; Larsen, J. C.; van Leeuwen, F. X.; Liem, A. K.; Nolt, C.; Peterson, R. E.; Poellinger, L.; Safe, S.; Schrenk, D.; Tillitt, D.; Tysklind, M.; Younes, M.; Waern, F.; Environ. Health Persp 1998, 106, 775.
- 30. Hülster, A.; Marschner, H.; Chemosphere 1993, 27, 439.
- 31. Prinz, B.; Krause, G. H. M.; Radermacher, L.; Chemosphere 1993, 27, 491.
- 32. Rappe, C.; Chemosphere 1993, 27, 211.
- 33. Malisch, R. In ref. 23.
- 34. Deutsche Institut für Normung (DIN), Report n° EN 1948-1, 1996.
-
35Verein Deutsche Ingenieure (VDI), Richtlinien 3957 – Bl2, 2090 – Bl1,Bl2, 2001.
- 36. US Environmental Protection Agency (USEPA), Methods n° 608, 1988b.
- 37. US Environmental Protection Agency (USEPA), Methods n° 505, 508, 508A, 1989c.
- 38. Lin, J. M. ; Que Hee, S. S.; Anal.Chem 1985, 57, 2130.
- 39. Mes, J.; Environ. Pollut 1994, 84, 261.
- 40. Sporring, S.; Wiberg, K.; Björklund, E.; Haglund, P.; Organohalogen Compnds 2003, 1,1.
- 41. Li, W.; Chen, Z-s.; Li, C-q.; Huang, P.; Liu, G-Y.; Zhou, Z.; Organohalogen Compnds 2003, 1, 3.
- 42. Sandau, C.; Sjodin, A.; Davis, M.; Waterman, A.; Patterson Jr., D. G.; Organohalogen Compnds 2003, 1, 4.
-
43US Agency for Toxic Substances and Diseases (ATSDR), Draft n° 205-93-0606, 1999.
- 44. Patterson Jr., D. G.; Organohalogen Compnds 1992, 8, 1.
- 45. Focant, J-F.; De Pauw, E.; Grainger, J.; Patterson Jr., D. G.; Dimandja, J-M. D.; Organohalogen Compnds 2001, 50, 25.
- 46. Grainger, J.; Green, V.; Liu, Z.; Barr, J.; McLure, C.; Patterson Jr., D. G.; Organohalogen Compnds 1996, 27, 354.
- 47. Dimandja, J-M.; Grainger, J.; Patterson Jr., D. G.; Organohalogen Compnds 1999, 40, 23.
- 48. Penteado, J. C. P; Vaz, J. M.; Quim. Nova 2001, 24, 390.
- 49. Pedersen-Bjerganrd, S.; Semb, S. I.; Brevik, E. M.; Greibrokk, T.; J. Chromatogr. 1996, 723, 337.
-
50http://www.chm.bris.ac.uk, acessada em Setembro 2003.
-
51http://www.getipm.com/articles/seveso-italy.htm, acessada em Setembro 2003.
- 52. Bertazzi, P. A.; Zocchetti, C.; Pesatori, A. C.; Guercilena, S.; Sanarico, M.; Radice, L.; Am. J. Epidemiol. 1989, 129, 1187.
- 53. Stellman, J. M.; Stellman, S. D.; Silbergeld, E. K.; Thomas, V. M. In Environmental and occupational medicine; 3rd ed.; Rom, N. W., ed.; Lippincottt-Raven Publishers: Philadelphia, 1998, cap. 84, 85.
-
54http://www.va.gov/agentorange/, acessada em Setembro 2003.
- 55. Kang, H. K.; Dalager, N. A.; Needham, L. L.; Patterson Jr., D. G.; Matanoski, G. M.; Kanchanaraksa, S.; Lees, P. S. J.; Chemosphere 2001, 43, 943.
- 56. Bernard, A.; Broeckaert, F.; De Poorter, G.; De Cock, A.; Hermans, C.; Saegerman, C.; Houins, G.; Environ. Res. 2002, 88, 1.
-
57http://www.unep.org/, acessada em Setembro 2003.
- 58. Ikeda, M.; Chemosphere 1996, 32, 559.
- 59. Yoshimura, T.; Industrial Health 2003, 41, 139.
- 60. Braga, A. M. C. B.; Krauss, T.; Santos, C. R. R.; Souza, P. M.; Chemosphere 2002, 46, 1329.
- 61. Pinheiro, S.; Nasr, N. Y.; Luz, D. In Agricultura ecológica e a máfia dos agrotóxicos no Brasil; 2Ş ed., CREA-RJ: Rio de Janeiro, 1993, cap. 4.
- 62. Malish, R.; Chemosphere 2000 40,1041.
- 63. Carvalhaes, G. K.; Brooks, P.; Krauss, T.; Chemosphere 1999 41, 137.
- 64. Carvalhaes, G. K.; Brooks, P.; Marques, C. G.; Azevedo, J. A. T.; Machado, M. C. S.; Azevedo, G. C.; Chemosphere 2002 46, 1409.
Publication Dates
-
Publication in this collection
30 Nov 2004 -
Date of issue
Dec 2004
History
-
Accepted
17 Mar 2004 -
Received
02 July 2003