Summary
Objective:
The aim was to evaluate the effectiveness of the experimental synergists muscle ablation model to promote muscle hypertrophy, determine the period of greatest hypertrophy and its influence on muscle fiber types and determine differences in bilateral and unilateral removal to reduce the number of animals used in this model.
Method:
Following the application of the eligibility criteria for the mechanical overload of the plantar muscle in rats, nineteen papers were included in the review.
Results:
The results reveal a greatest hypertrophy occurring between days 12 and 15, and based on the findings, synergist muscle ablation is an efficient model for achieving rapid hypertrophy and the contralateral limb can be used as there was no difference between unilateral and bilateral surgery, which reduces the number of animals used in this model.
Conclusion:
This model differs from other overload models (exercise and training) regarding the characteristics involved in the hypertrophy process (acute) and result in a chronic muscle adaptation with selective regulation and modification of fast-twitch fibers in skeletal muscle. This is an efficient and rapid model for compensatory hypertrophy.
Keywords:
ablation of synergists; compensatory hypertrophy; experimental models; muscle mass; skeletal muscle cross-sectional area
Resumo
Objetivo:
Avaliar a eficácia do modelo experimental de ablação dos sinergistas para promover a hipertrofia muscular, determinar o período de maior hipertrofia, sua influência sobre os tipos de fibras musculares e determinar diferenças na remoção unilateral ou bilateral para reduzir o número de animais utilizados nesse modelo.
Método:
Após a aplicação dos critérios de elegibilidade para sobrecarga mecânica do músculo plantar em ratos, 19 artigos foram incluídos na revisão.
Resultados:
Ocorre maior hipertrofia entre os dias 12 e 15, o que torna o modelo eficiente para alcançar a hipertrofia rapidamente. O membro contralateral também pode ser usado, pois não houve diferença entre a cirurgia unilateral e bilateral, o que reduz o número de animais usados no experimento.
Conclusão:
O modelo difere de outros modelos de sobrecarga (exercício e treinamento) em razão das características envolvidas no processo de sobrecarga imposta (aguda), resultando em uma adaptação crônica muscular com modificação de fibras de contração rápida do músculo esquelético. É um modelo rápido e eficiente para se estudar hipertrofia compensatória.
Palavras-chave:
ablação dos sinergistas; hipertrofia compensatória; modelos experimentais; massa muscular; área de secção transversa do músculo esquelético
Introduction
Skeletal muscle is highly adaptive and has a self-regulating capacity.11 DiPasquale DM, Cheng M, Billich W, Huang SA, Rooijen N, Hornberger TA, et al. Urokinase - type plasminogen activator and macrophages are required for skeletal muscle hypertrophy in mice. Am J Physiol Cell Physiol. 2007; 293(4):1278-85.
2 Pavaresh KC, Huber AM, Brochin RL, Bacon PL, McCall G.E, Huey KA, et al. Acute vascular endothelial growth factor expression during hypertrophy is muscle phenotype specific and localizes as a striated pattern within fibers. Exp Physiol. 2010; 95(11):1098-106.-33 McCarthy JJ, Mula J, Miyasaki M, Erfani R, Garrison K, Farooqui AB, et al. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development. 2011; 138(17):3657-66. Hypertrophy is an example of this plasticity and refers to the increase in muscle mass necessary to enable the muscle to optimize its response to the demands of sustaining and generating force.11 DiPasquale DM, Cheng M, Billich W, Huang SA, Rooijen N, Hornberger TA, et al. Urokinase - type plasminogen activator and macrophages are required for skeletal muscle hypertrophy in mice. Am J Physiol Cell Physiol. 2007; 293(4):1278-85.,22 Pavaresh KC, Huber AM, Brochin RL, Bacon PL, McCall G.E, Huey KA, et al. Acute vascular endothelial growth factor expression during hypertrophy is muscle phenotype specific and localizes as a striated pattern within fibers. Exp Physiol. 2010; 95(11):1098-106.,44 Adams GR, Haddad F, Baldwin KM. Time course of changes in markers of myogenesis in overloaded rat skeletal muscles. J Appl Physiol (1985). 1999; 87(5):1705-12.
5 Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001; 3(11):1014-9.-66 Marino JS, Taush BJ, Dearth CL, Manacci MV, McLoughlin TJ, Rakyta SJ, et al. Beta2-integrins contribute to skeletal muscle hypertrophy in mice. Am J Physiol Cell Physiol. 2008; 295(4):1026-36.
Skeletal muscle mass is regulated by a variety of stimuli, the best known of which is mechanical overload. The muscle adaptation process can be induced by stretching/ immobilization,4444 Allen DL, Harrison BC, Sartorius C, Byrnes WC, Leinwand LA. Mutation of the IIB myosin heavy chain gene results in muscle fiber loss and compensatory hypertrophy. Am J Physiol Cell Physiol. 2001; 280(3):C637-45.,4646 Fuller PM, Baldwin KM, Fuller CA. Parallel and divergent adaptations of rat soleus and plantaris to chronic exercise and hypergravity. Am J Physiol Regul Integr Comp Physiol. 2006; 290(2):R442-8. compensatory mechanisms (chronic).11 DiPasquale DM, Cheng M, Billich W, Huang SA, Rooijen N, Hornberger TA, et al. Urokinase - type plasminogen activator and macrophages are required for skeletal muscle hypertrophy in mice. Am J Physiol Cell Physiol. 2007; 293(4):1278-85.,22 Pavaresh KC, Huber AM, Brochin RL, Bacon PL, McCall G.E, Huey KA, et al. Acute vascular endothelial growth factor expression during hypertrophy is muscle phenotype specific and localizes as a striated pattern within fibers. Exp Physiol. 2010; 95(11):1098-106.
3 McCarthy JJ, Mula J, Miyasaki M, Erfani R, Garrison K, Farooqui AB, et al. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development. 2011; 138(17):3657-66.
4 Adams GR, Haddad F, Baldwin KM. Time course of changes in markers of myogenesis in overloaded rat skeletal muscles. J Appl Physiol (1985). 1999; 87(5):1705-12.
5 Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001; 3(11):1014-9.-66 Marino JS, Taush BJ, Dearth CL, Manacci MV, McLoughlin TJ, Rakyta SJ, et al. Beta2-integrins contribute to skeletal muscle hypertrophy in mice. Am J Physiol Cell Physiol. 2008; 295(4):1026-36.,88 Goodman CA, Kotecki JA, Jacobs BL, Hornberger TA. Muscle fiber type-dependent differences in the regulation of protein synthesis. PLoS One. 2012; 7(5):e37890.,99 Schuenke MD, Brooks NE, Hikida RS. Interactions of aging, overload and creatine supplementation in rat plantaris muscle. J Aging Res. 2011; 2011:393416.,1212 Yamaguchi A, Ikeda Y, Hirai T, Fujikawa T, Morita I. Local changes of IGF-1 mRNA, GH receptor MRNA, and fiber size in rat plantaris muscle following compensatory overload. Jpn J Physiol. 2003; 53:53-60.,1414 Pehme A, Alev K, Julkunen A, Seene T. The effect of mechanical loading on the MyHC synthesis rate and composition in rat plantaris muscle. Int J Sports Med. 2004; 25(5):332-8.,1818 Novack ML, Billich W, Smith S, Sukhija KB, McLoughlin TJ, Hornberger TA, et al. COX-2 inhibitor reduces skeletal muscle hypertrophy in mice. Am J Physiol Regul Integr Comp Physiol. 2009; 296(4):R1132-9.
19 Huey KA, Burdette S, Zhong H, Roy RR. Early response of heat shock proteins to functional overload of the soleus and plantaris in rats and mice. Exp Physiol. 2010; 95(12):1145-55.-2020 Gordon BS, Delgado Dias DC, White JP, Carson JA, Kostec MC. Six1 and Six1 cofactor expression is altered during early skeletal muscle overload in mice. J Physiol Sci. 2012; 62(5):393-401.,6161 Bentzinger CF, Lin S, Romanino K, Castets P, Guridi M, Summermatter S, et al. Differential response of skeletal muscles to mTORC1 signaling during atrophy and hypertrophy. Skelet Muscle. 2013; 3(1):6.
62 Lee WJ, Thompson RW, McClung JM, Carson JA. Regulation of androgen receptor expression at the onset of functional overload in rat plantaris muscle. Am J Physiol Reg Integr Comp Physiol. 2003; 285(5):R1076-85.
63 Sakuma K, Watanabe K, Sano M, Uramoto I, Totsuka T. Differential adaptation of growth and differentiation factor 8/myostatin, fibroblast growth factor 6 and leukemia inhibitory factor in overloaded, regenerating and denervated rat muscles. Biochim Biophys Acta. 2000; 1497(1):77-88.
64 Dunn SE, Burn JL, Michel RN. Calcineurin is required for skeletal muscle hypertrophy. J Biol Chem. 1999; 274:21908-12.-6565 Adams GR, Caiozzo VJ, Haddad F, Baldwin KM. Cellular and molecular responses to increased skeletal muscle loading after irradiation. Am J Physiol Cell Physiol. 2002; 283(4):C1182-95. and exercise/training.3333 Sakuma K, Yamaguchi A. Molecular determinants of skeletal muscle hypertrophy in animals. J Sport Medic Doping Studie. 2012; S1:002. Available from: https://www.omicsonline.org/2161-0673/2161-0673-S1-002.pdf.
https://www.omicsonline.org/2161-0673/21...
,4646 Fuller PM, Baldwin KM, Fuller CA. Parallel and divergent adaptations of rat soleus and plantaris to chronic exercise and hypergravity. Am J Physiol Regul Integr Comp Physiol. 2006; 290(2):R442-8. Evidence of this is derived from a large number of studies demonstrating that overload leads to an increase in muscle mass and cross-sectional area of the muscle fibers and induces chronic changes in the balance between the synthesis and degradation of proteins.22 Pavaresh KC, Huber AM, Brochin RL, Bacon PL, McCall G.E, Huey KA, et al. Acute vascular endothelial growth factor expression during hypertrophy is muscle phenotype specific and localizes as a striated pattern within fibers. Exp Physiol. 2010; 95(11):1098-106.,77 Goodman CA, Mayhew DL, Hornberger TA. Recent progress toward understanding the molecular mechanisms that regulate skeletal muscle mass. Cell Signal. 2011; 23(12):1896-906.
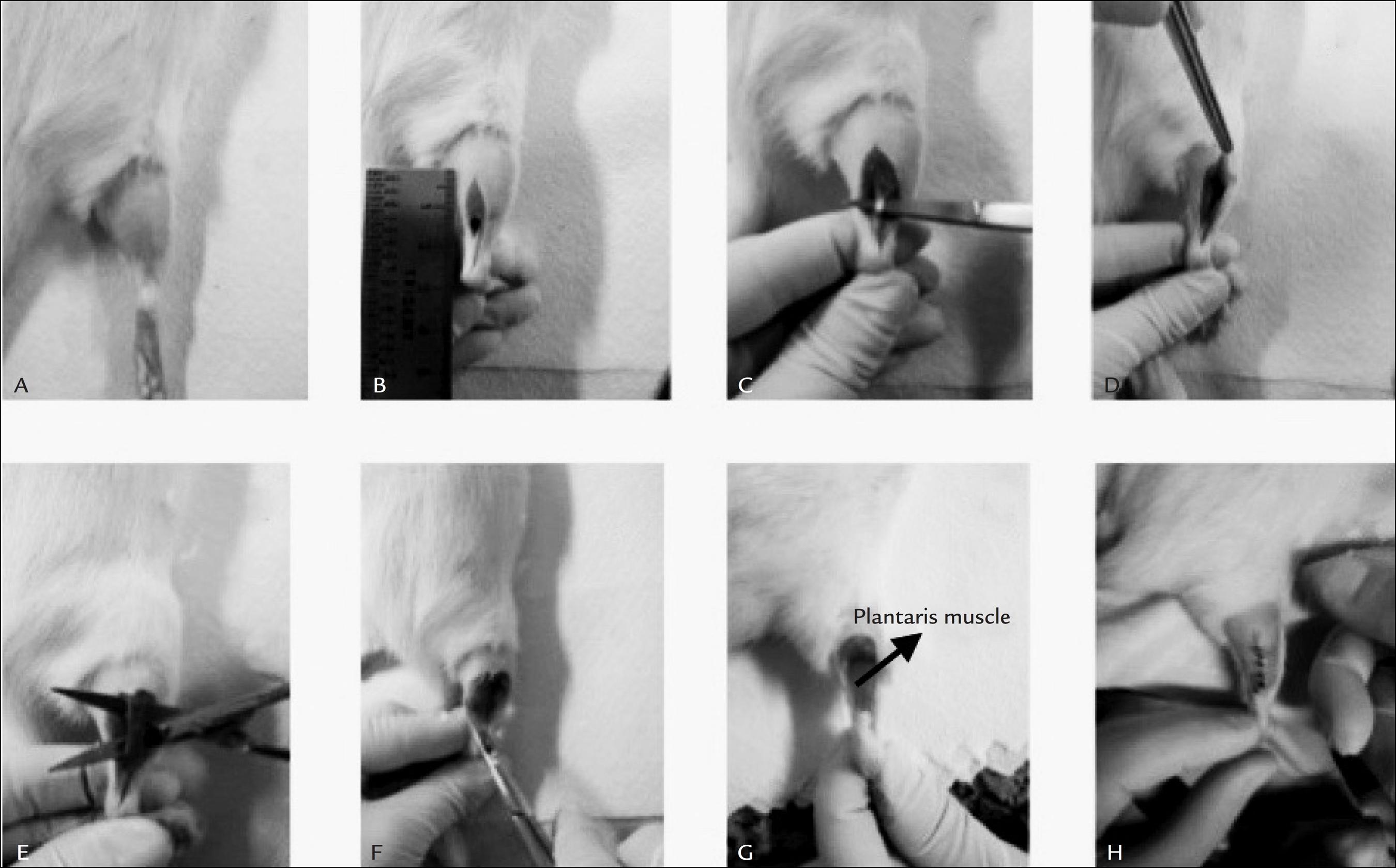
8 Goodman CA, Kotecki JA, Jacobs BL, Hornberger TA. Muscle fiber type-dependent differences in the regulation of protein synthesis. PLoS One. 2012; 7(5):e37890.-99 Schuenke MD, Brooks NE, Hikida RS. Interactions of aging, overload and creatine supplementation in rat plantaris muscle. J Aging Res. 2011; 2011:393416. Compensatory hypertrophy through the ablation of synergists of plantar flexion is one of the ways to produce chronic overload experimentally.33 McCarthy JJ, Mula J, Miyasaki M, Erfani R, Garrison K, Farooqui AB, et al. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development. 2011; 138(17):3657-66.,77 Goodman CA, Mayhew DL, Hornberger TA. Recent progress toward understanding the molecular mechanisms that regulate skeletal muscle mass. Cell Signal. 2011; 23(12):1896-906.,1212 Yamaguchi A, Ikeda Y, Hirai T, Fujikawa T, Morita I. Local changes of IGF-1 mRNA, GH receptor MRNA, and fiber size in rat plantaris muscle following compensatory overload. Jpn J Physiol. 2003; 53:53-60.,1313 Sakuma K, Nishikawa J, Nakao R, Nakano H, Sano M, Yasuhara M. Serum response factor plays an important role in the mechanically overload plantaris muscle of rats. Histochem Cell Biol. 2003; 119(2):149-60.,2020 Gordon BS, Delgado Dias DC, White JP, Carson JA, Kostec MC. Six1 and Six1 cofactor expression is altered during early skeletal muscle overload in mice. J Physiol Sci. 2012; 62(5):393-401.,5959 Terada M, Kawano F, Ohira T, Nakai N, Nishimoto N, Ohira Y. Effects of mechanical over-loading on the properties of soleus muscle fibers, with or without damage, in wild type and Mdx mice. PLoS One. 2011; 7(4):e.34557. The ablation of synergists for compensatory hypertrophy consists of the surgical removal of all or part of synergistic muscles, which can be either unilateral or bilateral, to generate chronic functional overload that causes hypertrophy.33 McCarthy JJ, Mula J, Miyasaki M, Erfani R, Garrison K, Farooqui AB, et al. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development. 2011; 138(17):3657-66.,77 Goodman CA, Mayhew DL, Hornberger TA. Recent progress toward understanding the molecular mechanisms that regulate skeletal muscle mass. Cell Signal. 2011; 23(12):1896-906.,1212 Yamaguchi A, Ikeda Y, Hirai T, Fujikawa T, Morita I. Local changes of IGF-1 mRNA, GH receptor MRNA, and fiber size in rat plantaris muscle following compensatory overload. Jpn J Physiol. 2003; 53:53-60.,1313 Sakuma K, Nishikawa J, Nakao R, Nakano H, Sano M, Yasuhara M. Serum response factor plays an important role in the mechanically overload plantaris muscle of rats. Histochem Cell Biol. 2003; 119(2):149-60.,2020 Gordon BS, Delgado Dias DC, White JP, Carson JA, Kostec MC. Six1 and Six1 cofactor expression is altered during early skeletal muscle overload in mice. J Physiol Sci. 2012; 62(5):393-401.,5959 Terada M, Kawano F, Ohira T, Nakai N, Nishimoto N, Ohira Y. Effects of mechanical over-loading on the properties of soleus muscle fibers, with or without damage, in wild type and Mdx mice. PLoS One. 2011; 7(4):e.34557. According to Parvaresh et al.,11 DiPasquale DM, Cheng M, Billich W, Huang SA, Rooijen N, Hornberger TA, et al. Urokinase - type plasminogen activator and macrophages are required for skeletal muscle hypertrophy in mice. Am J Physiol Cell Physiol. 2007; 293(4):1278-85. complete muscle removal can compromise the neurovascular supply, which increases edema and the recovery of the animal in the postoperative period. Thus, the removal of only the distal portion of synergist muscle is recommended (Figure 1).
Synergist ablation surgery of plantaris muscle. A. Shaving the back of the hind leg. B. Incision of 2 cm. C. Tendon of gastrocnemius muscle. D. Partial removal of the lateral gastrocnemius muscle. E. Soleus muscle - total removal. F. Partial removal of the medial gastrocnemius muscle. G. The plantaris muscle is isolated. H. Suture with seven points.
The synergist muscle ablation model induces muscle hypertrophy in only a few days, thereby facilitating the study of adaptive responses.22 Pavaresh KC, Huber AM, Brochin RL, Bacon PL, McCall G.E, Huey KA, et al. Acute vascular endothelial growth factor expression during hypertrophy is muscle phenotype specific and localizes as a striated pattern within fibers. Exp Physiol. 2010; 95(11):1098-106.,33 McCarthy JJ, Mula J, Miyasaki M, Erfani R, Garrison K, Farooqui AB, et al. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development. 2011; 138(17):3657-66.,77 Goodman CA, Mayhew DL, Hornberger TA. Recent progress toward understanding the molecular mechanisms that regulate skeletal muscle mass. Cell Signal. 2011; 23(12):1896-906.,1010 White JP, Reecy MJ, Washington TA, Sato, Le M, Davis JM, et al. Overload-induced skeletal muscle extracellular matrix remodeling and myofibre growth in mice lacking IL-6. Acta Physiol (Oxf). 2009; 197(4):321-32.
11 Almurshed KS, Grunewald KK. Dietary protein does not affect overloaded skeletal muscle in rat. J Nutr. 2000; 130(7):1743-8.
12 Yamaguchi A, Ikeda Y, Hirai T, Fujikawa T, Morita I. Local changes of IGF-1 mRNA, GH receptor MRNA, and fiber size in rat plantaris muscle following compensatory overload. Jpn J Physiol. 2003; 53:53-60.
13 Sakuma K, Nishikawa J, Nakao R, Nakano H, Sano M, Yasuhara M. Serum response factor plays an important role in the mechanically overload plantaris muscle of rats. Histochem Cell Biol. 2003; 119(2):149-60.
14 Pehme A, Alev K, Julkunen A, Seene T. The effect of mechanical loading on the MyHC synthesis rate and composition in rat plantaris muscle. Int J Sports Med. 2004; 25(5):332-8.
15 Young RE, Young JC. The effect of creatine supplementation on mass and performance of rat skeletal muscle. Life Sci. 2007; 81(9):710-6.
16 Locke M. Heat shock protein accumulation and heat shock transcription factor activation in rat skeletal muscle during compensatory hypertrophy. Acta Physiol (Oxf) 2008; 192(3):403-11.
17 Choi H, Selpides IPJ, Novell MM, Rourke BC. Functional overload in ground squirrel plantaris muscle fails to induce myosin isoform shifts. Am J Physiol Regul Integr Comp Physiol. 2009; 297(3):R578-86.
18 Novack ML, Billich W, Smith S, Sukhija KB, McLoughlin TJ, Hornberger TA, et al. COX-2 inhibitor reduces skeletal muscle hypertrophy in mice. Am J Physiol Regul Integr Comp Physiol. 2009; 296(4):R1132-9.
19 Huey KA, Burdette S, Zhong H, Roy RR. Early response of heat shock proteins to functional overload of the soleus and plantaris in rats and mice. Exp Physiol. 2010; 95(12):1145-55.-2020 Gordon BS, Delgado Dias DC, White JP, Carson JA, Kostec MC. Six1 and Six1 cofactor expression is altered during early skeletal muscle overload in mice. J Physiol Sci. 2012; 62(5):393-401. The most studied muscles are plantar flexors in the rear paw of rats. As skeletal muscle has different types of fibers (type I [slow-twitch] and type II [fast-twitch - IIa, IIb, IIx/IId]),2121 Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M. Mechanisms regulating skeletal muscle grow and atrophy. FEBS J. 2013; 280(17):4294-314. a number of authors justify the choice of the plantaris muscle due to its diversity of fiber types (type I: 8 ± 2%; type IIA: 19 ± 3%; type IIB/D: 74 ± 4%) and its different adaptation possibilities.99 Schuenke MD, Brooks NE, Hikida RS. Interactions of aging, overload and creatine supplementation in rat plantaris muscle. J Aging Res. 2011; 2011:393416. Compensatory hypertrophy induced by the functional elimination of synergistic muscles results in an increase in muscle fiber diameter and muscle mass as well as the regulation of protein synthesis in different types of muscle fibers.
The present systematic review of the literature discusses the results found in studies using this experimental model to cause overload in the plantar muscle of rats, comparing the findings with regard to the percentage increase in the mass of the plantar muscle, the period of greatest muscle mass gain and differences between unilateral and bilateral surgery. The aim of this review was to evaluate the effectiveness of the experimental synergist muscle ablation model to promote muscle hypertrophy in different overload models, to determine the period of greatest hypertrophy and its influence on muscle fiber types, and to determine differences in bilateral and unilateral removal to reduce the number of animals used in this model, thereby facilitating its reproduction and its choice among different chronic hypertrophy models.
Method
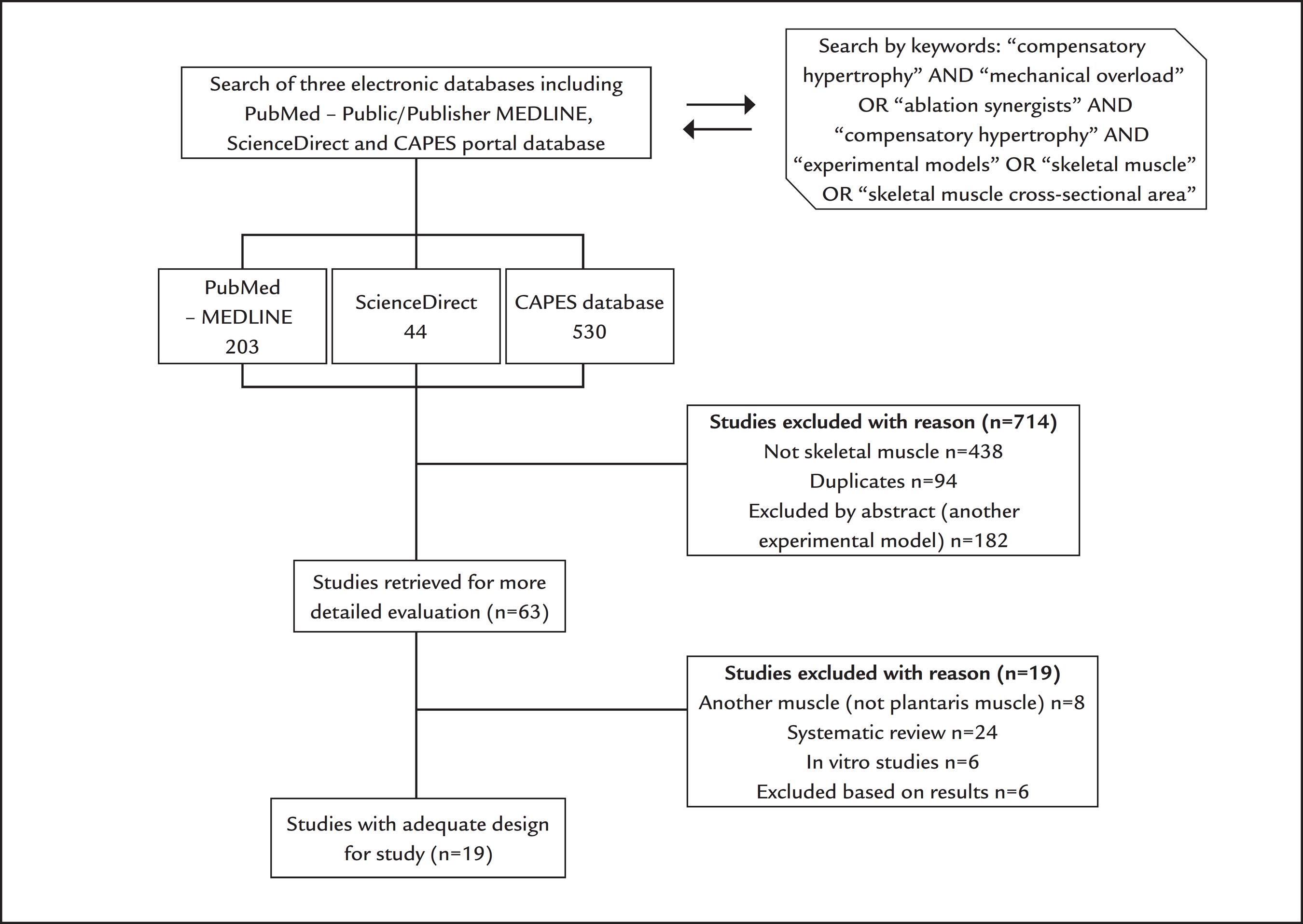
The methods were based on PRISMA guidelines. Searches were performed in the PubMed, ScienceDirect, MEDLINE and CAPES Portal databases for articles published between January 1999 and July 2013 using the keywords "compensatory hypertrophy" AND "mechanical overload" OR "ablation of synergists" AND "compensatory hypertrophy" AND "experimental models" OR "skeletal muscle cross-sectional area." The following criteria were used for the selection of papers: (1) the use of a rat model; (2) the use of synergist ablation to overload the plantaris muscle; (3) bilateral or unilateral muscle removal; and (4) determination of the cross-sectional area of muscle fibers or muscle mass. Review articles were excluded, as well as other experimental models and in vitro studies. Articles that used overload in another muscle and did not report on their studies the cross-sectional area (CSA) or muscle mass were also excluded.
A total of 63 articles were retrieved using combinations of the keywords. Twenty-four papers were review articles;77 Goodman CA, Mayhew DL, Hornberger TA. Recent progress toward understanding the molecular mechanisms that regulate skeletal muscle mass. Cell Signal. 2011; 23(12):1896-906.,99 Schuenke MD, Brooks NE, Hikida RS. Interactions of aging, overload and creatine supplementation in rat plantaris muscle. J Aging Res. 2011; 2011:393416.,2121 Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M. Mechanisms regulating skeletal muscle grow and atrophy. FEBS J. 2013; 280(17):4294-314.
22 Bismuth K, Relaix F. Genetic regulation of skeletal muscle development. Exp Cell Res. 2010; 316(18):3081-6.
23 Elliott B, Renshaw D, Getting S, Mackenzie R. The central role of myostatin in skeletal muscle and whole body homeostasis. Acta Physiol (Oxf). 2012; 205(3):324-40.
24 Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol. 2005; 37(10):1974-84.
25 Aline G, Sotiropoulos A. A key factor controlling muscle hypertrophy by enhance the recruitment of muscle stem cells. Bioarchitecture. 2012; 2:88-90.
26 Martin NRW, Lewis MP. Satellite cell activation and number following acute and chronic exercise: a mini review. Cell Mol Exerc Physiol. 2012; 1(1):e3.
27 Otto A, Patel K. Signalling and the control of skeletal muscle size. Exp Cell Res. 2010; 316(18):3059-66.
28 Ohira Y, Kawano F, Wang XD, Nakai N, Ohira T, Okabe H, et al. Role(s) of mechanical load and satellite cells in the regulation of the size of soleus muscle fiber in rats. Biol Sci Space. 2010; 24(3-4):135-44.
29 Kawano F, Nakai N, Ohira Y. Regulation of soleus muscle properties by mechanical stress and/or neural activity. J Phys Sports Med. 2012; 1(1):29-36.
30 Morgan JE, Partridge TA. Muscle satellite cells. Int J Biochem Cell Biol. 2003; 35(8):1151-6.
31 Stewart CE, Pell JM. Point: IGF is the major physiological regulation of muscle mass. J Appl Physiol. 2010; 108:1820-4.
32 Sakuma K, Yamaguchi A. The functional role of calcineurin in hypertrophy, regeneration, and disorders of skeletal muscle. J Biomed Biotechnol. 2010; 2010:72129.
33 Sakuma K, Yamaguchi A. Molecular determinants of skeletal muscle hypertrophy in animals. J Sport Medic Doping Studie. 2012; S1:002. Available from: https://www.omicsonline.org/2161-0673/2161-0673-S1-002.pdf.
https://www.omicsonline.org/2161-0673/21...
34 Scharner J, Zammit PS. The muscle satellite cell at 50: the formative years. Skelet Muscle. 2011; 1(1):28.
35 Schadrach JL, Wagers AJ. Stem cells for skeletal muscle repair. Phil Trans R Soc B. 2011; 366:2297-306.
36 Schmalbruch H. The satellite cell of skeletal muscle fibers. Braz J Morphol Sci. 2006; 23(2):159-72.
37 Teixeira CE, Duarte J.A. Myonuclear domain in skeletal muscle fibers. A critical review. Arch Exerc Health. 2011; 2(2):92-101.
38 Yabolnka-Reuveni Z. The skeletal muscle satellite cell: still young and fascinating at 50. J Histochem Cytochem. 2011; 59(12):1041-59.
39 West DWD, Burd NA, Staples AW, Phillips SM. Human exercise-mediated skeletal muscle hypertrophy is an intrinsic process. Int J Biochem Cell Biol. 2010; 42(9):1371-5.
40 van Wessel T, de Haan A, van der Laarse WJ, Jaspers RT. The muscle fiber type-fiber paradox: hypertrophy or oxidative metabolism? Eur J Appl Physiol. 2010; 110(4):665-94.
41 Zanou N, Gailly P. Skeletal muscle hypertrophy and regeneration: interplay between the myogenic regulatory factors (MRFs) and insulin-like growth factors (IGFs) pathways. Cell Mol Life Sci. 2013; 70(21):4117-30.-4242 Yan Z, Okutsu M, Alhtar YN, Lira V. Regulation of exercise-induced fiber type transformation, mitochondrial biogenesis, and angiogenesis in skeletal muscle. J Appl Physiol (1985). 2011; 110(1):264-74. eight studies used a model other than the ablation of synergists to cause hypertrophy;4343 Allouh MZ, Yabolnka-Reuveni Z, Rosser BWC. Pax7 reveals a greater frequency and concentration of satellite cells at the ends of growing skeletal muscle fibers. J Histochem Cytochem. 2008; 56(1):77-87.
44 Allen DL, Harrison BC, Sartorius C, Byrnes WC, Leinwand LA. Mutation of the IIB myosin heavy chain gene results in muscle fiber loss and compensatory hypertrophy. Am J Physiol Cell Physiol. 2001; 280(3):C637-45.
45 Aoki MS, Miyabara EH, Soares AG, Salvini TF, Moriscot AS. Cyclosporin-A does not affect skeletal muscle mass during disuse and recovery. Braz J Med Biol Res. 2006; 39(2):243-51.
46 Fuller PM, Baldwin KM, Fuller CA. Parallel and divergent adaptations of rat soleus and plantaris to chronic exercise and hypergravity. Am J Physiol Regul Integr Comp Physiol. 2006; 290(2):R442-8.
47 Qaisar R, Renaud G, Morine K, Barton ER, Sweeney HL, Larsson L. Is functional hypertrophy and specific force coupled with the addition of myonuclei at the single muscle fiber level? FASEB J. 2012; 26(3):1077-85.
48 Kawano F, Goto K, Wang XD, Terada M, Oshira T, Nakai N, et al. Role(s) of gravitational loading during developing period on the growth of rat soleus muscle fibers. J Appl Physiol (1985). 2010; 108(3):676-85.
49 Seene T, Pehme A, Alev K, Kassik P, Umnova M, Aru M. Effects of resistance training on fast- and slow-twitch muscles in rats. Biol Sport. 2010; 27:221-9.-5050 Zhang BT, Yeung SS, Liu Y, Wang HH, Wan YM, Ling SK, et al. The effects of low frequency electrical stimulation on satellite cell activity in rat skeletal muscle during hindlimb suspension. BMC Cell Biol. 2010; 11:87. and six were in vitro studies.5151 Blaauw B, Canato M, Agatea L, Toniolo L, Mammucari C, Masiero E, et al. Inducible activation of Akt increases skeletal muscle mass and force without satellite cell activation. FASEB J. 2009; 23(11):3896-905.
52 Chen JF, Tao Y, Li J, Deng Z, Yan Z, Xiao X, et al. microRNA-1 and microRNA-206 regulate skeletal muscle satellite cell proliferation and differentiation by repressing Pax7. J Cell Biol. 2010; 190(:867-79.
53 Jacquemim V, Furling AB, Butler-Browne GS, Mouly V. IGF-1 induces human myotube hypertrophy by increasing cell recruitment. Exp Cell Res. 2004; 299(1):148-58.
54 Shefer G, Wleklinski-Lee M, Yabolnka-Reuveni Z. Skeletal muscle satellite cells can spontaneously enter an alternative mesenchymal pathway. J Cell Sci. 2004; 117(Pt 22):5393-404.
55 Liadaki K, Casar JC, Wessen M, Luth EC, Jun S, Gussoni E, Kunkel LM. β4 integrin marks intersticial myogenic progenitor cells in adult murine skeletal muscle. J Histochem Cytochem. 2012; 60(1):31-44.-5656 Wang M, Yu H, Kim YS, Bidwell CA, Kuang S. Myostatin facilities slow and inhibits fast myosin heavy chain expression during myogenic differentiation. Biochem Biophys Res Commun. 2012; 426(1):83-8. All these studies were excluded. Among the remaining 25 studies, seven did not compare the cross-sectional area of the muscle and/or muscle mass to a control group and were excluded.33 McCarthy JJ, Mula J, Miyasaki M, Erfani R, Garrison K, Farooqui AB, et al. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development. 2011; 138(17):3657-66.,1313 Sakuma K, Nishikawa J, Nakao R, Nakano H, Sano M, Yasuhara M. Serum response factor plays an important role in the mechanically overload plantaris muscle of rats. Histochem Cell Biol. 2003; 119(2):149-60.,1515 Young RE, Young JC. The effect of creatine supplementation on mass and performance of rat skeletal muscle. Life Sci. 2007; 81(9):710-6.,4848 Kawano F, Goto K, Wang XD, Terada M, Oshira T, Nakai N, et al. Role(s) of gravitational loading during developing period on the growth of rat soleus muscle fibers. J Appl Physiol (1985). 2010; 108(3):676-85.,5757 Ishido M, Kami K, Masuhara M. Localization of MyoD, myogenin and cell cycle regulatory factors in hypertrophying rat skeletal muscle. Acta Physiol Scand. 2004; 180(3):281-9.,5959 Terada M, Kawano F, Ohira T, Nakai N, Nishimoto N, Ohira Y. Effects of mechanical over-loading on the properties of soleus muscle fibers, with or without damage, in wild type and Mdx mice. PLoS One. 2011; 7(4):e.34557.,6060 Reynolds TH 4th, Bodine SC, Lawrence JC Jr. Control of Ser2448 phosphorylation in the mammalian target of rapamycin by insulin and skeletal muscle load. J Biol Chem. 2002; 277(20):17657-62. Thus, 19 studies met the inclusion criteria and were selected for the present review (Figure 2).
Statistical analysis
The data from graphs were grouped based on collection time and percentage of increase in mass of the plantaris muscle with error propagation. A scatter plot was created to show the distribution of muscle mass gain in function of the number of days following the ablation procedure. Two regressions were employed: one for less than 15 days of data and another for more than 15 days of data. A slope of the regression line coefficient of 0.042 ± 0.002% and linear coefficient of 0.095 ± 0.021% was used for this calculation (slope of the regression line coefficient + x linear coefficient, in which x is the number of days). R2 values demonstrate how the data approaches the progression and form a straight line (R2 = 0.52 in the first 15 days following the ablation of synergist muscles and R2 = 0.06, 15 days after surgery). Values greater than 50% demonstrate that the linear fit is adequate. Chebyshev's inequality test was used to compare muscle mass following unilateral or bilateral removal. This test makes no assumptions regarding the normality of the data distribution and only requires the means and standard errors as inputs. Only periods of 14 and 28 days were compared, which were the periods used by most authors. The results were p=0.2996 for 14 days and p=0.2584 for 28 days.
Results
Table 1 summarizes the findings of the 19 articles analyzed in the present systematic review. Considerable variation was found in the analysis period following the ablation of synergists. Increases in muscle mass (g) and fiber cross-sectional area (µm2) of the plantar muscle were reported in all studies evaluated, demonstrating compensatory hypertrophy.
Studies selected for review using the synergist ablation model for compensatory hypertrophy.
The data were grouped based on collection time and percentage of increase in mass of the plantar muscle with error propagation. The trend line revealed linear progression up to 15 days, with stabilization of the data after this period. The method of least squares was used, including the error of the data reported by the authors. For studies that did not provide such information, the mean error was used. The trend line in Figure 3 shows the percentage (± error) of increase in muscle mass according to days after surgery as follows: 13.6 ± 2.1% one day after ablation, 38.7 ± 2.6% seven days after ablation and 68.0 ± 3.6% 14 days after ablation.
Distribution of plantaris muscle hypertrophy according to number of days after ablation of synergists. Dark gray dots represent data collected in less than 15 days after ablation. Light gray dots represent data collected 15 days after ablation of synergists. The trend line demonstrates linear progression up to 15 days, with stabilization thereafter.
Only periods of 14 and 28 days were compared, which were the periods used by most authors. Both groups presented a large effect size (1.15 and 1.39 for 14 and 28 days, respectively) but since the authors made no assumptions regarding the data's distribution, the p-values were higher than the significance level (p=0.2996 for 14 days and p=0.2584 for 28 days), thus no significant differences were found between unilateral and bilateral surgery in the two periods. At 28 days, there is an overlap between both 95% confidence intervals ([68%, 85%] and [45%, 61%] for unilateral and bilateral, respectively) but no overlap was found at 14 days ([38%, 55%] and [45%, 61%] for unilateral and bilateral, respectively). By not assuming the normality of the data's distribution, the authors guarantee the probability of the type I error at α = 0.05 at the expenses of an increased probability of the type II error. Therefore, despite the lack of statistical significance, the power of the test was low due to the limited data in the literature on unilateral synergist ablation reporting the percentage gain in plantaris muscle mass.
All data were grouped based on the period after the ablation of synergists (Figure 4). Greatest hypertrophy occurred between 12 and 15 days postoperatively. The increase in the cross-sectional area of the muscle and muscle fibers was studied using histological techniques.11 DiPasquale DM, Cheng M, Billich W, Huang SA, Rooijen N, Hornberger TA, et al. Urokinase - type plasminogen activator and macrophages are required for skeletal muscle hypertrophy in mice. Am J Physiol Cell Physiol. 2007; 293(4):1278-85.,77 Goodman CA, Mayhew DL, Hornberger TA. Recent progress toward understanding the molecular mechanisms that regulate skeletal muscle mass. Cell Signal. 2011; 23(12):1896-906.
8 Goodman CA, Kotecki JA, Jacobs BL, Hornberger TA. Muscle fiber type-dependent differences in the regulation of protein synthesis. PLoS One. 2012; 7(5):e37890.-99 Schuenke MD, Brooks NE, Hikida RS. Interactions of aging, overload and creatine supplementation in rat plantaris muscle. J Aging Res. 2011; 2011:393416.,6060 Reynolds TH 4th, Bodine SC, Lawrence JC Jr. Control of Ser2448 phosphorylation in the mammalian target of rapamycin by insulin and skeletal muscle load. J Biol Chem. 2002; 277(20):17657-62. The mean increase in cross-sectional area in comparison to the control was 66 ± 4% at day 14, demonstrating that compensatory hypertrophy is an effective model for increasing muscle mass.
Mean gain in muscle mass according to time after the ablation of synergists based on the literature.
Discussion
Compensatory hypertrophy occurs in response to a sustained increase in the mechanical load of skeletal muscle. Although the mechanisms involved in compensatory hypertrophy are not yet fully understood, this is an intense topic of research, which includes the definition, measuring, loading stimulus parameters, acute responses, hyperplasia, experimental models, adaptations of muscle fiber types, the involvement of satellite cells and endocrinology. The purpose of the present systematic review was to gather results reported by researchers who have used the standard ablation of synergists (gastrocnemius and soleus muscles) model to determine the induction of hypertrophy in the plantar muscle of rats, comparing the percentage of muscle gain to facilitate and standardize the use of this model for the study of muscle plasticity following functional overload. Increasing interest in the molecular and cellular mechanisms responsible for hypertrophy in recent years11 DiPasquale DM, Cheng M, Billich W, Huang SA, Rooijen N, Hornberger TA, et al. Urokinase - type plasminogen activator and macrophages are required for skeletal muscle hypertrophy in mice. Am J Physiol Cell Physiol. 2007; 293(4):1278-85.,66 Marino JS, Taush BJ, Dearth CL, Manacci MV, McLoughlin TJ, Rakyta SJ, et al. Beta2-integrins contribute to skeletal muscle hypertrophy in mice. Am J Physiol Cell Physiol. 2008; 295(4):1026-36.
7 Goodman CA, Mayhew DL, Hornberger TA. Recent progress toward understanding the molecular mechanisms that regulate skeletal muscle mass. Cell Signal. 2011; 23(12):1896-906.
8 Goodman CA, Kotecki JA, Jacobs BL, Hornberger TA. Muscle fiber type-dependent differences in the regulation of protein synthesis. PLoS One. 2012; 7(5):e37890.-99 Schuenke MD, Brooks NE, Hikida RS. Interactions of aging, overload and creatine supplementation in rat plantaris muscle. J Aging Res. 2011; 2011:393416. underscores the need for a reliable and easily reproducible model. Rats are often used due to their considerable activity and their larger size in comparison to mice. The mean weight of the animals used in the studies analyzed was 220 ± 12 g.11 DiPasquale DM, Cheng M, Billich W, Huang SA, Rooijen N, Hornberger TA, et al. Urokinase - type plasminogen activator and macrophages are required for skeletal muscle hypertrophy in mice. Am J Physiol Cell Physiol. 2007; 293(4):1278-85.
2 Pavaresh KC, Huber AM, Brochin RL, Bacon PL, McCall G.E, Huey KA, et al. Acute vascular endothelial growth factor expression during hypertrophy is muscle phenotype specific and localizes as a striated pattern within fibers. Exp Physiol. 2010; 95(11):1098-106.
3 McCarthy JJ, Mula J, Miyasaki M, Erfani R, Garrison K, Farooqui AB, et al. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development. 2011; 138(17):3657-66.
4 Adams GR, Haddad F, Baldwin KM. Time course of changes in markers of myogenesis in overloaded rat skeletal muscles. J Appl Physiol (1985). 1999; 87(5):1705-12.-55 Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001; 3(11):1014-9.,77 Goodman CA, Mayhew DL, Hornberger TA. Recent progress toward understanding the molecular mechanisms that regulate skeletal muscle mass. Cell Signal. 2011; 23(12):1896-906.,1414 Pehme A, Alev K, Julkunen A, Seene T. The effect of mechanical loading on the MyHC synthesis rate and composition in rat plantaris muscle. Int J Sports Med. 2004; 25(5):332-8.
15 Young RE, Young JC. The effect of creatine supplementation on mass and performance of rat skeletal muscle. Life Sci. 2007; 81(9):710-6.-1616 Locke M. Heat shock protein accumulation and heat shock transcription factor activation in rat skeletal muscle during compensatory hypertrophy. Acta Physiol (Oxf) 2008; 192(3):403-11.,1818 Novack ML, Billich W, Smith S, Sukhija KB, McLoughlin TJ, Hornberger TA, et al. COX-2 inhibitor reduces skeletal muscle hypertrophy in mice. Am J Physiol Regul Integr Comp Physiol. 2009; 296(4):R1132-9.
Among the models described in the literature for changes in muscle demand, different protocols of mechanical loading have been used: resistance training (RT) and compensatory hypertrophy after ablation and tenotomy.22 Pavaresh KC, Huber AM, Brochin RL, Bacon PL, McCall G.E, Huey KA, et al. Acute vascular endothelial growth factor expression during hypertrophy is muscle phenotype specific and localizes as a striated pattern within fibers. Exp Physiol. 2010; 95(11):1098-106.,33 McCarthy JJ, Mula J, Miyasaki M, Erfani R, Garrison K, Farooqui AB, et al. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development. 2011; 138(17):3657-66.,55 Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001; 3(11):1014-9.
6 Marino JS, Taush BJ, Dearth CL, Manacci MV, McLoughlin TJ, Rakyta SJ, et al. Beta2-integrins contribute to skeletal muscle hypertrophy in mice. Am J Physiol Cell Physiol. 2008; 295(4):1026-36.-77 Goodman CA, Mayhew DL, Hornberger TA. Recent progress toward understanding the molecular mechanisms that regulate skeletal muscle mass. Cell Signal. 2011; 23(12):1896-906.,1313 Sakuma K, Nishikawa J, Nakao R, Nakano H, Sano M, Yasuhara M. Serum response factor plays an important role in the mechanically overload plantaris muscle of rats. Histochem Cell Biol. 2003; 119(2):149-60.,1818 Novack ML, Billich W, Smith S, Sukhija KB, McLoughlin TJ, Hornberger TA, et al. COX-2 inhibitor reduces skeletal muscle hypertrophy in mice. Am J Physiol Regul Integr Comp Physiol. 2009; 296(4):R1132-9.,6565 Adams GR, Caiozzo VJ, Haddad F, Baldwin KM. Cellular and molecular responses to increased skeletal muscle loading after irradiation. Am J Physiol Cell Physiol. 2002; 283(4):C1182-95. Current theories suggest differences between mechanisms that induce hypertrophy through exercise and compensatory hypertrophy. Both methods cause changes in the muscle, but the molecular signaling pathways seem to be different.2626 Martin NRW, Lewis MP. Satellite cell activation and number following acute and chronic exercise: a mini review. Cell Mol Exerc Physiol. 2012; 1(1):e3.,4949 Seene T, Pehme A, Alev K, Kassik P, Umnova M, Aru M. Effects of resistance training on fast- and slow-twitch muscles in rats. Biol Sport. 2010; 27:221-9. Compensatory hypertrophy due to the ablation of synergists and tenotomy differ in terms of phases. The former has two distinct phases: an inflammatory phase, followed by the response of the muscle to the demand for a functional increase. Tenotomy has the disadvantage of the rapid reconnection of the cut tendon, which limits functionality.6565 Adams GR, Caiozzo VJ, Haddad F, Baldwin KM. Cellular and molecular responses to increased skeletal muscle loading after irradiation. Am J Physiol Cell Physiol. 2002; 283(4):C1182-95.
Most commonly studied muscles
Compensatory hypertrophy by the ablation of synergists is an efficient model for studies on muscle hypertrophy,22 Pavaresh KC, Huber AM, Brochin RL, Bacon PL, McCall G.E, Huey KA, et al. Acute vascular endothelial growth factor expression during hypertrophy is muscle phenotype specific and localizes as a striated pattern within fibers. Exp Physiol. 2010; 95(11):1098-106.,33 McCarthy JJ, Mula J, Miyasaki M, Erfani R, Garrison K, Farooqui AB, et al. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development. 2011; 138(17):3657-66.,77 Goodman CA, Mayhew DL, Hornberger TA. Recent progress toward understanding the molecular mechanisms that regulate skeletal muscle mass. Cell Signal. 2011; 23(12):1896-906.,1010 White JP, Reecy MJ, Washington TA, Sato, Le M, Davis JM, et al. Overload-induced skeletal muscle extracellular matrix remodeling and myofibre growth in mice lacking IL-6. Acta Physiol (Oxf). 2009; 197(4):321-32.
11 Almurshed KS, Grunewald KK. Dietary protein does not affect overloaded skeletal muscle in rat. J Nutr. 2000; 130(7):1743-8.
12 Yamaguchi A, Ikeda Y, Hirai T, Fujikawa T, Morita I. Local changes of IGF-1 mRNA, GH receptor MRNA, and fiber size in rat plantaris muscle following compensatory overload. Jpn J Physiol. 2003; 53:53-60.
13 Sakuma K, Nishikawa J, Nakao R, Nakano H, Sano M, Yasuhara M. Serum response factor plays an important role in the mechanically overload plantaris muscle of rats. Histochem Cell Biol. 2003; 119(2):149-60.
14 Pehme A, Alev K, Julkunen A, Seene T. The effect of mechanical loading on the MyHC synthesis rate and composition in rat plantaris muscle. Int J Sports Med. 2004; 25(5):332-8.
15 Young RE, Young JC. The effect of creatine supplementation on mass and performance of rat skeletal muscle. Life Sci. 2007; 81(9):710-6.
16 Locke M. Heat shock protein accumulation and heat shock transcription factor activation in rat skeletal muscle during compensatory hypertrophy. Acta Physiol (Oxf) 2008; 192(3):403-11.
17 Choi H, Selpides IPJ, Novell MM, Rourke BC. Functional overload in ground squirrel plantaris muscle fails to induce myosin isoform shifts. Am J Physiol Regul Integr Comp Physiol. 2009; 297(3):R578-86.
18 Novack ML, Billich W, Smith S, Sukhija KB, McLoughlin TJ, Hornberger TA, et al. COX-2 inhibitor reduces skeletal muscle hypertrophy in mice. Am J Physiol Regul Integr Comp Physiol. 2009; 296(4):R1132-9.
19 Huey KA, Burdette S, Zhong H, Roy RR. Early response of heat shock proteins to functional overload of the soleus and plantaris in rats and mice. Exp Physiol. 2010; 95(12):1145-55.-2020 Gordon BS, Delgado Dias DC, White JP, Carson JA, Kostec MC. Six1 and Six1 cofactor expression is altered during early skeletal muscle overload in mice. J Physiol Sci. 2012; 62(5):393-401. as the fast increase in muscle mass reduces the duration of the experiment. In recent years, changes have occurred in the standard of surgery (bilateral or unilateral) and the relationship between the number of days and increase in muscle mass, which justifies this systematic review.
In rats, the most commonly studied muscles are the tibial anterior, digitorum longus, soleus and plantar muscles.11 DiPasquale DM, Cheng M, Billich W, Huang SA, Rooijen N, Hornberger TA, et al. Urokinase - type plasminogen activator and macrophages are required for skeletal muscle hypertrophy in mice. Am J Physiol Cell Physiol. 2007; 293(4):1278-85.
2 Pavaresh KC, Huber AM, Brochin RL, Bacon PL, McCall G.E, Huey KA, et al. Acute vascular endothelial growth factor expression during hypertrophy is muscle phenotype specific and localizes as a striated pattern within fibers. Exp Physiol. 2010; 95(11):1098-106.
3 McCarthy JJ, Mula J, Miyasaki M, Erfani R, Garrison K, Farooqui AB, et al. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development. 2011; 138(17):3657-66.
4 Adams GR, Haddad F, Baldwin KM. Time course of changes in markers of myogenesis in overloaded rat skeletal muscles. J Appl Physiol (1985). 1999; 87(5):1705-12.-55 Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001; 3(11):1014-9.,77 Goodman CA, Mayhew DL, Hornberger TA. Recent progress toward understanding the molecular mechanisms that regulate skeletal muscle mass. Cell Signal. 2011; 23(12):1896-906.,1414 Pehme A, Alev K, Julkunen A, Seene T. The effect of mechanical loading on the MyHC synthesis rate and composition in rat plantaris muscle. Int J Sports Med. 2004; 25(5):332-8.
15 Young RE, Young JC. The effect of creatine supplementation on mass and performance of rat skeletal muscle. Life Sci. 2007; 81(9):710-6.-1616 Locke M. Heat shock protein accumulation and heat shock transcription factor activation in rat skeletal muscle during compensatory hypertrophy. Acta Physiol (Oxf) 2008; 192(3):403-11.,1818 Novack ML, Billich W, Smith S, Sukhija KB, McLoughlin TJ, Hornberger TA, et al. COX-2 inhibitor reduces skeletal muscle hypertrophy in mice. Am J Physiol Regul Integr Comp Physiol. 2009; 296(4):R1132-9. In the 1980s and 1990s, the model most often employed was the entire removal of the tibialis anterior to generate overload of the digitorum longus.1515 Young RE, Young JC. The effect of creatine supplementation on mass and performance of rat skeletal muscle. Life Sci. 2007; 81(9):710-6. Currently, the most used muscles in such models are the soleus and plantar muscle and compensatory hypertrophy commonly involves the removal of the distal portion of the gastrocnemius. Despite displaying anatomical proximity in rats, these muscles are distinct in their architecture and biochemistry.66 Marino JS, Taush BJ, Dearth CL, Manacci MV, McLoughlin TJ, Rakyta SJ, et al. Beta2-integrins contribute to skeletal muscle hypertrophy in mice. Am J Physiol Cell Physiol. 2008; 295(4):1026-36.,99 Schuenke MD, Brooks NE, Hikida RS. Interactions of aging, overload and creatine supplementation in rat plantaris muscle. J Aging Res. 2011; 2011:393416.,1616 Locke M. Heat shock protein accumulation and heat shock transcription factor activation in rat skeletal muscle during compensatory hypertrophy. Acta Physiol (Oxf) 2008; 192(3):403-11.,1919 Huey KA, Burdette S, Zhong H, Roy RR. Early response of heat shock proteins to functional overload of the soleus and plantaris in rats and mice. Exp Physiol. 2010; 95(12):1145-55.,5858 Kawano F, Matsuoka Y, Oke Y, Higo Y, Terada M, Wang XD, et al. Role(s) of nucleoli and phosphorylation of ribosomal protein S6 and/or HSP27 in the regulation of muscle mass. Am J Physiol Cell Physiol. 2006; 293(1):C35-44.
Considering the different types of muscle fiber (slow-twitch [type I] and fast-twitch [type IIa, IIb, IIx and IId]),2121 Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M. Mechanisms regulating skeletal muscle grow and atrophy. FEBS J. 2013; 280(17):4294-314. a number of authors justify the choice of the plantar muscle for studies on adaptation due to its composition of different fiber types. The plantar muscle is predominantly composed of fibers IId and therefore has a smaller amount of mitochondria as such fibers use the glycolytic pathway for a faster response during gait. Authors attribute this adaptation feature of the plantar muscle to its constant activation during the stance phase and weight bearing in quadrupeds, which use this muscle to resume ambulation.99 Schuenke MD, Brooks NE, Hikida RS. Interactions of aging, overload and creatine supplementation in rat plantaris muscle. J Aging Res. 2011; 2011:393416.
Unilateral vs. bilateral surgery
Based on the data analyzed, both unilateral and bilateral synergist ablation lead to an increase in muscle mass, with no statistically significant difference between the two types of surgery. Researchers working with unilateral surgery report a mean increase in muscle mass of 46.8 ± 2.6% 14 days following synergist ablation,44 Adams GR, Haddad F, Baldwin KM. Time course of changes in markers of myogenesis in overloaded rat skeletal muscles. J Appl Physiol (1985). 1999; 87(5):1705-12.,1212 Yamaguchi A, Ikeda Y, Hirai T, Fujikawa T, Morita I. Local changes of IGF-1 mRNA, GH receptor MRNA, and fiber size in rat plantaris muscle following compensatory overload. Jpn J Physiol. 2003; 53:53-60.,6161 Bentzinger CF, Lin S, Romanino K, Castets P, Guridi M, Summermatter S, et al. Differential response of skeletal muscles to mTORC1 signaling during atrophy and hypertrophy. Skelet Muscle. 2013; 3(1):6.,6363 Sakuma K, Watanabe K, Sano M, Uramoto I, Totsuka T. Differential adaptation of growth and differentiation factor 8/myostatin, fibroblast growth factor 6 and leukemia inhibitory factor in overloaded, regenerating and denervated rat muscles. Biochim Biophys Acta. 2000; 1497(1):77-88. whereas those working with bilateral surgery report an increase of 52.3 ± 3.1% in the same period.44 Adams GR, Haddad F, Baldwin KM. Time course of changes in markers of myogenesis in overloaded rat skeletal muscles. J Appl Physiol (1985). 1999; 87(5):1705-12.,1212 Yamaguchi A, Ikeda Y, Hirai T, Fujikawa T, Morita I. Local changes of IGF-1 mRNA, GH receptor MRNA, and fiber size in rat plantaris muscle following compensatory overload. Jpn J Physiol. 2003; 53:53-60.,6161 Bentzinger CF, Lin S, Romanino K, Castets P, Guridi M, Summermatter S, et al. Differential response of skeletal muscles to mTORC1 signaling during atrophy and hypertrophy. Skelet Muscle. 2013; 3(1):6.,6363 Sakuma K, Watanabe K, Sano M, Uramoto I, Totsuka T. Differential adaptation of growth and differentiation factor 8/myostatin, fibroblast growth factor 6 and leukemia inhibitory factor in overloaded, regenerating and denervated rat muscles. Biochim Biophys Acta. 2000; 1497(1):77-88. Although the number of studies involving unilateral ablation (n=4) was smaller than the number involving bilateral ablation (n=14), the similar increase in muscle mass demonstrates the benefits of unilateral surgery, which reduces the number of animals used in experiments and is in line with the goals of the International Council for Laboratory Animal Sciences.
Expected time for hypertrophy
The data collection period varied considerably among the studies analyzed. Moreover, it is important to determine how the data are distributed for adequate visualization of the period of greatest hypertrophy. The synergist ablation model led to an increase in muscle mass in the first three days due to inflammation and edema caused by the surgical procedure.11 DiPasquale DM, Cheng M, Billich W, Huang SA, Rooijen N, Hornberger TA, et al. Urokinase - type plasminogen activator and macrophages are required for skeletal muscle hypertrophy in mice. Am J Physiol Cell Physiol. 2007; 293(4):1278-85.,22 Pavaresh KC, Huber AM, Brochin RL, Bacon PL, McCall G.E, Huey KA, et al. Acute vascular endothelial growth factor expression during hypertrophy is muscle phenotype specific and localizes as a striated pattern within fibers. Exp Physiol. 2010; 95(11):1098-106.,66 Marino JS, Taush BJ, Dearth CL, Manacci MV, McLoughlin TJ, Rakyta SJ, et al. Beta2-integrins contribute to skeletal muscle hypertrophy in mice. Am J Physiol Cell Physiol. 2008; 295(4):1026-36.,2020 Gordon BS, Delgado Dias DC, White JP, Carson JA, Kostec MC. Six1 and Six1 cofactor expression is altered during early skeletal muscle overload in mice. J Physiol Sci. 2012; 62(5):393-401.,6262 Lee WJ, Thompson RW, McClung JM, Carson JA. Regulation of androgen receptor expression at the onset of functional overload in rat plantaris muscle. Am J Physiol Reg Integr Comp Physiol. 2003; 285(5):R1076-85.,6363 Sakuma K, Watanabe K, Sano M, Uramoto I, Totsuka T. Differential adaptation of growth and differentiation factor 8/myostatin, fibroblast growth factor 6 and leukemia inhibitory factor in overloaded, regenerating and denervated rat muscles. Biochim Biophys Acta. 2000; 1497(1):77-88. This disadvantage in the compensatory hypertrophy model by synergist ablation is due to the inflammation process that occurs after surgery. However, Novack et al.1818 Novack ML, Billich W, Smith S, Sukhija KB, McLoughlin TJ, Hornberger TA, et al. COX-2 inhibitor reduces skeletal muscle hypertrophy in mice. Am J Physiol Regul Integr Comp Physiol. 2009; 296(4):R1132-9. demonstrated that components of the acute inflammatory response are required in the muscle repair and remodeling process and the intensity of the inflammatory response is related to the magnitude of hypertrophy. With synergist ablation, the increase in prostaglandin-endoperoxide synthase 2 (COX-2) seems to be related to the considerable increase in muscle mass that occurs in this model and the inflammatory response enables and facilitates the activity of extracellular proteases, the accumulation of macrophages and cell proliferation, including the activation and proliferation of satellite cells, which seems to exert an influence on the greater hypertrophy achieved with this model in comparison to exercise-induced hypertrophy.
According to Marino et al.,66 Marino JS, Taush BJ, Dearth CL, Manacci MV, McLoughlin TJ, Rakyta SJ, et al. Beta2-integrins contribute to skeletal muscle hypertrophy in mice. Am J Physiol Cell Physiol. 2008; 295(4):1026-36. no statistically significant difference in the cross-sectional area of the muscle fibers was found in the first three days following ablation. At 3 to 5 days, the edema is reduced, followed by an increase in the cross-sectional area of the muscle fibers as well as enzyme activity and protein synthesis, which constitute hypertrophy as an adaptation to the new condition of chronic overload.11 DiPasquale DM, Cheng M, Billich W, Huang SA, Rooijen N, Hornberger TA, et al. Urokinase - type plasminogen activator and macrophages are required for skeletal muscle hypertrophy in mice. Am J Physiol Cell Physiol. 2007; 293(4):1278-85.,66 Marino JS, Taush BJ, Dearth CL, Manacci MV, McLoughlin TJ, Rakyta SJ, et al. Beta2-integrins contribute to skeletal muscle hypertrophy in mice. Am J Physiol Cell Physiol. 2008; 295(4):1026-36.,1818 Novack ML, Billich W, Smith S, Sukhija KB, McLoughlin TJ, Hornberger TA, et al. COX-2 inhibitor reduces skeletal muscle hypertrophy in mice. Am J Physiol Regul Integr Comp Physiol. 2009; 296(4):R1132-9.,6161 Bentzinger CF, Lin S, Romanino K, Castets P, Guridi M, Summermatter S, et al. Differential response of skeletal muscles to mTORC1 signaling during atrophy and hypertrophy. Skelet Muscle. 2013; 3(1):6.,6363 Sakuma K, Watanabe K, Sano M, Uramoto I, Totsuka T. Differential adaptation of growth and differentiation factor 8/myostatin, fibroblast growth factor 6 and leukemia inhibitory factor in overloaded, regenerating and denervated rat muscles. Biochim Biophys Acta. 2000; 1497(1):77-88. The period of 12 to 15 days was identified as that with the greatest percentage increase in muscle mass in comparison to the control (Figure 3), demonstrating a linear progression (i.e., a progressive gain in muscle mass over the first 15 days after ablation). At 28 days, the authors found no further increase in gene expression related to increased muscle mass,99 Schuenke MD, Brooks NE, Hikida RS. Interactions of aging, overload and creatine supplementation in rat plantaris muscle. J Aging Res. 2011; 2011:393416.,6464 Dunn SE, Burn JL, Michel RN. Calcineurin is required for skeletal muscle hypertrophy. J Biol Chem. 1999; 274:21908-12.,6565 Adams GR, Caiozzo VJ, Haddad F, Baldwin KM. Cellular and molecular responses to increased skeletal muscle loading after irradiation. Am J Physiol Cell Physiol. 2002; 283(4):C1182-95. as demonstrated by the cessation of linear progression and stabilization of the data (Figure 2). Thus, peak hypertrophy (greatest increase in muscle mass and cross-sectional area of the muscle fibers) occurs between the second and third week following synergist ablation. Concentrating studies on this period is fundamental to determining the impact of novel therapies and interventions designed either to diminish or potentiate the effects of compensatory muscle hypertrophy.
Cross-sectional area and types of muscle fiber
The increase in the cross-sectional area of the muscle and muscle fibers was studied using histological techniques.11 DiPasquale DM, Cheng M, Billich W, Huang SA, Rooijen N, Hornberger TA, et al. Urokinase - type plasminogen activator and macrophages are required for skeletal muscle hypertrophy in mice. Am J Physiol Cell Physiol. 2007; 293(4):1278-85.,77 Goodman CA, Mayhew DL, Hornberger TA. Recent progress toward understanding the molecular mechanisms that regulate skeletal muscle mass. Cell Signal. 2011; 23(12):1896-906.
8 Goodman CA, Kotecki JA, Jacobs BL, Hornberger TA. Muscle fiber type-dependent differences in the regulation of protein synthesis. PLoS One. 2012; 7(5):e37890.-99 Schuenke MD, Brooks NE, Hikida RS. Interactions of aging, overload and creatine supplementation in rat plantaris muscle. J Aging Res. 2011; 2011:393416.,6161 Bentzinger CF, Lin S, Romanino K, Castets P, Guridi M, Summermatter S, et al. Differential response of skeletal muscles to mTORC1 signaling during atrophy and hypertrophy. Skelet Muscle. 2013; 3(1):6. The mean increase in cross-sectional area in comparison to the control was 18.66% in 14 days, demonstrating that compensatory hypertrophy is an effective model for increasing muscle mass. The trend line in Figure 2 shows the percentage increase in muscle mass according to days following surgery: approximately 10% one day after ablation, 38% seven days after ablation and 68% 14 days after ablation.
The increase in the cross-sectional area of muscle is related to protein synthesis of the muscle fibers and the activation of satellite cells. Studies suggest that satellite cells are responsible for both the growth of muscle fibers and the regulation of the muscle fiber phenotype.88 Goodman CA, Kotecki JA, Jacobs BL, Hornberger TA. Muscle fiber type-dependent differences in the regulation of protein synthesis. PLoS One. 2012; 7(5):e37890.,1414 Pehme A, Alev K, Julkunen A, Seene T. The effect of mechanical loading on the MyHC synthesis rate and composition in rat plantaris muscle. Int J Sports Med. 2004; 25(5):332-8.,1919 Huey KA, Burdette S, Zhong H, Roy RR. Early response of heat shock proteins to functional overload of the soleus and plantaris in rats and mice. Exp Physiol. 2010; 95(12):1145-55.,2020 Gordon BS, Delgado Dias DC, White JP, Carson JA, Kostec MC. Six1 and Six1 cofactor expression is altered during early skeletal muscle overload in mice. J Physiol Sci. 2012; 62(5):393-401.,3737 Teixeira CE, Duarte J.A. Myonuclear domain in skeletal muscle fibers. A critical review. Arch Exerc Health. 2011; 2(2):92-101.,4646 Fuller PM, Baldwin KM, Fuller CA. Parallel and divergent adaptations of rat soleus and plantaris to chronic exercise and hypergravity. Am J Physiol Regul Integr Comp Physiol. 2006; 290(2):R442-8. At the onset of compensatory hypertrophy, the muscle fiber alters its response. The relationship among the cross-sectional area, hypertrophy and fiber type3737 Teixeira CE, Duarte J.A. Myonuclear domain in skeletal muscle fibers. A critical review. Arch Exerc Health. 2011; 2(2):92-101. indicates that chronic overload induces changes in the expression of heavy chain myosin.
Goodman et al.88 Goodman CA, Kotecki JA, Jacobs BL, Hornberger TA. Muscle fiber type-dependent differences in the regulation of protein synthesis. PLoS One. 2012; 7(5):e37890. demonstrated a significant increase in protein synthesis in four types of muscle fiber (slow-twitch [type I] and fast-twitch [type IIa, IIb and IIx]) in the plantaris muscle in rats submitted to synergist ablation. Type IIb fibers exhibited the least amount of protein synthesis, whereas IIa fibers exhibited the most amount of protein synthesis, which did not differ significantly from that found in type I fibers. In the cross-sectional area, type IIb fibers were shorter than IIa fibers, which also exceeded the area found in type I fibers. These findings suggest that this model results in the selective regulation and modification of fast-twitch fibers in skeletal muscle.
Conclusion
Based on the findings of the present systematic review, the following conclusions may be drawn: 1. the synergist ablation model differs from other overload models regarding the characteristics involved in the hypertrophy process; 2. 12 to 15 days following ablation is the period of greatest muscle hypertrophy; 3. the lack of a significant difference in the gain in muscle mass between unilateral and bilateral ablation demonstrates that contralateral limb can be used as the control, which reduces the number of animals used in this model; and 4. synergist muscle ablation is an efficient reproducible model for achieving rapid hypertrophy and results in the selective regulation and modification of fast-twitch fibers in skeletal muscle.
-
Study conducted by the Graduate Program in Biophotonics applied to Health Sciences, Universidade Nove de Julho (Uninove), São Paulo, SP, Brazil
Acknowledgment
Funding for this study was provided by Universidade Nove de Julho, São Paulo, Brazil.
References
-
1DiPasquale DM, Cheng M, Billich W, Huang SA, Rooijen N, Hornberger TA, et al. Urokinase - type plasminogen activator and macrophages are required for skeletal muscle hypertrophy in mice. Am J Physiol Cell Physiol. 2007; 293(4):1278-85.
-
2Pavaresh KC, Huber AM, Brochin RL, Bacon PL, McCall G.E, Huey KA, et al. Acute vascular endothelial growth factor expression during hypertrophy is muscle phenotype specific and localizes as a striated pattern within fibers. Exp Physiol. 2010; 95(11):1098-106.
-
3McCarthy JJ, Mula J, Miyasaki M, Erfani R, Garrison K, Farooqui AB, et al. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development. 2011; 138(17):3657-66.
-
4Adams GR, Haddad F, Baldwin KM. Time course of changes in markers of myogenesis in overloaded rat skeletal muscles. J Appl Physiol (1985). 1999; 87(5):1705-12.
-
5Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001; 3(11):1014-9.
-
6Marino JS, Taush BJ, Dearth CL, Manacci MV, McLoughlin TJ, Rakyta SJ, et al. Beta2-integrins contribute to skeletal muscle hypertrophy in mice. Am J Physiol Cell Physiol. 2008; 295(4):1026-36.
-
7Goodman CA, Mayhew DL, Hornberger TA. Recent progress toward understanding the molecular mechanisms that regulate skeletal muscle mass. Cell Signal. 2011; 23(12):1896-906.
-
8Goodman CA, Kotecki JA, Jacobs BL, Hornberger TA. Muscle fiber type-dependent differences in the regulation of protein synthesis. PLoS One. 2012; 7(5):e37890.
-
9Schuenke MD, Brooks NE, Hikida RS. Interactions of aging, overload and creatine supplementation in rat plantaris muscle. J Aging Res. 2011; 2011:393416.
-
10White JP, Reecy MJ, Washington TA, Sato, Le M, Davis JM, et al. Overload-induced skeletal muscle extracellular matrix remodeling and myofibre growth in mice lacking IL-6. Acta Physiol (Oxf). 2009; 197(4):321-32.
-
11Almurshed KS, Grunewald KK. Dietary protein does not affect overloaded skeletal muscle in rat. J Nutr. 2000; 130(7):1743-8.
-
12Yamaguchi A, Ikeda Y, Hirai T, Fujikawa T, Morita I. Local changes of IGF-1 mRNA, GH receptor MRNA, and fiber size in rat plantaris muscle following compensatory overload. Jpn J Physiol. 2003; 53:53-60.
-
13Sakuma K, Nishikawa J, Nakao R, Nakano H, Sano M, Yasuhara M. Serum response factor plays an important role in the mechanically overload plantaris muscle of rats. Histochem Cell Biol. 2003; 119(2):149-60.
-
14Pehme A, Alev K, Julkunen A, Seene T. The effect of mechanical loading on the MyHC synthesis rate and composition in rat plantaris muscle. Int J Sports Med. 2004; 25(5):332-8.
-
15Young RE, Young JC. The effect of creatine supplementation on mass and performance of rat skeletal muscle. Life Sci. 2007; 81(9):710-6.
-
16Locke M. Heat shock protein accumulation and heat shock transcription factor activation in rat skeletal muscle during compensatory hypertrophy. Acta Physiol (Oxf) 2008; 192(3):403-11.
-
17Choi H, Selpides IPJ, Novell MM, Rourke BC. Functional overload in ground squirrel plantaris muscle fails to induce myosin isoform shifts. Am J Physiol Regul Integr Comp Physiol. 2009; 297(3):R578-86.
-
18Novack ML, Billich W, Smith S, Sukhija KB, McLoughlin TJ, Hornberger TA, et al. COX-2 inhibitor reduces skeletal muscle hypertrophy in mice. Am J Physiol Regul Integr Comp Physiol. 2009; 296(4):R1132-9.
-
19Huey KA, Burdette S, Zhong H, Roy RR. Early response of heat shock proteins to functional overload of the soleus and plantaris in rats and mice. Exp Physiol. 2010; 95(12):1145-55.
-
20Gordon BS, Delgado Dias DC, White JP, Carson JA, Kostec MC. Six1 and Six1 cofactor expression is altered during early skeletal muscle overload in mice. J Physiol Sci. 2012; 62(5):393-401.
-
21Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M. Mechanisms regulating skeletal muscle grow and atrophy. FEBS J. 2013; 280(17):4294-314.
-
22Bismuth K, Relaix F. Genetic regulation of skeletal muscle development. Exp Cell Res. 2010; 316(18):3081-6.
-
23Elliott B, Renshaw D, Getting S, Mackenzie R. The central role of myostatin in skeletal muscle and whole body homeostasis. Acta Physiol (Oxf). 2012; 205(3):324-40.
-
24Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol. 2005; 37(10):1974-84.
-
25Aline G, Sotiropoulos A. A key factor controlling muscle hypertrophy by enhance the recruitment of muscle stem cells. Bioarchitecture. 2012; 2:88-90.
-
26Martin NRW, Lewis MP. Satellite cell activation and number following acute and chronic exercise: a mini review. Cell Mol Exerc Physiol. 2012; 1(1):e3.
-
27Otto A, Patel K. Signalling and the control of skeletal muscle size. Exp Cell Res. 2010; 316(18):3059-66.
-
28Ohira Y, Kawano F, Wang XD, Nakai N, Ohira T, Okabe H, et al. Role(s) of mechanical load and satellite cells in the regulation of the size of soleus muscle fiber in rats. Biol Sci Space. 2010; 24(3-4):135-44.
-
29Kawano F, Nakai N, Ohira Y. Regulation of soleus muscle properties by mechanical stress and/or neural activity. J Phys Sports Med. 2012; 1(1):29-36.
-
30Morgan JE, Partridge TA. Muscle satellite cells. Int J Biochem Cell Biol. 2003; 35(8):1151-6.
-
31Stewart CE, Pell JM. Point: IGF is the major physiological regulation of muscle mass. J Appl Physiol. 2010; 108:1820-4.
-
32Sakuma K, Yamaguchi A. The functional role of calcineurin in hypertrophy, regeneration, and disorders of skeletal muscle. J Biomed Biotechnol. 2010; 2010:72129.
-
33Sakuma K, Yamaguchi A. Molecular determinants of skeletal muscle hypertrophy in animals. J Sport Medic Doping Studie. 2012; S1:002. Available from: https://www.omicsonline.org/2161-0673/2161-0673-S1-002.pdf
» https://www.omicsonline.org/2161-0673/2161-0673-S1-002.pdf -
34Scharner J, Zammit PS. The muscle satellite cell at 50: the formative years. Skelet Muscle. 2011; 1(1):28.
-
35Schadrach JL, Wagers AJ. Stem cells for skeletal muscle repair. Phil Trans R Soc B. 2011; 366:2297-306.
-
36Schmalbruch H. The satellite cell of skeletal muscle fibers. Braz J Morphol Sci. 2006; 23(2):159-72.
-
37Teixeira CE, Duarte J.A. Myonuclear domain in skeletal muscle fibers. A critical review. Arch Exerc Health. 2011; 2(2):92-101.
-
38Yabolnka-Reuveni Z. The skeletal muscle satellite cell: still young and fascinating at 50. J Histochem Cytochem. 2011; 59(12):1041-59.
-
39West DWD, Burd NA, Staples AW, Phillips SM. Human exercise-mediated skeletal muscle hypertrophy is an intrinsic process. Int J Biochem Cell Biol. 2010; 42(9):1371-5.
-
40van Wessel T, de Haan A, van der Laarse WJ, Jaspers RT. The muscle fiber type-fiber paradox: hypertrophy or oxidative metabolism? Eur J Appl Physiol. 2010; 110(4):665-94.
-
41Zanou N, Gailly P. Skeletal muscle hypertrophy and regeneration: interplay between the myogenic regulatory factors (MRFs) and insulin-like growth factors (IGFs) pathways. Cell Mol Life Sci. 2013; 70(21):4117-30.
-
42Yan Z, Okutsu M, Alhtar YN, Lira V. Regulation of exercise-induced fiber type transformation, mitochondrial biogenesis, and angiogenesis in skeletal muscle. J Appl Physiol (1985). 2011; 110(1):264-74.
-
43Allouh MZ, Yabolnka-Reuveni Z, Rosser BWC. Pax7 reveals a greater frequency and concentration of satellite cells at the ends of growing skeletal muscle fibers. J Histochem Cytochem. 2008; 56(1):77-87.
-
44Allen DL, Harrison BC, Sartorius C, Byrnes WC, Leinwand LA. Mutation of the IIB myosin heavy chain gene results in muscle fiber loss and compensatory hypertrophy. Am J Physiol Cell Physiol. 2001; 280(3):C637-45.
-
45Aoki MS, Miyabara EH, Soares AG, Salvini TF, Moriscot AS. Cyclosporin-A does not affect skeletal muscle mass during disuse and recovery. Braz J Med Biol Res. 2006; 39(2):243-51.
-
46Fuller PM, Baldwin KM, Fuller CA. Parallel and divergent adaptations of rat soleus and plantaris to chronic exercise and hypergravity. Am J Physiol Regul Integr Comp Physiol. 2006; 290(2):R442-8.
-
47Qaisar R, Renaud G, Morine K, Barton ER, Sweeney HL, Larsson L. Is functional hypertrophy and specific force coupled with the addition of myonuclei at the single muscle fiber level? FASEB J. 2012; 26(3):1077-85.
-
48Kawano F, Goto K, Wang XD, Terada M, Oshira T, Nakai N, et al. Role(s) of gravitational loading during developing period on the growth of rat soleus muscle fibers. J Appl Physiol (1985). 2010; 108(3):676-85.
-
49Seene T, Pehme A, Alev K, Kassik P, Umnova M, Aru M. Effects of resistance training on fast- and slow-twitch muscles in rats. Biol Sport. 2010; 27:221-9.
-
50Zhang BT, Yeung SS, Liu Y, Wang HH, Wan YM, Ling SK, et al. The effects of low frequency electrical stimulation on satellite cell activity in rat skeletal muscle during hindlimb suspension. BMC Cell Biol. 2010; 11:87.
-
51Blaauw B, Canato M, Agatea L, Toniolo L, Mammucari C, Masiero E, et al. Inducible activation of Akt increases skeletal muscle mass and force without satellite cell activation. FASEB J. 2009; 23(11):3896-905.
-
52Chen JF, Tao Y, Li J, Deng Z, Yan Z, Xiao X, et al. microRNA-1 and microRNA-206 regulate skeletal muscle satellite cell proliferation and differentiation by repressing Pax7. J Cell Biol. 2010; 190(:867-79.
-
53Jacquemim V, Furling AB, Butler-Browne GS, Mouly V. IGF-1 induces human myotube hypertrophy by increasing cell recruitment. Exp Cell Res. 2004; 299(1):148-58.
-
54Shefer G, Wleklinski-Lee M, Yabolnka-Reuveni Z. Skeletal muscle satellite cells can spontaneously enter an alternative mesenchymal pathway. J Cell Sci. 2004; 117(Pt 22):5393-404.
-
55Liadaki K, Casar JC, Wessen M, Luth EC, Jun S, Gussoni E, Kunkel LM. β4 integrin marks intersticial myogenic progenitor cells in adult murine skeletal muscle. J Histochem Cytochem. 2012; 60(1):31-44.
-
56Wang M, Yu H, Kim YS, Bidwell CA, Kuang S. Myostatin facilities slow and inhibits fast myosin heavy chain expression during myogenic differentiation. Biochem Biophys Res Commun. 2012; 426(1):83-8.
-
57Ishido M, Kami K, Masuhara M. Localization of MyoD, myogenin and cell cycle regulatory factors in hypertrophying rat skeletal muscle. Acta Physiol Scand. 2004; 180(3):281-9.
-
58Kawano F, Matsuoka Y, Oke Y, Higo Y, Terada M, Wang XD, et al. Role(s) of nucleoli and phosphorylation of ribosomal protein S6 and/or HSP27 in the regulation of muscle mass. Am J Physiol Cell Physiol. 2006; 293(1):C35-44.
-
59Terada M, Kawano F, Ohira T, Nakai N, Nishimoto N, Ohira Y. Effects of mechanical over-loading on the properties of soleus muscle fibers, with or without damage, in wild type and Mdx mice. PLoS One. 2011; 7(4):e.34557.
-
60Reynolds TH 4th, Bodine SC, Lawrence JC Jr. Control of Ser2448 phosphorylation in the mammalian target of rapamycin by insulin and skeletal muscle load. J Biol Chem. 2002; 277(20):17657-62.
-
61Bentzinger CF, Lin S, Romanino K, Castets P, Guridi M, Summermatter S, et al. Differential response of skeletal muscles to mTORC1 signaling during atrophy and hypertrophy. Skelet Muscle. 2013; 3(1):6.
-
62Lee WJ, Thompson RW, McClung JM, Carson JA. Regulation of androgen receptor expression at the onset of functional overload in rat plantaris muscle. Am J Physiol Reg Integr Comp Physiol. 2003; 285(5):R1076-85.
-
63Sakuma K, Watanabe K, Sano M, Uramoto I, Totsuka T. Differential adaptation of growth and differentiation factor 8/myostatin, fibroblast growth factor 6 and leukemia inhibitory factor in overloaded, regenerating and denervated rat muscles. Biochim Biophys Acta. 2000; 1497(1):77-88.
-
64Dunn SE, Burn JL, Michel RN. Calcineurin is required for skeletal muscle hypertrophy. J Biol Chem. 1999; 274:21908-12.
-
65Adams GR, Caiozzo VJ, Haddad F, Baldwin KM. Cellular and molecular responses to increased skeletal muscle loading after irradiation. Am J Physiol Cell Physiol. 2002; 283(4):C1182-95.
Publication Dates
-
Publication in this collection
Feb 2017
History
-
Received
21 Feb 2016 -
Accepted
26 June 2016