Abstracts
BACKGROUND AND OBJECTIVES: The analgesic effect of intraperitoneal administration of local anesthetics after laparoscopic cholecystectomy is a controversial issue, and the results described vary from considerable pain relief to little reduction in pain. The objective of this study was to evaluate the efficacy of the intraperitoneal administration of 50% enantiomeric excess bupivacaine (S75-R25) for the postoperative pain relief of laparoscopic cholecystectomy. METHODS: A randomized, double blind, placebo controlled study was conducted with 40 patients undergoing laparoscopic cholecystectomy, who were divided in two groups: GI (n = 20) received 80 mL of intraperitoneal 0,125% S75-R25 bupivacaine at the end of the procedure; and GII (n = 20) received 80 mL of intraperitoneal normal saline. Both groups received 40 mg of tenoxicam and 30 mg.kg-1 of intravenous dypirone shortly before the end of the surgery. Tramadol was used for postoperative analgesia (PO). Pain scores were evaluated at rest, sitting up, and during the Valsalva maneuver, according to a numeric scale upon waking up and 2, 4, 8, 12, and 24 hours postoperatively; the presence of shoulder pain was assessed, as well as the length of time until the first request of analgesic and its cumulative consumption. RESULTS: There was a statistically significant difference in resting pain scores at 12 hours PO (GI < GII). The length of time until the first request of tramadol was greater in GI and this group presented smaller consumption of this drug, but these differences were not statistically significant. CONCLUSIONS: The intraperitoneal instillation of 80 mL of 0.125% S75-R25 bupivacaine provided for lower resting pain scores, which was statistically significant only at the 12th postoperative hour.
ANALGESIA; ANESTHETICS, Local; ANALGESIC TECHNIQUE; SURGERY, Abdominal
JUSTIFICATIVA E OBJETIVOS: O efeito analgésico de infusões intraperitoneais de anestésicos locais após colecistectomia videolaparoscópica é controverso e os resultados descritos vão de alívio considerável à pequena redução da dor. O objetivo deste estudo foi avaliar a eficácia da administração intraperitoneal da mistura com excesso enantiomérico de 50% de bupivacaína (S75-R25) para o alívio da dor no pós-operatório de colecistectomia videolaparoscópica. MÉTODO: Estudo aleatório, placebo-controlado e duplamente encoberto com 40 pacientes submetidos à colecistectomia videolaparoscópica divididos em dois grupos: GI (n = 20) que recebeu 80 mL de solução de bupivacaína S75-R25 a 0,125% intraperitoneal no fim da operação; GII (n = 20) que recebeu 80 mL de solução fisiológica a 0,9%. Ambos os grupos receberam 40 mg de tenoxicam e 30 mg.kg-1 de dipirona, por via venosa, pouco antes do fim da operação. A analgesia no pós-operatório (PO) foi feita com tramadol. Foram avaliados os escores de dor em repouso, ao sentar e à manobra de Valsalva, segundo a escala numérica ao despertar e 2, 4, 8, 12 e 24 horas no PO; a presença de dor no ombro; o tempo para a primeira solicitação do analgésico; e o seu consumo cumulativo. RESULTADOS: Houve diferença estatística significativa entre os escores de dor às 12 horas no PO com o paciente em repouso (GI < GII). O tempo da primeira solicitação de tramadol foi maior no GI e o seu consumo menor neste grupo, porém essas diferenças não foram significativas. CONCLUSÕES: A instilação intraperitoneal de 80 mL de bupivacaína S75-R25 a 0,125% proporcionou baixos escores de dor em repouso de forma significativa somente às 12 horas de PO de colecistectomia videolaparoscópica.
ANALGESIA; ANESTÉSICOS, Local; TÉCNICA ANALGÉSICA; CIRURGIA, Abdominal
JUSTIFICATIVA Y OBJETIVOS: El efecto analgésico de infusiones intraperitoneales de anestésicos locales después colecistectomía videolaparoscópica es controvertido y los resultados descritos van desde el alivio considerable a la pequeña reducción del dolor. El objetivo de este estudio fue evaluar la eficacia de la administración intraperitoneal de la mezcla con exceso enantiomérico de 50% de bupivacaína (S75-R25) para el alivio del dolor en el postoperatorio de colecistectomía videolaparoscópica. MÉTODO: Estudio aleatorio, placebo-controlado y doblemente encubierto con 40 pacientes sometidos a colecistectomía videolaparoscópica divididos en dos grupos: GI (n = 20) que recibió 80 mL de solución de bupivacaína S75-R25 a 0,125% intraperitoneal al final de la operación; y GII (n = 20) que recibió 80 mL de solución fisiológica a 0,9%. Los dos grupos recibieron 40 mg de tenoxican y 30 mg.kg-1 de dipirona, por vía venosa, poco antes del final de la operación. La analgesia en el postoperatorio (PO) se hizo con tramadol. Se evaluaron las puntuaciones de dolor en reposo, al sentarse y en la maniobra de Valsalva, según la escala numérica al despertar y 2, 4, 8, 12 y 24 horas en el PO; la presencia de dolor en el hombro; el tiempo para la primera solicitación del analgésico y su consumo acumulativo. RESULTADOS: Hubo una diferencia estadística significativa entre los puntajes de dolor a las 12 horas en el PO con el paciente en reposo (GI < GII). El tiempo de la primera solicitación de tramadol fue mayor en el GI y en su consumo menor en este grupo, sin embargo esas diferencias no fueron significativas. CONCLUSIONES: La instilación intraperitoneal de 80 mL de bupivacaína S75-R25 a 0,125%, proporcionó bajos puntajes de dolor en reposo de forma significativa solamente a las 12 horas de PO de colecistectomía videolaparoscópica.
SCIENTIFIC ARTICLE
Intraperitoneal administration of 50% enantiomeric excess (S75-R25) bupivacaine in postoperative analgesia of laparoscopic cholecystectomy* * Received from Hospital Universitário Materno-Infantil da Universidade Federal do Maranhão (HU/UFMA), São Luís, MA
Administración intraperitoneal de la mezcla con exceso enantiomérico de 50% de bupivacaína (S75-R25) para analgesia postoperatoria en colecistectomías videolaparoscópicas
João Batista Santos Garci, TSAI; Antônio M. Alencar JúniorII; Carlos Eduardo Claro dos Santos, TSAIII
IProfessor Adjunto Doutor da Disciplina de Anestesiologia da UFMA. Responsável pelo Serviço de Dor do HU/UFMA
IIMédico Residente do HU/UFMA; Membro da Liga de Dor do Maranhão
IIIMédico Assistente do Serviço de Anestesiologia do Maranhão
Correspondence to Correspondence to: Dr. João Batista S. Garcia Av. dos Holandeses, 213/701 Ponta D'Areia 65085-450 São Luís, MA E-mail: jbgarcia@uol.com.br
SUMMARY
BACKGROUND AND OBJECTIVES: The analgesic effect of intraperitoneal administration of local anesthetics after laparoscopic cholecystectomy is a controversial issue, and the results described vary from considerable pain relief to little reduction in pain. The objective of this study was to evaluate the efficacy of the intraperitoneal administration of 50% enantiomeric excess bupivacaine (S75-R25) for the postoperative pain relief of laparoscopic cholecystectomy.
METHODS: A randomized, double blind, placebo controlled study was conducted with 40 patients undergoing laparoscopic cholecystectomy, who were divided in two groups: GI (n = 20) received 80 mL of intraperitoneal 0,125% S75-R25 bupivacaine at the end of the procedure; and GII (n = 20) received 80 mL of intraperitoneal normal saline. Both groups received 40 mg of tenoxicam and 30 mg.kg-1 of intravenous dypirone shortly before the end of the surgery. Tramadol was used for postoperative analgesia (PO). Pain scores were evaluated at rest, sitting up, and during the Valsalva maneuver, according to a numeric scale upon waking up and 2, 4, 8, 12, and 24 hours postoperatively; the presence of shoulder pain was assessed, as well as the length of time until the first request of analgesic and its cumulative consumption.
RESULTS: There was a statistically significant difference in resting pain scores at 12 hours PO (GI < GII). The length of time until the first request of tramadol was greater in GI and this group presented smaller consumption of this drug, but these differences were not statistically significant.
CONCLUSIONS: The intraperitoneal instillation of 80 mL of 0.125% S75-R25 bupivacaine provided for lower resting pain scores, which was statistically significant only at the 12th postoperative hour.
Key Words: ANALGESIA: postoperative; ANESTHETICS, Local: Bupivacaine S75-R25; ANALGESIC TECHNIQUE: intraperitoneal; SURGERY, Abdominal: laparoscopic cholecystectomy
RESUMEN
JUSTIFICATIVA Y OBJETIVOS: El efecto analgésico de infusiones intraperitoneales de anestésicos locales después colecistectomía videolaparoscópica es controvertido y los resultados descritos van desde el alivio considerable a la pequeña reducción del dolor. El objetivo de este estudio fue evaluar la eficacia de la administración intraperitoneal de la mezcla con exceso enantiomérico de 50% de bupivacaína (S75-R25) para el alivio del dolor en el postoperatorio de colecistectomía videolaparoscópica.
MÉTODO: Estudio aleatorio, placebo-controlado y doblemente encubierto con 40 pacientes sometidos a colecistectomía videolaparoscópica divididos en dos grupos: GI (n = 20) que recibió 80 mL de solución de bupivacaína S75-R25 a 0,125% intraperitoneal al final de la operación; y GII (n = 20) que recibió 80 mL de solución fisiológica a 0,9%. Los dos grupos recibieron 40 mg de tenoxican y 30 mg.kg-1 de dipirona, por vía venosa, poco antes del final de la operación. La analgesia en el postoperatorio (PO) se hizo con tramadol. Se evaluaron las puntuaciones de dolor en reposo, al sentarse y en la maniobra de Valsalva, según la escala numérica al despertar y 2, 4, 8, 12 y 24 horas en el PO; la presencia de dolor en el hombro; el tiempo para la primera solicitación del analgésico y su consumo acumulativo.
RESULTADOS: Hubo una diferencia estadística significativa entre los puntajes de dolor a las 12 horas en el PO con el paciente en reposo (GI < GII). El tiempo de la primera solicitación de tramadol fue mayor en el GI y en su consumo menor en este grupo, sin embargo esas diferencias no fueron significativas.
CONCLUSIONES: La instilación intraperitoneal de 80 mL de bupivacaína S75-R25 a 0,125%, proporcionó bajos puntajes de dolor en reposo de forma significativa solamente a las 12 horas de PO de colecistectomía videolaparoscópica.
INTRODUCTION
Compared with the conventional surgery, laparoscopic cholecystectomy, a minimally invasive technique, is associated with a smaller surgical trauma 1. But it is associated with intra-abdominal and incisional pain, as well as pain in the supraclavicular 2 region, especially in the immediate postoperative period 3. Since this surgery entails a short hospital stay, pain control in this period is paramount 4.
The analgesic effects of intraperitoneal infusion of local anesthetics after laparoscopic cholecystectomy is controversial, and the results described in the literature range from considerable pain relief to absence of pain relief 5.
Bupivacaine is one of the local anesthetics used more often in this setting, but it has considerable cardiac and central nervous system toxicity 6. Levobupivacaine, the S(-) enantiomer of bupivacaine, whose intensity and duration of the analgesic effect is similar to the racemic mixture, with decreased systemic toxicity, is an alternative for this problem 7.
The change in the enantiomeric ratio of racemic bupivacaine made it possible to create a new anesthetic, the 50% enantiomeric excess mixture, S75-R25, whose toxicity is similar to that of levobupivacaine, therefore smaller than that of the racemic mixture, while maintaining the same efficacy regarding the nervous blockade 8. We have not found in the literature studies evaluating the intraperitoneal instillation of this drug for pain control.
The objective of this study was to evaluate the efficacy of the administration of intraperitoneal S75-R25 bupivacaine for relief of postoperative pain in laparoscopic cholecystectomy.
METHODS
Forty patients, physical status ASA I and II, of both genders, who underwent elective laparoscopic cholecystectomy, were included in this study.
The study population was randomly divided in two groups: Group I (GI) and Group II (GII), both with 20 patients each, for a double-blind study, using sealed envelopes chosen immediately before the procedure. The investigator was unaware which group the patient belonged to until the end of the study. The study protocol was approved by the Ethics Committee of the Hospital Universitário and patients signed an informed consent.
Patients with a history of allergies to non-steroidal anti-inflammatories and to the local anesthetic, cardiac arrhythmias or cardiopathy, and cognitive deficits were excluded from the study, as well as those who refused to participate.
Patients in both groups received intravenous midazolam (1 mg) in the operating room. Titrated doses of propofol (2.5 mg.kg-1) were used for the anesthetic induction until the patient lost consciousness and alfentanyl (30 to 50 µg.kg-1). Tracheal intubation was done after the administration of cisatracurium (0.15 mg.kg-1). Anesthesia was maintained with sevoflurane and a muscle relaxant, which was repeated at one third of the original dose if needed. Metochlopramide was administered at the end of the surgical procedure.
Monitoring included pulse oximetry, cardioscope on the DII derivation, capnograph, and non-invasive blood pressure, which were measured at 5-minute intervals.
Patients in Group I received the intraperitoneal instillation of 80 mL of 0.125% S75-R25 bupivacaine at the end of the surgical procedure. Patients in Group II received 80 mL of normal saline at 0,9% for intraperitoneal instillation at the end of the surgery.
The solution for intraperitoneal instillation was prepared by a member of the team who had no other role in the study. The intraperitoneal instillation was performed in the vesical bed, in the supra-hepatic region, and adjacent areas.
Both groups also received intravenous dypirone (30 mg.kg-1) and tenoxicam (40 mg) shortly before the end of the procedure.
Postoperative analgesia was composed of intravenous tramadol administered upon the request of the patient or when the patient experienced pain equal or greater than 3 in the numeric scale, up to a four-hour interval, at a dose of 100 mg.
The nursing staff was oriented to administer the doses of the analgesics according to the needs of each patient, unaware of which group the patient belonged to.
Intra-abdominal pain immediately after the surgery was measured by a numeric scale at the following times: shortly after the patient regained consciousness, and 2, 4, 6, 8, 12, and 24 hours after the surgery with the patient at rest, raising to the sitting position, and during the Valsalva maneuver. In the numeric scale, the patient attributed numbers to the pain intensity that varied from 0 to 10, where 0 was the absence of pain and 10 the worse pain imaginable. The presence or absence of shoulder pain at 24 hours and the length of time between extubation and the first request for pain medication were also recorded.
The total and cumulative consumption of analgesics (tramadol) were recorded after 24 hours and compared between both groups. Other side effects, such as nausea, vomiting, tinnitus, muscle spasms, tingling, and etc. were recorded.
The data gathered were organized in tables using the Excel software and posteriorly analyzed statistically by the Stata software using the following tests: test t Student for age, height and duration of the surgery; Chi-square test, for race, gender, ASA classification, presence of supraclavicular pain, and side effects; Mann-Whitney test, for weight, intra-abdominal pain scores (numerical scale), length of time until the first request for pain medication (tramadol), and total consumption of tramadol during the 24 hours the patient remained in the hospital; and the Kolmogorov-Smirnov test, to analyze the cumulative consumption of tramadol.
Mean and standard deviation were used to express the results of the statistical analysis. A p < 0.05 was considered statistically significant.
RESULTS
The final study population consisted of 32 patients, 19 in Group I (GI), who received 0.125% S75-R25 bupivacaine, and 13 in Group II (GII), who received normal saline (placebo). One patient in GI and 7 in GII were excluded due to the impossibility of completing the protocol.
There were no statistically significant differences between GI and GII regarding age, weight, height, gender, and race (Table I).
In GI, 63.16% of the patients were classified as physical status ASA I and 36.84%, ASA II; in GII, 100% of the patients were ASA I (p = 0.013).
The mean duration of the surgery was 44.21 minutes in GI and 41.15 minutes in GII, which did not show a statistically significant difference (p = 0.6028).
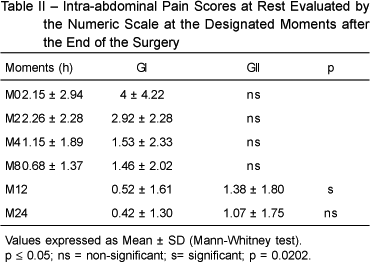
The intra-abdominal pain scores (numeric scale) at rest in both groups, evaluated upon waking up (M0), 2 hours (M2), 4 hours (M4), 8 hours (M8), 12 hours (M12), and 24 hours (M24) after the surgery were significantly lower in GI only at M12 (Table II).
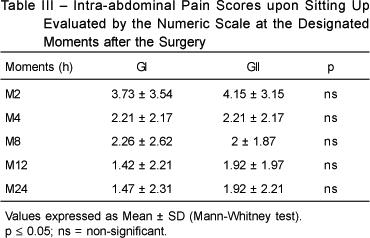
The intra-abdominal pain scores on sitting up and during the Valsalva maneuver 2 hours (M2), 4 hours (M4), 8 hours (M8), 12 hours (M12), and 24 hours (M24) after the surgical procedure did not show any statistically significant differences between both groups (Tables III and IV).
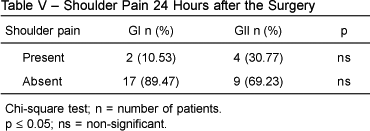
Two patients in GI (10.53%) and 4 patients in GII (30.77%) experienced shoulder pain, but this was not statistically significant (Table V).
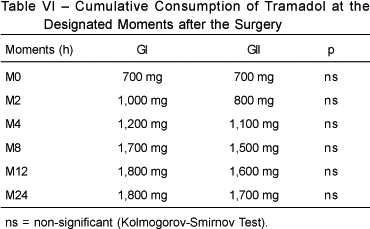
The length of time between the end of the surgery until the first request of analgesic (tramadol) and the total mean consumption of tramadol at the end of the 24 hours of hospital stay did not show statistically significant differences between both groups. The first request occurred a mean of 92.63 minutes after the surgery in Group I, and 31.15 minutes in Group II (p = 0.2397); the mean total consumption of tramadol in Group I was 94.73 mg, and 130.76 mg in Group II (p = 0.3380).
The difference in the cumulative consumption of tramadol between both groups was not statistically significant (Table VI).
Side effects were observed in 6 (31.58%) patients in Group I, with 5 (26.31%) experiencing nausea, 3 (15.78%) vomiting, 2 (10.52%) dizziness, and 1 (5.26%), epigastric pain. In GII, 6 patients (46.15%) experienced side effects; 5 of those (38.46%) experienced nausea, and 4 (30.76%) vomiting. There were no statistically significant differences between both groups (p = 0.403; Chi-square). Patients in both groups did not show any changes in cardiac and CNS functions.
DISCUSSION
There were no statistically significant differences between GI and GII regarding the demographic data, demonstrating the homogeneity of the study population. Besides, the mean age and gender distribution were in line to that presented in the literature, which reports the incidence of gallbladder stones in 20% of the patients between 40 and 60 years, with a predominance of females. It is seven times more frequent in young women than young men, but only twice as frequent in women than man above 60 years 9. Therefore, the study population was a good representation of the general population. In GI, 63.16% were classified as ASA I and 36.84%, ASA II, while in GII, 100% of the patients were ASA I (p = 0.013); however, this did not hinder the study.
There were no statistical differences between both groups regarding the duration of the surgical procedure (p = 0.6028). The mean time was 1 hour, which conforms to the literature, demonstrating that laparoscopic cholecystectomy is a fast technique, decreasing the duration of the surgical trauma. However, it is associated with intra-abdominal, incisional, and shoulder pain 2, which is usually treated using a multimodal approach. It includes the use of opioids, non-steroidal anti-inflammatories (NSAIDs), paracetamol, and local anesthetics 10.
Although NSAIDs have a morphine-sparing effect, by themselves they cannot produce enough postoperative analgesia for laparoscopic interventions 11. Besides, they cause gastric irritation, decrease the number of platelets, and decrease renal function postoperatively, when patients are at risk for developing anyone of those disorders due to forced fasting, dehydration, and tissue trauma 10.
Local anesthetics do not possess the same side effects of opioids that can delay recovery and increase hospital stay. These effects include nausea, delayed return of gastrointestinal motility, and pruritus 10.
Local anesthetics have been administered in the peritoneal cavity during procedures such as laparoscopic cholecystectomy, and gynecological laparoscopies for sterilization and diagnosis 12. The reason for this route of administration is that the peritoneum is exposed to the blockade of the visceral nociceptive conduction, with an additional mechanism of analgesia. However, the local anesthetic can also be absorbed through this large peritoneal surface, which may be responsible for the prolongation of the analgesia 10.
The use of local anesthetics is an attractive method because, in theory, it improves pain control during this period, besides decreasing the consumption of opioids 12.
A systematic revision of 13 clinical studies on the intraperitoneal administration of 50 to 200 mg of bupivacaine in volumes of 10 to 100 mL after laparoscopic cholecystectomy demonstrated a significant reduction in global, abdominal, and shoulder pain scores in seven studies, but not in the remaining six. Besides, in five of those studies the consumption of analgesics was reduced 12. This analysis revealed a statistically significant difference, but a questionable clinical relevance. Thus, it seems that the intraperitoneal administration of local anesthetics in laparoscopic laparotomies produces moderate analgesia that may not be adequate for routine use 10. Its use in gynecological laparoscopy demonstrated a more effective analgesia, possibly because this procedure is less traumatic than cholecystectomy 12.
It is interesting to observe that in a study, the intraperitoneal instillation of a combination of 200 mg of lidocaine and 20 mg of tenoxicam reduced pain scores and opioid consumption when compared to placebo or intraperitoneal lidocaine associated with intravenous tenoxicam 13.
The differences in the results of intraperitoneal instillation of local anesthetics may result from the type and location of the surgery, and dose, type and time of instillation. The failure of some studies to demonstrate an analgesic effect can be due to the fast dilution of the local anesthetic in the peritoneal cavity 14. However, it is not possible to increase the dose without increasing the risk of systemic toxicity. Although potentially more toxic than lidocaine, the action of bupivacaine is more prolonged. However, clinical studies have demonstrated that the analgesic effects of bupivacaine are short acting 10.
Levobupivacaine, the S(-) enantiomer of bupivacaine, whose intensity and duration of the analgesic effect is similar to that of the racemic mixture, with reduced systemic toxicity, may be an alternative 7. The 50% enantiomeric excess bupivacaine, S75-R25, is the most recent alternative. Its toxicity is similar to that of levobupivacaine and has the same efficacy of the racemic mixture regarding the nervous blockade 8.
This new anesthetic showed longer duration of analgesia and decreased motor blockade compared with the racemic bupivacaine group in patients who underwent epidural anesthesia for vascular or orthopedic surgical procedures of the lower limbs 15. Another study did not demonstrate any differences in the duration or in the length of time necessary to achieve maximal motor blockade between racemic and S75-R25 bupivacaine. However, the intensity of the motor blockade was significantly smaller in the S75-R25 group 16.
Comparing ropivacaine in epidural anesthesia for cesarean sections with S75-R25 and racemic bupivacaine (all in association with fentanyl), had a smaller duration of analgesia and, at the end of the procedure, the S75-R25 group had a significant reduction in motor blockade than the other two groups 17.
However, we did not find in the literature studies on the intraperitoneal instillation of S75-R25 bupivacaine, making this a pioneer study.
In this study, the intra-abdominal pain scores at rest upon regaining consciousness, 2, 4, 8, and 24 hours after the surgery did not show statistically significant differences. In a controlled study using the intraperitoneal instillation of bupivacaine in patients who underwent gynecological laparoscopy, the comparison of the pain scores between the group that received this anesthetic and the control group did not show a statistically significant difference 4 and 24 hours after the surgery 18. Another study, in which 0.5% bupivacaine was administered to patients undergoing laparoscopic cholecystectomy, did not demonstrate significant differences in pain scores between the study and control groups 1, 2, 4, and 14 hours after the procedure 4.
In the present study, this difference occurred 12 hours after the surgical procedure. At 24 hours, the difference was close to the statistic significance (p = 0.0942). One study using intraperitoneal bupivacaine for laparoscopic cholecystectomy demonstrated that the pain was more severe at every evaluation in the first 24 postoperative hours in the placebo group and that, after the first eight hours, there was a statistically significant difference between both groups 19. On the other hand, a placebo-controlled study using intraperitoneal bupivacaine in patients undergoing laparoscopic cholecystectomy demonstrated that the pain was reduced significantly in the patients who received bupivacaine only in the first 6 postoperative hours 20.
However, one could observe at each moment that the mean intra-abdominal pain scores in GI were always lower than in GII.
Intra-abdominal pain scores upon sitting up and during the Valsalva maneuver were not significantly different in both groups at any moment (M2, M4, M8, M12, and M24).
In this study, the prevalence of shoulder pain was almost 3 times greater in GII than in GI (30.77% versus 10.53%), but this difference was not statistically significant. A placebo-controlled study of intra-peritoneal instillation of 80 mL of 0.125% bupivacaine in patients undergoing laparoscopic cholecystectomy demonstrated a 30% incidence of shoulder pain, without statistically significant differences between groups 21.
The length of time (in minutes) until the first request for tramadol showed no statistically significant differences between both groups, but one could observe that in GI this request occurred an average of more than 1 hour (61.48 minutes) after the first request in GII (92.63 minutes versus 31.15 minutes).
Some authors demonstrated that the length of time until the first dose of analgesic did not decrease after the surgical intervention in patients undergoing laparoscopic cholecystectomy who received intraperitoneal bupivacaine 22. In another study, with the same type of surgery, 200 mg of lidocaine diluted in 200 mL of solution instilled intraperitoneally, in the right diaphragmatic surface, increased the length of time until the first request for analgesics from 25 to 105 minutes 23.
The difference between the mean total tramadol consumption between both groups during the 24 hours of hospitalization was not statistically significant, although it was smaller in GI (94.73 mg) than in GII (130.76 mg).
One study demonstrated that the postoperative request for analgesics by patients who did not receive local anesthesia was significantly higher than by patients who received 0.5% bupivacaine in the subcutaneous tissue, fascia, and parietal peritoneum. In this last group, the consumption of analgesics was greater in patients whose infiltration was done before the insertion of the trocar when compared with the groups who received bupivacaine after the surgery 24.
On the other hand, in another placebo-controlled study with patients who received intraperitoneal bupivacaine, the total morphine consumption for postoperative analgesia was not significantly different between the study groups 25.
Other authors demonstrated that, in patients undergoing laparoscopic cholecystectomy, the cumulative consumption of metamizol was smaller in the group that received 30 mL of 0.25% bupivacaine than in the other two groups who received the same amount of normal saline or 0.25% bupivacaine associated with morphine 26. In the current study, the cumulative consumption of tramadol was not significantly different between GI and GII.
The toxicity of local anesthetics is a serious problem, which limits its dose and efficacy. Bupivacaine is used traditionally due to its long action; however, it has cardiovascular and CNS toxicity, and accidental death and cardiac arrest have been reported 27. Plasma concentrations between 0.92 and 1.14 µg.mL-1 achieved after the intraperitoneal instillation of 100 to 150 mg of bupivacaine are below the toxic concentration of 3 µg.mL-1. Similar systemic concentrations produced neurological symptoms, such as paresthesia and tingling sensation in non-anesthetized volunteers during intravenous infusion of bupivacaine. However, it remains unknown whether these concentrations produce effective postoperative analgesia 10.
Levobupivacaine, the S(-) enantiomer of racemic bupivacaine, has reduced CNS toxicity and decreased incidence of fatal arrhythmias than racemic bupivacaine in animal studies 28. In studies with human volunteers, levobupivacaine was related with a smaller reduction in the rate of infarction, acceleration index, and ejection fraction than bupivacaine 29. S75-R25 bupivacaine demonstrates a response pattern similar to that of levobupivacaine, without the cardiovascular depression induced by the racemic mixture 8.
We did not observe arrhythmias, cardiac arrest, convulsion, or other CNS or cardiovascular symptoms, and the side effects observed (nausea, vomiting, dizziness, and epigastric pain) were within the acceptable levels of incidence. It was not possible to evaluate the systemic concentrations of the S75-R25 enantiomeric excess mixture.
The results of the current study confirm those found in the literature regarding other local anesthetics. However, one can suppose that a larger study population could have provided more positive results, because we could observe the close relationship of some significant data, even though it was not statistically significant. Therefore, we suggest that similar studies should be undertaken with this new anesthetic (S75-R25 bupivacaine), including larger study populations, for more accurate results, and bupivacaine plasma concentration analysis.
We concluded that the intraperitoneal instillation of 80 mL of S75-R25 bupivacaine, at a concentration of 0.125%, provided low pain scores at rest in the bupivacaine group, which were significant only 12 hours after the surgical procedure of laparoscopic cholecystectomy.
REFERENCES
Submitted em 28 de junho de 2006
Accepted para publicação em 08 de março de 2007
- 01. Widdison AL Systematic review of the effectiveness and safety of laparoscopic cholecystectomy. Ann R Coll Surg Engl, 1996;78:476.
- 02. Labaille T, Mazoit JX, Paqueron X et al. The clinical efficacy and pharmacokinetics of intraperitoneal ropivacaine for laparoscopic cholecystectomy. Anesth Analg, 2002;94:100-105.
- 03. Ure BM, Troidl H, Spangenberger W et al. Pain after laparoscopic cholecystectomy. Intensity and localization of pain and analysis of predictors in preoperative symptoms and intraoperative events. Surg Endosc, 1994;8:90-96.
- 04. Zmora O, Stolik-Dollberg O, Bar-Zakai B et al. Intraperitoneal bupivacaine does not attenuate pain following laparoscopic cholecystectomy. J Soc Laparosc Surg, 2000;4:301-304.
- 05. Elfberg BA, Sjovall-Mjoberg S Intraperitoneal bupivacaine does not effectively reduce pain after laparoscopic cholecystectomy: a randomized, placebo-controlled and double-blind study. Surg Laparosc Endosc Percutan Tech, 2000;10:357-359.
- 06. McLeod GA, Burke D Levobupivacaine. Anaesthesia, 2001;56:331-341.
- 07. Foster RH, Markham A Levobupivacaine: a review of its pharmacology and use as a local anaesthetic. Drugs, 2000; 59:551-579.
- 08. Simonetti MPB, Ferreira FMC Does the D-isomer of bupivacaine contribute to the improvement of efficacy in neural block? Reg Anesth and Pain Med, 1999;24(suppl):43.
- 09. Macedo EP, Ferrari Jr AP Litíase Biliar, em: Prado FC, Ramos J, Valle JR Atualização Terapêutica 2003, 21Ş ed. São Paulo, Artes Médicas, 2003;477-479.
- 10. Ng A, Smith G Intraperitoneal administration of analgesia: is this practice of any utility? Br J Anaesth, 2002;89:535-537.
- 11. Alexander JI Pain after laparoscopy. Br J Anaesth, 1997; 79 :369-378.
- 12. Moiniche S, Jorgensen H, Wetterslev J et al. Local anesthetic infiltration for postoperative pain relief after laparoscopy: a qualitative and quantitative systematic review of intraperitoneal, port-site infiltration and mesosalpinx block. Anesth Analg, 2000;90:899-912.
- 13. Elhakim M, Amine H, Kamel S et al. Effects of intraperitoneal lidocaine combined with intravenous or intraperitoneal tenoxican on pain relief and bowel recovery after laparoscopic cholecystectomy. Acta Anaesthesiol Scand, 2000;44:929-933.
- 14. Schulte-Steinberg H, Weninger E, Jokisch D et al. Intraperitoneal versus interpleural morphine or bupivacaine for pain after laparoscopic cholecystectomy. Anesthesiology, 1995; 82:634-640.
- 15. Gonçalves RF, Lauretti, GR, Mattos AL Estudo comparativo entre bupivacaína a 0,5% e mistura enantiomérica de bupivacaína (S75-R25) a 0,5% em anestesia peridural. Rev Bras Anestesiol, 2003;53:169-176.
- 16. Tanaka PP, Souza RO, Salvalaggio MFO et al. Estudo comparativo entre a bupivacaína a 0,5% e a mistura enantiomérica de bupivacaína (S75-R25) a 0,5% em anestesia peridural em pacientes submetidos a cirurgia ortopédica de membros inferiores. Rev Bras Anestesiol, 2003;53:331-337.
- 17. Cortês CAF, Oliveira AS, Castro LFL et al. Estudo comparativo entre a bupivacaína a 0,5%, mistura enantiomérica de bupivacaína S75-R25 a 0,5% e ropivacaína a 0,75%, associadas ao fentanil em anestesia peridural para cesarianas. Rev Bras Anestesiol, 2003;53:177-187.
- 18. Buck L, Varras MN, Miskry T et al. Intraperitoneal bupivacaine for the reduction of postoperative pain following operative laparoscopy: a pilot study and review of the literature. J Obstet Gynaecol, 2004;24:448-451.
- 19. Mraovi B, Jurisi T, Kogler-Majeric V et al. Intraperitoneal bupivacaine for analgesia after laparoscopic cholecystectomy. Acta Anaesthesiol Scand, 1997;41:193-196.
- 20. Szem JW, Hydo L, Barie PS A double-blinded evaluation of intraperitoneal bupivacaine vs saline for the reduction of postoperative pain and nausea after laparoscopic cholecystectomy. Surg Endosc, 1996;10:44-48.
- 21. Lepner U, Goroshina J, Samarutel J Postoperative pain relief after laparoscopic cholecystectomy: a randomised prospective double-blind clinical trial. Scand J Surg, 2003;92:121-124.
- 22. Jiranantarat V, Rushatamukayanunt W, Lert-akyamanee N et al. Analgesic effect of intraperitoneal instillation of bupivacaine for postoperative laparoscopic cholecystectomy. J Med Assoc Thai, 2002;85(suppl):s897-s903.
- 23. Elhakim M, Elkott M, Ali NM et al. Intraperitoneal lidocaine for postoperative pain after aparoscopy. Acta Anaesthesiol Scand, 2000;44:280-284.
- 24. Inan A, Sen M, Dener C Local anesthesia use for laparoscopic cholecystectomy. World J Surg, 2004;28:741-744.
- 25. Fuhrer Y, Charpentier C, Boulanger G et al. Analgesie pres cholecystectomie par voie coelioscopique par administration intraperitoneale de bupivacaine. Ann Fr Anesth Reanim, 1996; 15:128-134.
- 26. Hernandez-Palazon J, Tortosa JA, Nuno de la Rosa V et al. Intraperitoneal application of bupivacaine plus morphine for pain relief after laparoscopic cholecystectomy. Eur J Anaesthesiol, 2003;20:891-896.
- 27. Albright GA Cardiac arrest following regional anesthesia with etidocaine or bupivacaine. Anesthesiology, 1979;51:285-287.
- 28. Huang YF, Pryor ME, Mather LE et al. Cardiovascular and central nervous system effects of intravenous levobupivacaine and bupivacaine in sheep. Anesth Analg, 1998;86:797-804.
- 29. Bardsley H, Gristwood R, Baker H et al. A comparison of the cardiovascular effects of levobupivacaine and rac-bupivacaine following intravenous administration to healthy volunteers. Br J Clin Pharmacol, 1998;46:245-249.
Publication Dates
-
Publication in this collection
31 July 2007 -
Date of issue
Aug 2007
History
-
Received
28 June 2006 -
Accepted
28 June 2006