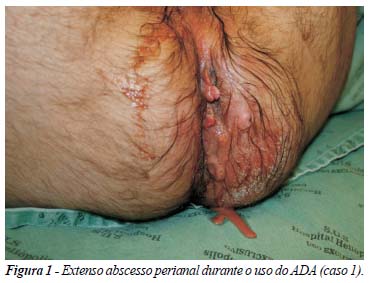
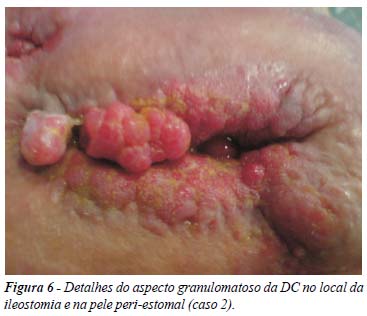
Introduction: Adalimumab (ADA) is a fully-human anti-TNF-alpha antibody approved for the management of Crohn´s disease (CD). In situations in which interruption of the treatment is mandatory, rescue therapy is controversial. Reinduction of remission with full dose may be an alternative in severe CD cases. There are no descriptions of this alternative in the literature. The aim of this study was to describe the experience with two patients treated with reinduction of remission with ADA, after interruption of treatment. Method: retrospective analysis of two patients in a cohort of 24 CD patients treated with ADA, with case reports. Results: description of two young patients with fistulizing CD, with the need of interruption of ADA therapy due to different reasons (perianal abscess and difficulties in receiving the medication). Both were treated with reinduction of remission with full dose ADA regimen (160/80 mg), with good results and no adverse events. Conclusions: there are no studies in the literature comparing reinduction of remission with other possibilities of rescue therapy after interruption of treatment with any anti-TNF agent. Small case series like this, show good results with this alternative in selected and severe cases of CD.
Crohn´s disease; Biological Therapy; Adalimumab; Tumor-necrosis-factor Alpha; Reinduction