ABSTRACT
The present study was undertaken to investigate the effects of Flammulinavelutipes stem base (FVS) on growth performance, microbial flora and volatile fatty acids of growing layers under heat stress condition. A total of 72 ISA Brown hens were randomly divided into six treatments: thermoneutral temperature control group (CON), heat stress control group (HS), heat stress antibiotic group (ANT) as positive control and heat stress FVS groups (20, 40 or 60 g/kg FVS). The experimental period had a duration of 28 d (days 84-112). On day 98, daily gain average was significantly higher (p<0.05) in the FVS groups than in the HS group. The number of bands in the FVS groups were higher (p<0.05) than in the HS group on day 98. The microbial similarity between the 60 g/kg FVS group and the HS group were the lowest on day 98. FVS group’s specific bacteria were mainly Coprococcus comes, [Clostridium] papyrosolvens, Butyricicoccuspullicaecorumon day 98. Whereas on day 112, the FVS groups specific bacteria were mainly Parabacteroides distasonis, Coprobacterfastidiosus, Elusimicrobiumminutum. The content of acetic acid and butyric acid were higher (p<0.05) in 20 g/kg FVS group than in the CON group on day 112. In conclusion, FVS can lighten the adverse effect of heat stress by increasing the diversity of intestinal flora in growing layers.
Keywords:
Laying hens; thermal stress; microbial flora; mushroomstembase; short chain fatty acids
INTRODUCTION
The condition of the gut system affects the nutrients utilization for organ development, tissue growth and immune system maturation in the host. Cecal microbial populations are the indication of gut health in animals (Mahfuz et al., 2017Mahfuz SU, Song H, Liu Z. Improved production performance and health status with winter mushroom stem (Flammulinavelutipes) in laying chicken:review. International Journal of Poultry Science 2017;16:112-117.). The cecum is a complex ecosystem of microbial colonization in poultry. Volatile fatty acids (VFA) usually is produced through bacterial fermentation in the cecum that is necessary for the intestinal function and intestinal integrity (Meimandipour et al., 2010Meimandipour A, Shuhaimi M, Soleimani AF, Azha K, Hair-Bejo M, Kabeir BM, et al. Selected microbial groups and short-chain fatty acids profile in a simulated chicken cecum supplemented with two strains of Lactobacillus. Poultry Science 2010;89:470-476.). In recent years, high-through put sequencing has been used by many researchers to investigate the gut microbial diversity in animals (Wang et al., 2017Wang Y, Sun J, Zhong H, Li N, Xu H, Zhu Q, et al. Effect of probiotics on the meat flavour and gut microbiota of chicken. Scientific Reports 2017;7:6400.). Intestinal flora has an important influence on host health (Chang et al., 2016Chang CL, Chung CY, Kuo CH, Kuo TF, Yang CW, Yang WC. Beneficial effect of Bidenspilosa on body weight gain, food conversion ratio, gut bacteria and coccidiosis in chickens. Plos One 2016;11:e146141.). The composition and activities of intestinal microflora can be altered by dietary patterns, such as feed additives (Maesschalck et al., 2015Maesschalck CD, Eeckhaut V, Maertens L, Lange LD, Marchal L, Nezer C, et al. Effects of Xylo-oligosaccharides on broiler chicken performance and microbiota. Applied & Environmental Microbiology 2015;81:5880-5888.) and antibiotics (Kalter et al., 2010Kalter HD, Gilman RH, Moulton LH, Cullotta AR, Cabrera L, Velapatino B. Risk factors for antibiotic-resistant Escherichia coli carriage in young children in Peru:community-based cross-sectional prevalence study. American Journal of Tropical Medicine and Hygiene 2010;82:879-888.; Zou et al., 2016Zou F, Zeng D, Wen B, Sun H, Zhou Y, Yang M, et al. Illumina Miseq platform analysis caecum bacterial communities of rex rabbits fed with different antibiotics. AMB Express 2016;6:100.).
Since the last few decades, antibiotic shave been used in the poultry to promote growth performance. These antibiotics products include bambermycin, avilamycin, and the flavomycin (Butaye et al., 2003Butaye P, Devriese LA, Haesebrouck F. Antimicrobial growth promoters used in animal feed:effects of less wellknown antibiotics on gram-positive bacteria. Clinical Microbiology Reviews 2003;16:175.). The product flavomycin (synonyms: moenomycin, flavophospholipol and bambermycin) is a glycolipid antibiotic produced by Streptomyces species including S. bambergiensis, S. ghanaensis, S. geysirensis, and S. ederensis (Huber et al., 1965Huber G, Schacht U, Weidenmüller HL, Schmidtthomé J, Duphorn J, Tschesche R. Moenomycin, a new antibiotic. II. Characterization and chemistry. Antimicrobial Agents & Chemotherapy 1965;5:737.; Wallhausser et al., 1965Wallhausser KH, Nesemann G, Prave P, Steigler A. Moenomycin, a new antibiotic. I. Fermentation and isolation. Antimicrobial Agents & Chemotherapy 1965;5:734.). In addition, this antibiotic as been commonly used in poultry industry to prevent infectious diseases (Osweiler et al., 2010Osweiler GD, Jagannatha S, Trampel DW, Imerman PM, Ensley SM, Yoon I, et al. Evaluation of XPC and prototypes on aflatoxin-challenged broilers. Poultry Science 2010;89:1887-1893.). However, the overuse of antibiotics may lead to antibiotic-resistant genes which spread extensively by promoting the selection of antibiotic-resistant bacteria in host animals (Zhou et al., 2016Zhou M, Zeng D, Ni X, Tu T, Yin Z, Pan K, et al. Effects of Bacillus licheniformis on the growth performance and expression of lipid metabolism-related genes in broiler chickens challenged with Clostridium perfringens-induced necrotic enteritis. Lipids in Health and Disease 2016;15:48.). In addition, the constant application of antibiotics in poultry feed with the purpose to improve production performance has led to human health hazards (Mahfuz et al., 2018Mahfuz SU, Song H, Wei J, Chen M, Zhen D, Nahar J, et al. Organic Egg Production, Egg Quality, Calcium Utilization, and Digestibility in Laying Hens Fed with Mushroom (Flammulinavelutipes) Stem Waste. Brazilian Journal of Poultry Science 2018;20:717-724.). Therefore, it was urgent to find antibiotic substitutes, optimize the composition of intestinal flora, and promote the health of the birds.
Heat stress caused by extreme hot weather was a very common issue in poultry breeding. Heat stress can damage the intestinal micro ecological balance of poultry, may cause great economic losses by declining production performance, and even by the death of the host. At high ambient temperature (36±1°C, minimum temperature 31±1°C), it can lead to minor cracks in the duodenum, jejunum, ileum, and may lead to the rupture of the villi, and even severe absence of the villi in chicken (Quinteiro-Filho et al., 2010Quinteiro-Filho WM, Ribeiro A, Ferraz-de-Paula V, Pinheiro ML, Sakai M, Sa LR, et al. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poultry Science 2010;89:1905-1914.). Ramnath et al. (2008Ramnath V, Rekha PS, Sujatha KS. Amelioration of heat stress induced disturbances of antioxidant defense system in chicken by brahma rasayana. Evidence-based Complementary and Alternative Medicine 2008;5:77-84.) reported that heat stress (40±1°C, non-heat stressed temperature 30±1°C) affected concentrations of certain oxidative stress markers. The study of Abidin (2013Abidin Z, Khatoon A. Heat stress in poultry and the beneficial effects of ascorbic acid (vitamin C) supplementation during periods of heat stress. Worlds Poultry Science Journal 2013;69:135-152.) showed that temperature ranging from 18°C to 26°C was comfortable for broilers.
Flammulinavelutipess tem base (FVS) is known as waste material commonly found in the mushroom industry. FVS is an agricultural byproduct having nutritional and medicinal values and its availability is abundant but up to now its utilization is limited for animal production (Mahfuz et al., 2017Mahfuz SU, Song H, Liu Z. Improved production performance and health status with winter mushroom stem (Flammulinavelutipes) in laying chicken:review. International Journal of Poultry Science 2017;16:112-117.). Flammulinavelutipes is a saprophytic fungus. The optimum temperature for its growth is 22-25°C. Flammulinavelutipes is widely distributed in nature, including China, Japan, Russia, Europe, North America and Australia (Yoshihama et al., 1994Yoshihama Y, Kusakabe K, Matsui S, Morita H, inventor. Biologically pure mushroom culture and method for mushroom cultivation. United States Patent 5349121; 1994.). Flammulinavelutipes contain dietary fiber, are low in calories, have a high content of protein consisting of all the essential amino acids, minerals and vitamins and are free of cholesterol (Karaman et al., 2010Karaman M, Jovin E, Malbasa R, Matavuly M, Popovic M. Medicinal and edible lignicolous fungi as natural sources of antioxidative and antibacterial agents. Phytotherapy Research 2010;24:1473-1481.). It has been highly valued as a functional food for its good antioxidant, anti-inflammatory, immunomodulatory, anti-tumour, and cholesterol-lowering effects (Wu et al., 2014Wu M, Luo X, Xu X, Wei W, Yu M, Jiang N, et al. Antioxidant and immunomodulatory activities of a polysaccharide from Flammulinavelutipes. Journal of Traditional Chinese Medicine 2014;34:733-740.; Yan et al., 2014Yan ZF, Liu NX, Mao XX, Li Y, Li CT. Activation effects of polysaccharides of Flammulinavelutipes mycorrhizae on the Tlymphocyte immune function. Journal of Immunology Research 2014;2014:285421.; Chen et al., 2015Chen P, Yong Y, Gu Y, Wang Z, Zhang S, Lu L. Comparison of antioxidant and antiproliferation activities of polysaccharides from eight species of medicinal mushrooms. International Journal of Medicinal Mushrooms 2015;17:287-295.; Xia, 2015Xia Z. Preparation of the oligosaccharides derived from Flammulinavelutipes and their antioxidant activities. Carbohydrate Polymers 2015;118:41-43.). Laying hens fed with FVS (3-5%) could reduce the pathogenic bacteria like Salmonella spp., E. coli and Clostridium spp., as well as increase the number of beneficial bacteria of Lactobacillus spp., and Bifidobacterium spp.(Lee et al., 2012Lee S, Choi Y, Cho S, Shin T, Cho B, Kang H, et al. Effects of dietary Flammulinavelutipesmycelium on broiler chick performance, pathogenic bacterial counts in caecal contents and amount of NH3 in excreta. Journal of Animal Science & Technology 2012;54:341-347.; Lee et al., 2014). However, there was no previous study on the effect of Flammulinavelutipess tem base on animals submitted to heat stress.
This study examined the effect of FVS on growth performance, caecal microflora and VFA concentration in ISA Brown growing layers under heat stress condition.
MATERIALS AND METHODS
Test materials, experimental condition and feeding management
Experimental chickens (ISA Brown) were purchased from Changchun Octavia Farms and FVS was collected from the local domestic mushroom farm in Changchun City, Jilin, China.
The experiment was carried out at the Animal Unit, College of Chinese Medicine Materials, Jilin Agricultural University, and all the procedures were approved by the animal care and use committee of Jilin Agricultural University. A total of 72 hens, aged 84d derived from ISA Brown strain were divided into 6 groups, with 3 replications having 4 chickens each. The birds were housed into a wire cage (100cm, 60cm, 50cm, length, width, height) and an average homogeneous not significant body weight (1192±15.32 g; Table 2) was considered for each replication. Dietary treatment included thermoneutral temperature control group (CON, basal diet, 28±1°C), heat stress control group (HS, basal diet, 38±1°C), heat stress antibiotic group (ANT, basal diet supplemented with 5 mg/kg flavomycin, 38±1°C) as positive control and heat stress FVS group (basal diet supplemented with 20, 40, and 60 g/kg FVS, 38±1°C). Heat stress was not constant throughout the experimental period. During the experimental period, room temperature was maintained at 38±1°C from 8:00-18:00, after the heat stress time, the room temperature was 28±1°C, until the next morning. The incandescent lamps with room heater were used to maintain the heat stress and the spray method was used to control the relative humidity at 50%-60%. A wet and dry bulb thermometer were used to record the temperature and humidity throughout the experimental period. The trial lasted for 28 days from day 84 to day 112. All procedures were applied for heat stress, only. Feed and water were provided ad libitum throughout the whole period. Mushroom and antibiotics were mixed with the growing layers diet formulated according to NRC (1994) specification in Feed Mill (Jilin Hanghong Animal Husbandry Co. Ltd, China). The analyzed nutritional composition of the experimental diet and FVS are presented in Table 1.
Sample collection
Mushroom stem was harvested from a domestic mushroom farm at Changchun city, and sun dried properly. Then the sub sample was grinded and was prepared (0.01mm) for proximate component analysis. Feed sample and FVS were analyzed (n=6) following the method of AOAC (2000). Dry matter, ether extract, crude fiber, and total ash were analyzed according to the procedures of AOAC (2000). Nitrogen was determined using an FP528 nitrogen determinator (LECO Corporation, Joseph, MI, USA).The analyzed results were presented in Table 1. Analyzed compositions of Flammulinavelutipes mushroom stem were dry matter=88.50±0.80g/kg, crude protein=13.55±0.42g/kg, crude fiber=21.05±0.11g/kg, ether extract=2.3±0.014g/kg, Ash=11.4±0.085g/kg, calcium=4.0±0.1 g/kg and phosphorus=6.2±0.28g/kg. A total of 36 birds (two from each replicate, n=6) were slaughtered on day 98 and day 112 to collect cecum and samples were kept in a freezer(-80°C) for further analysis.
Growth performance
Body weight and feed intake per pen were recorded from day 84 to day 98 and day98 to 112 and used to calculate average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (FCR).
Analysis of the cecal digesta microbial flora
Cecal chime were taken to extract total bacterial DNA by using AxyPrep genomic DNA mini kit following the instructions of the manufacture Co. The 16SrDNA V3 region of the total bacterial DNA was amplified by PCR. JY-TD331A PCR-DGGE (denaturing gradient gel electrophoresis) and Vilber gel scanning imaging system were used for DGGE test. The common and specific bands in the DGGE map were recovered by gel cutting and amplified where DGGE electrophoresis was carried out according to the above PCR method to confirm the correctness of the retrieved target fragments. DNA was amplified and purified by universal primers (without GC clamps). The PCR product of the target template was connected by a carrier linked conversion kit (pMD18-TVector). The competent cell of E. coli DH5 was transferred into the junction product coated agar plates that contained amplicillin. Cloning sequencing was completed by Shanghai Biological Engineering Co. ltd. Chromas software was used to analyze and sort out the sequence and then compared the sequence of close relatives with the GenBank database.
Determination of VFA content in the cecal digesta
The experimental conditions were as follows; the chromatographic column was DB-FFAP capillary column (30m×250 µm×0.25µm); the inlet temperature of 220°C; FID detector temperature of 250°C ; column temperature program: initial temperature of 65°C, and then to 20°C/min to 190°C. Split ratio 25: 1; gas flow rate; N2 25 mL/min; hydrogen H2 40 mL/min; air 400 mL/min. Agilent gas chromatograph 7890A was used to detect the concentration of acetic acid, propionic acid and butyric acid in the cecal digesta.
Statistical analysis
The experimental data were processed by Microsoft Excel, and then subjected to one-way analysis of variance using SPSS software. Multiple comparisons were performed using Tukey-kramer’s test. Cluster analysis was performed using Quantity one. p<0.05 indicates significant difference.
RESULTS
Effect of FVS on growth performance
The effects of FVS on growth performance growing layers were presented in Table 2. On day 98, the ADG in the FVS groups were higher (p<0.05) than that of the HS group. On day112, the ADG was higher (p<0.05) in the 60 g/kg FVS group than in the HS group. The ADG in the FVS groups had no significant difference with the CON group and ANT group in the whole period of the test. On day 98, the ADFI was higher (p<0.05) both in the 20 g/kg FVS group and 40 g/kg FVS group than in the HS group. On day 112, the ADFI was lower (p<0.05) in the FVS groups than in the CON group. There was no significant difference in FCR among groups throughout the test period.
Effect of FVS on the microbial diversity
The number of microbial flora bands was shown in Table 3. On day98, the band number was higher (p<0.05) in the FVS groups than in the HS group, and there was no significant difference in the number of bands both in the FVS groups with the CON group. The number of the bands in the 20 g/kg FVS groups and 60 g/kg had no significant difference with the ANT group. On day 112, there was no significant difference among the groups.
The results of sequence alignment of bands in DGGE maps (Fig. 1) are shown in Table 4. Under heat stress on day 98, the specific bacteria in the CON group were Ruminococcuslactaris, Alistipes senegalensis (1,3). The specific bacteria in the HS group were Helicobacter pullorum, Stomatobaculumlongum (5, 7). The specific bacteria in the FVS groups were Selenomonasruminantium strain, Coprococcus comes, Intestinimonasbutyriciproducens strain, Merdimonasfaecisstrain, [Clostridium] papyrosolvens, Butyricicoccuspullicaecorum, Terasakiellapusilla (16, 18, 22, 23, 28, 29, 32, 33, 34). All the groups contain bacteria Bacteroides uniformis (4, 13, 24, 26) except HS group. The ANT group and the FVS groups contain the bacteria Alistipesinops strain, [Clostridium] termitidis (12, 19, 27).
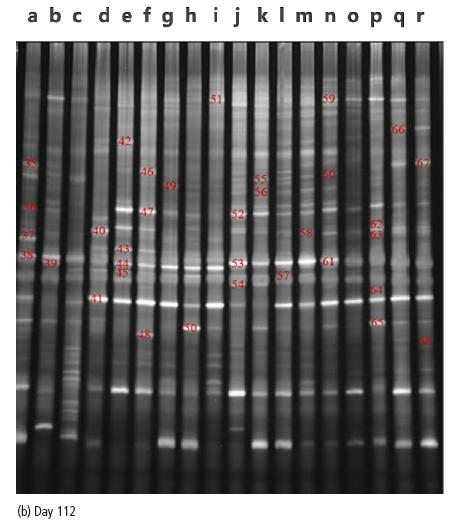
Under heat stress on day 112, the specific bacteria in the CON group were Olivibactersitiensis and Leeuwenhoekiella sp. (36, 37). The specific bacteria in the HS group were Prevotelladentasini and Alistipesfinegoldii (42, 43). The specific bacteria in the FVS groups were Odoribactersplanchnicus, Parabacteroides distasonis, Rikenellamicrofusus, Tyzzerellanexilis, Coprobacterfastidiosus, Bacteroides ovatus strain and Elusimicrobiumminutum (54, 58, 60, 63, 64, 66, 67). All the groups contain bacteria Arenibacteralgicola strain and Bacteroides coprocola (39, 50, 53, 56, 59, 61, 65) except the HS group. All the groups contain bacteria of Bacteroides stercoris (44, 46, 52) except the CON group. The ANT group and the FVS groups contain the bands of Ruminococcusbicirculans and Oscillibacter sp. (49, 57).
Effect of FVS on the microbial similarity
As shown in Fig. 2 (a), on day 98, the highest similarity between the 20 g/kg FVS group and the ANT group was 70.47%. The lowest similarity between the 60 g/kg FVS group and the HS group was 55.13%. Among the heat stress groups, the highest similarity was in the 40 g/kg FVS group with the CON group. As shown in Fig. 2 (b), on day 112, the highest similarity between the 20 g/kg FVS group and the ANT group was 62.58%. Among the heat stress groups, the ANT group had the highest results, similar to the CON group and followed by the 60 g/kg FVS group.
Effects of FVS on VFA content
As shown in Table 5, on day 98, the content of VFA in the FVS groups was lower(p<0.05) than in the HS group. The content of acetic acid and butyric acid in the 20 g/kg FVS group and the 60 g/kg FVS group had no significant differences with the CON group on day 98. There were no significant differences in the content of propionic acid in the FVS groups with the CON group on day 98. On day 112, the content of acetic acid and butyric acid in the 40 g/kg FVS group and the 60 g/kg FVS group had no significant difference with the CON group and the HS group respectively. The content of VFA in the 20 g/kg FVS group was higher (p<0.05) than in the CON group.
DISCUSSION
Effect of FVS on growth performance
Heat stress is a common problem, specially in most of the tropical countries. Heat stress is a hazard to commercial poultry production in most areas of China, especially in the summer season. This study highlighted that FVS could alleviate the effect of heat stress on the growth performance in laying hens. This may be related to FVS rich in crude fiber speeding up the intestinal peristalsis of the chicken under heat stress and promoting digestion and absorption. He et al. (2014) reported that heat stress could reduce the egg weight, growth performance, digestive enzyme activities, beneficial bacteria and increase harmful bacteria in the cecum of chicken. Similarly, this study found that found that heat stress can lead to the decline of growth performance of growing layers. The study of Ai (2008Ai Q. Effect of early age thermal acclimation on adaptability acquisition in broilers during heat exposure. Beijing: Chinese Academy of Agricultural Sciences; 2008.) showed that the early heat acclimatization to poultry could effectively alleviate the decline in production performance at the later stage of growth. On day 112, there were no significant differences in the ADG, ADFI and FCR between the HS group and CON group, which may be the result of heat stress adaptation in laying hens. Garriga et al. (2006Garriga C, Hunter RR, Amat C, Planas JM, Mitchell MA, Moreto M. Heat stress increases apical glucose transport in the chicken jejunum. American Journal of Physiology Regulatory Integrative & Comparative Physiology 2006;290:R195-R201.) showed that stress can cause intestinal villus injury on the intestinal mucosa associated with poor nutrient absorption. This may be the reason for the decrease of ADG in the HS group.
Effect of FVS on the microbial diversity
This study found that FVS could alleviate the effect of heat stress in the intestinal flora of growing layers, increase the diversity of intestinal flora, mainly beneficial bacteria and reduce the colonization of intestinal harmful bacteria. These findings were similar to Zeng et al. (2016Zeng QL, Song H, Guo HH, Guo C, Zhen D, Liang F, Zhang TY. Effect of supplementation of Flammulinavelutipesstembaseon the caecal microflora, short chain fatty acid of broiler. Journal of China Agriculture University 2016;21:104-114.), who reported that the diversity of cecal microflora in broilers was significantly higher in FVS supplemented diets than in the control. Some past studies found that FVS could increase the beneficial bacteria and could decrease the harmful bacteria (Lee et al., 2012Lee S, Choi Y, Cho S, Shin T, Cho B, Kang H, et al. Effects of dietary Flammulinavelutipesmycelium on broiler chick performance, pathogenic bacterial counts in caecal contents and amount of NH3 in excreta. Journal of Animal Science & Technology 2012;54:341-347.; Lee et al., 2014). In this study, the FVS groups did not contain the pathogenic species Helicobacter pullorum (Steinbrueckner et al., 1997Steinbrueckner B, Haerter G, Pelz K, Weiner S, Rump JA, Deissler W, et al. Isolation of Helicobacter pullorum from patients with enteritis. Scandinavian Journal of Infectious Diseases 1997;29:315-318.) and Alistipesfinegoldii (Dziarski et al.,2016Dziarski R, Park SY, Kashyap DR, Dowd SE, Gupta D. Pglyrp-regulated gut microflora Prevotella falsenii, Parabacteroides distasonis and Bacteroides eggerthii enhance and Alistipes finegoldii attenuates colitis in mice. Plos One 2016;11:e146162.) which were found in the HS group only. This indicated that FVS can optimize the composition of the intestinal flora and inhibit harmful bacteria. On day 98, Bacteroides uniformis was found in the CON group, ANT group and FVS groups. However, Bacteroides uniformis was not found in the HS group. Bacteroides uniformis has an important role in the breakdown of complex polysaccharides, starch and cellulose to simple ingredients (Lan et al., 2006Lan PT, Sakamoto M, Sakata S, Benno Y. Bacteroides barnesiaesp.nov., Bacteroides salanitronissp. nov. and Bacteroides gallinarum sp. nov., isolated from chicken caecum. International Journal of Systematic and Evolutionary Microbiology 2006;56:2853-2859.), and then promote digestion and absorption of the intestinal tract.
Effect of FVS on the microbial similarity
This study found that FVS could alleviate the effect of heat stress on the similarity of intestinal flora, and the effect was similar to that of antibiotics. This study showed that the highest similarity in results were between the 20 g/kg FVS group and the ANT group. This ensured that the role of the 20 g/kg FVS group and the ANT group were the most similar to the composition of the intestinal flora. On day 98, the lowest similarity was found between the 60 g/kg FVS group and the HS group. The 40 g/kg FVS group was the highest similarity among the heat stress groups with the CON group. These indicated that feeding FVS may alleviate the effect of heat stress on the composition of the intestinal flora. On day 112, among the heat stress groups, the ANT group was the highest similar to the CON group and followed by the 60 g/kg FVS group. This showed that feeding high doses of FVS could alleviate the effect of heat stress on the composition of the intestinal flora. These results were similar to the study by Guo et al. (2004Guo FC, Kwakkel RP, Williams BA, Parmentier HK, Li WK, Yang ZQ, et al. Effects of mushroom and herb polysaccharides on cellular and humoral immune responses of Eimeria tenella-infected chickens. Poultry Science 2004;83:1124-32.), who reported that the cecal viscosity and microbial populations were significantly improved by feeding mushroom extracts.
Effects of FVS on VFA content
VFA and other organic acids (such as lactic acid and succinic acid) are the key metabolites of carbohydrate fermentation in the large intestine. Previous studies have shown that acetate and propionate have a good therapeutic effect on colitis (Tedelind et al., 2007Tedelind S, Westberg F, Kjerrulf M, Vidal A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate:A study with relevance to inflammatory bowel disease. World Journal of Gastroenterology 2007;13:2826-2832.). Van Der Wielen et al. (2000Wielen PW, Biesterveld S, Notermans S, Hofstra H, Urlings BA, van Knapen F. Role of volatile fatty acids in development of the cecal microflora in broiler chickens during growth. Applied & Environmental Microbiology 2000;66:2536-2540.) stated that VFA are responsible for the reduction of Enterobacteriaceae in the ceca of broiler chickens during growth. Among the VFA, butyric acid stands out as a preferred energy source for enterocytes and takes part in cellular differentiation and proliferation within the intestinal mucosa (Rinttilä et al., 2013Rinttilä T, Apajalahti J. Intestinal microbiota and metabolites-Implications for broiler chicken health and performance1. Journal of Applied Poultry Research 2013;22:647-658.). In addition, the butyrogenic effect of different prebiotics in the broiler cecum has been previously reported (Rehman et al., 2008Rehman H, Bohm J, Zentek J. Effects of differentially fermentable carbohydrates on the microbial fermentation profile of the gastrointestinal tract of broilers. Journal of Animal Physiology & Animal Nutrition 2008;92:471-480.). This study found that FVS could alleviate the effect of heat stress on the content of VFA in the intestine. This may be due to the fact that the FVS groups contained specific bacterial flora that were different from the other groups and resulted in changes in the VFA content. The concentration of VFA was related to the composition of feed, number and type of anaerobic bacteria (Rehman et al., 2007). The FVS groups contained a lot of beneficial bacteria that could increase the VFA concentration directly and indirectly. Coprococcus comes produces butyric acid primarily. Intestinimonasbutyriciproducens strain is mainly responsible for the production of acetic acid and butyric acid. Alistipesinops strain mainly produces acetic acid and succinic acid. Merdimonasfaecis strain can ferment glucose to produce acetic acid. [Clostridium] Papyrosolvens mainly produces volatile fatty acids, acetic acid, propionic acid, and butyric acid. Butyricicoccuspullicaecorum belongs to the genus Clostridium and has the potential of probiotics whose metabolites are acetic acid and butyric acid.
CONCLUSIONS
FVS could alleviate the effect of heat stress on growth performance, intestinal flora and VFA in growing layers. In conclusion, this study provides a new way to find environmentally friendly alternatives to antibiotics, by the utilization of edible and medicinal fungi wastes, which has great significance for alleviating heat stress in poultry production. In order to achieve better performance and sound gut health, FVS may be considered an alternative potential feed supplement for growing layers under heat stress condition.
ACKNOWLEDGEMENTS
This work was supported by “Innovation Platform for Economic Fungi in Jilin Province” [Grant No. 2014-2016] Changchun, P.R. China.
REFERENCES
- Abidin Z, Khatoon A. Heat stress in poultry and the beneficial effects of ascorbic acid (vitamin C) supplementation during periods of heat stress. Worlds Poultry Science Journal 2013;69:135-152.
- Ai Q. Effect of early age thermal acclimation on adaptability acquisition in broilers during heat exposure. Beijing: Chinese Academy of Agricultural Sciences; 2008.
- AOAC - Association of Official Analytical Chemistry. Official methods o fanalysis of AOAC international. 17th ed. Maryland; 2000.
- Butaye P, Devriese LA, Haesebrouck F. Antimicrobial growth promoters used in animal feed:effects of less wellknown antibiotics on gram-positive bacteria. Clinical Microbiology Reviews 2003;16:175.
- Chang CL, Chung CY, Kuo CH, Kuo TF, Yang CW, Yang WC. Beneficial effect of Bidenspilosa on body weight gain, food conversion ratio, gut bacteria and coccidiosis in chickens. Plos One 2016;11:e146141.
- Chen P, Yong Y, Gu Y, Wang Z, Zhang S, Lu L. Comparison of antioxidant and antiproliferation activities of polysaccharides from eight species of medicinal mushrooms. International Journal of Medicinal Mushrooms 2015;17:287-295.
- Dziarski R, Park SY, Kashyap DR, Dowd SE, Gupta D. Pglyrp-regulated gut microflora Prevotella falsenii, Parabacteroides distasonis and Bacteroides eggerthii enhance and Alistipes finegoldii attenuates colitis in mice. Plos One 2016;11:e146162.
- Guo FC, Kwakkel RP, Williams BA, Li WK, Li HS, Luo JY, Li XP, et al. Effects of mushroom and herb polysaccharides, as alternatives for an antibiotic, on growth performance of broilers. British Poultry Science 2004;45:684-694.
- Guo FC, Kwakkel RP, Williams BA, Parmentier HK, Li WK, Yang ZQ, et al. Effects of mushroom and herb polysaccharides on cellular and humoral immune responses of Eimeria tenella-infected chickens. Poultry Science 2004;83:1124-32.
- Garriga C, Hunter RR, Amat C, Planas JM, Mitchell MA, Moreto M. Heat stress increases apical glucose transport in the chicken jejunum. American Journal of Physiology Regulatory Integrative & Comparative Physiology 2006;290:R195-R201.
- He SJ, Zhao SJ, Li J, Che CY, Dai SF, Liu DY. Effect of betaine on growth performance activities of duodenum digestive enzymes and cecal microflora of heat-stressed broilers. Chinese Journal of Animal Nutrition 2014;3731-3739.
- Huber G, Schacht U, Weidenmüller HL, Schmidtthomé J, Duphorn J, Tschesche R. Moenomycin, a new antibiotic. II. Characterization and chemistry. Antimicrobial Agents & Chemotherapy 1965;5:737.
- Kalter HD, Gilman RH, Moulton LH, Cullotta AR, Cabrera L, Velapatino B. Risk factors for antibiotic-resistant Escherichia coli carriage in young children in Peru:community-based cross-sectional prevalence study. American Journal of Tropical Medicine and Hygiene 2010;82:879-888.
- Karaman M, Jovin E, Malbasa R, Matavuly M, Popovic M. Medicinal and edible lignicolous fungi as natural sources of antioxidative and antibacterial agents. Phytotherapy Research 2010;24:1473-1481.
- Lan PT, Sakamoto M, Sakata S, Benno Y. Bacteroides barnesiaesp.nov., Bacteroides salanitronissp. nov. and Bacteroides gallinarum sp. nov., isolated from chicken caecum. International Journal of Systematic and Evolutionary Microbiology 2006;56:2853-2859.
- Lee S, Choi Y, Cho S, Shin T, Cho B, Kang H, et al. Effects of dietary Flammulinavelutipesmycelium on broiler chick performance, pathogenic bacterial counts in caecal contents and amount of NH3 in excreta. Journal of Animal Science & Technology 2012;54:341-347.
- Lee SB, Im J, Kim SK, Kim YC, Kim MJ, Lee JS, et al. Effects of dietary fermented Flammulinavelutipesmycelium on performance and egg quality in laying hens. International Journal of Poultry Science 2014;13:637-644.
- Maesschalck CD, Eeckhaut V, Maertens L, Lange LD, Marchal L, Nezer C, et al. Effects of Xylo-oligosaccharides on broiler chicken performance and microbiota. Applied & Environmental Microbiology 2015;81:5880-5888.
- Mahfuz SU, Song H, Liu Z. Improved production performance and health status with winter mushroom stem (Flammulinavelutipes) in laying chicken:review. International Journal of Poultry Science 2017;16:112-117.
- Mahfuz SU, Song H, Wei J, Chen M, Zhen D, Nahar J, et al. Organic Egg Production, Egg Quality, Calcium Utilization, and Digestibility in Laying Hens Fed with Mushroom (Flammulinavelutipes) Stem Waste. Brazilian Journal of Poultry Science 2018;20:717-724.
- Meimandipour A, Shuhaimi M, Soleimani AF, Azha K, Hair-Bejo M, Kabeir BM, et al. Selected microbial groups and short-chain fatty acids profile in a simulated chicken cecum supplemented with two strains of Lactobacillus. Poultry Science 2010;89:470-476.
- NRC- National Research Council. Nutrient requirements of poultry. 9th ed. Washington: National Academy Press;1994.
- Osweiler GD, Jagannatha S, Trampel DW, Imerman PM, Ensley SM, Yoon I, et al. Evaluation of XPC and prototypes on aflatoxin-challenged broilers. Poultry Science 2010;89:1887-1893.
- Quinteiro-Filho WM, Ribeiro A, Ferraz-de-Paula V, Pinheiro ML, Sakai M, Sa LR, et al. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poultry Science 2010;89:1905-1914.
- Ramnath V, Rekha PS, Sujatha KS. Amelioration of heat stress induced disturbances of antioxidant defense system in chicken by brahma rasayana. Evidence-based Complementary and Alternative Medicine 2008;5:77-84.
- Rehman H, Bohm J, Zentek J. Effects of differentially fermentable carbohydrates on the microbial fermentation profile of the gastrointestinal tract of broilers. Journal of Animal Physiology & Animal Nutrition 2008;92:471-480.
- Rehman HU, Vahjen W, Awad WA, Zentek J. Indigenous bacteria and bacterial metabolic products in the gastrointestinal tract of broiler chickens. Archives of Animal Nutrition 2007;61:319-335.
- Rinttilä T, Apajalahti J. Intestinal microbiota and metabolites-Implications for broiler chicken health and performance1. Journal of Applied Poultry Research 2013;22:647-658.
- Steinbrueckner B, Haerter G, Pelz K, Weiner S, Rump JA, Deissler W, et al. Isolation of Helicobacter pullorum from patients with enteritis. Scandinavian Journal of Infectious Diseases 1997;29:315-318.
- Tedelind S, Westberg F, Kjerrulf M, Vidal A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate:A study with relevance to inflammatory bowel disease. World Journal of Gastroenterology 2007;13:2826-2832.
- Wielen PW, Biesterveld S, Notermans S, Hofstra H, Urlings BA, van Knapen F. Role of volatile fatty acids in development of the cecal microflora in broiler chickens during growth. Applied & Environmental Microbiology 2000;66:2536-2540.
- Wallhausser KH, Nesemann G, Prave P, Steigler A. Moenomycin, a new antibiotic. I. Fermentation and isolation. Antimicrobial Agents & Chemotherapy 1965;5:734.
- Wang Y, Sun J, Zhong H, Li N, Xu H, Zhu Q, et al. Effect of probiotics on the meat flavour and gut microbiota of chicken. Scientific Reports 2017;7:6400.
- Wu M, Luo X, Xu X, Wei W, Yu M, Jiang N, et al. Antioxidant and immunomodulatory activities of a polysaccharide from Flammulinavelutipes. Journal of Traditional Chinese Medicine 2014;34:733-740.
- Xia Z. Preparation of the oligosaccharides derived from Flammulinavelutipes and their antioxidant activities. Carbohydrate Polymers 2015;118:41-43.
- Yan ZF, Liu NX, Mao XX, Li Y, Li CT. Activation effects of polysaccharides of Flammulinavelutipes mycorrhizae on the Tlymphocyte immune function. Journal of Immunology Research 2014;2014:285421.
- Yoshihama Y, Kusakabe K, Matsui S, Morita H, inventor. Biologically pure mushroom culture and method for mushroom cultivation. United States Patent 5349121; 1994.
- Zeng QL, Song H, Guo HH, Guo C, Zhen D, Liang F, Zhang TY. Effect of supplementation of Flammulinavelutipesstembaseon the caecal microflora, short chain fatty acid of broiler. Journal of China Agriculture University 2016;21:104-114.
- Zhou M, Zeng D, Ni X, Tu T, Yin Z, Pan K, et al. Effects of Bacillus licheniformis on the growth performance and expression of lipid metabolism-related genes in broiler chickens challenged with Clostridium perfringens-induced necrotic enteritis. Lipids in Health and Disease 2016;15:48.
- Zou F, Zeng D, Wen B, Sun H, Zhou Y, Yang M, et al. Illumina Miseq platform analysis caecum bacterial communities of rex rabbits fed with different antibiotics. AMB Express 2016;6:100.
Publication Dates
-
Publication in this collection
20 Dec 2019 -
Date of issue
2019
History
-
Received
24 May 2019 -
Accepted
18 Oct 2019


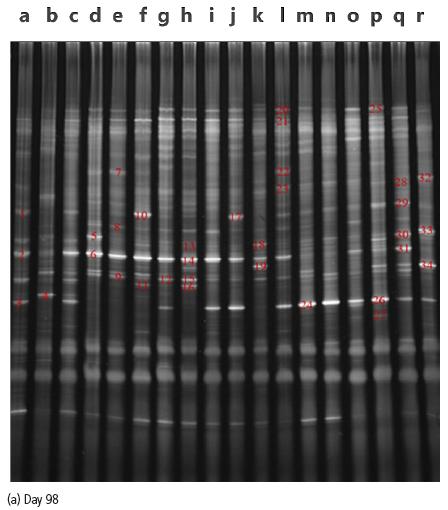 1CON=thermoneutraltemperature control group (basal diet,28±1°C), HS=heat stress control group (basal diet, 38±1°C), ANT=antibiotic group (basal diet supplemented with 5 mg/kg flavomycin, 38±1°C ), FVS=FVS group (basal diet supplemented with 20, 40 or 60 g/kg FVS, 38±1°C).2The number presented inside the figure is the stripe number.3a-c=CON group, d-f=HS group, g-i=ANT group, j-l=20 g/kgFVS group, m-o=40 g/kg FVS group, p-r=60 g/kg FVS group.
1CON=thermoneutraltemperature control group (basal diet,28±1°C), HS=heat stress control group (basal diet, 38±1°C), ANT=antibiotic group (basal diet supplemented with 5 mg/kg flavomycin, 38±1°C ), FVS=FVS group (basal diet supplemented with 20, 40 or 60 g/kg FVS, 38±1°C).2The number presented inside the figure is the stripe number.3a-c=CON group, d-f=HS group, g-i=ANT group, j-l=20 g/kgFVS group, m-o=40 g/kg FVS group, p-r=60 g/kg FVS group.
 1CON=thermoneutral temperature control group (basal diet,28±1°C), HS=heat stress control group (basal diet, 38±1°C), ANT=antibiotic group (basal diet supplemented with 5 mg/kg flavomycin, 38±1°C), FVS=FVS group (basal diet supplemented with 20, 40 or 60 g/kg FVS, 38±1 °C).2#1-#3=CON group, #4-#6=HS group, #7-#9=ANT group, #10-#12=20 g/kg FVS group, #13-#15=40 g/kg FVS group, #16-#18=60 g/kg FVS group.
1CON=thermoneutral temperature control group (basal diet,28±1°C), HS=heat stress control group (basal diet, 38±1°C), ANT=antibiotic group (basal diet supplemented with 5 mg/kg flavomycin, 38±1°C), FVS=FVS group (basal diet supplemented with 20, 40 or 60 g/kg FVS, 38±1 °C).2#1-#3=CON group, #4-#6=HS group, #7-#9=ANT group, #10-#12=20 g/kg FVS group, #13-#15=40 g/kg FVS group, #16-#18=60 g/kg FVS group.
