Abstract
Seventy-eight chickens from a very high poultry density (approximately eight million) region and twelve backyard chickens from neighboring areas were analyzed by histopathology and additional techniques for the presence of the infectious laryngotracheitis virus. The virus distribution was determined in different tissues using immunohistochemistry (IHC) and polymerase chain reaction (PCR). The disease was histopathologically diagnosed in 41.0% (32/78) of the commercial layers. Lesions were mainly characterized by syncytial cells with eosinophilic intranuclear inclusion body formed from the hyperplastic epithelium of the upper respiratory tract, primary and secondary bronchi, and conjunctiva. IHC showed 70% (21/30) positive signal in the larynx/trachea and, 53.8% (14/26) in the lungs, either in epithelial cells or syncytia. In the turbinates and paranasal sinuses, 29.6% (8/27) of samples showed positive signal. PCR detected the following gallid herpesvirus 1-positive percentages: conjunctiva 63.2% (31/49), lungs 57.6% (30/52), turbinates and paranasal sinuses 56% (28/50), and larynx/trachea 50% (39/78). IHC showed to be a useful additional tool for definitive ILT diagnosis, especially during the subacute phase of the disease when syncytial cells with intranuclear inclusion bodies are no longer observed. PCR using specific primers from ICP4 gene, generating a product of 237 base pairs, was sensitive for ILT diagnosis, and very useful for rapid detection of GaHV-1 in chickens. Fixed tissues allowing histopatological examination and detection of GaHV-1 by PCR, are a good option in areas where farms are located several hundred kilometers away from a diagnostic center, reducing problems with conservation of fresh samples and the risk of virus spread.
Avian infectious laryngotracheitis; conventional PCR; histopathology; immunohistochemistry; laying hen
Preis ISI; Fiúza ATLI; Silva CCI; Braga JFVI; Couto RMI; Martins NR da SII; Ecco RI
ISetor de Patologia Veterinária, Departamento de Clínica e Cirurgia Veterinárias, Universidade Federal de Minas Gerais, Av. Antônio Carlos 6627, Belo Horizonte, MG 30123-970, Brazil
IISetor de Doenças das Aves, Departamento de Medicina Veterinaria Preventiva, Escola de Veterinaria, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, 30123-970, Brazil
CorrespondenceCorrespondence: Universidade Federal de Minas Gerais Escola de Veterinária Av. Antônio Carlos 6627 Belo Horizonte, MG 30123-970, Brazil E-mail: ecco@vet.ufmg.br, eccoro.ufmg@gmail.com
ABSTRACT
Seventy-eight chickens from a very high poultry density (approximately eight million) region and twelve backyard chickens from neighboring areas were analyzed by histopathology and additional techniques for the presence of the infectious laryngotracheitis virus. The virus distribution was determined in different tissues using immunohistochemistry (IHC) and polymerase chain reaction (PCR). The disease was histopathologically diagnosed in 41.0% (32/78) of the commercial layers. Lesions were mainly characterized by syncytial cells with eosinophilic intranuclear inclusion body formed from the hyperplastic epithelium of the upper respiratory tract, primary and secondary bronchi, and conjunctiva. IHC showed 70% (21/30) positive signal in the larynx/trachea and, 53.8% (14/26) in the lungs, either in epithelial cells or syncytia. In the turbinates and paranasal sinuses, 29.6% (8/27) of samples showed positive signal. PCR detected the following gallid herpesvirus 1-positive percentages: conjunctiva 63.2% (31/49), lungs 57.6% (30/52), turbinates and paranasal sinuses 56% (28/50), and larynx/trachea 50% (39/78). IHC showed to be a useful additional tool for definitive ILT diagnosis, especially during the subacute phase of the disease when syncytial cells with intranuclear inclusion bodies are no longer observed. PCR using specific primers from ICP4 gene, generating a product of 237 base pairs, was sensitive for ILT diagnosis, and very useful for rapid detection of GaHV-1 in chickens. Fixed tissues allowing histopatological examination and detection of GaHV-1 by PCR, are a good option in areas where farms are located several hundred kilometers away from a diagnostic center, reducing problems with conservation of fresh samples and the risk of virus spread.
Keywords: Avian infectious laryngotracheitis, conventional PCR, histopathology, immunohistochemistry, laying hen
INTRODUCTION
Infectious laryngotracheitis (ILT) is a viral disease of the respiratory tract of chickens that has a worldwide distribution, and it is responsible for large economic losses. The disease occurs in areas where there is intensive poultry production and economic losses are mainly caused by a drop in egg production and increased mortality (Asheg et al., 2011). The etiological agent is the gallid herpesvirus 1 (GaHV-1), which belongs to the family Herpesviridae, subfamily Alphaherpesvirinae (Guy & Garcia 2008). The primary hosts are chickens, but other species of birds such as pheasants, peacocks (Crawshaw & Boycott 1982), and turkeys (Winterfield & So, 1968, Portz et al., 2008) may be infected. The GaHV-1 causes acute respiratory disease and clinical signs usually are nasal discharge, coughing, sneezing, conjunctivitis, and dyspnea (Kernohan, 1931). Diagnosis should be based on one or more confirmatory laboratory tests, in addition to histopathology, which is the most common and relatively fast (Crespo et al., 2007). The etiologic agent can be identified by viral isolation, direct immunofluorescence, DNA hybridization, and Polymerase Chain Reaction (PCR) (Guy & Garcia, 2008). The GaVH-1 was detected in Brazil by Hipólito et al. (1974), Beltrão et al. (2004), Chacón et al. (2007), and recently by Preis et al. (2013) in multi-age egg laying chickens in a high poultry density farm. The aim of this study was to describe lesions and virus distribution in different tissues of chickens naturally infected with GaHV-1 using IHC and PCR.
MATERIAL AND METHODS
Chickens
In this this study, a total of 78 chickens were analyzed. Layers were selected from seven commercial farms located in four different municipalities of south Minas Gerais state, Brazil. All layers were HyLine strain and samples were collected at different stages of lay, between 19 to 70 weeks of age. Eighteen out the 78 layer chickens had been previously evaluated by Preis et al. (2013), and were submitted to additional testing and were re-analyzed in the present study. Five to six birds were sampled during every clinical episode of the acute respiratory diseases. The number of chickens and the time of each collection are shown in Table 1. In addition, eight backyard chickens from neighboring infected farms were included.
Sample collection for histopathology
Larynx, trachea, lungs, air sacs, paranasal sinuses and turbinates, brain, trigeminal ganglia, and conjunctiva of the 78 layer chickens were collected and fixed in 10% buffered formalin for a maximum of 52 hours. Tissue samples were then transferred to 70% ethanol and routinely processed. The larynx and cranial part of trachea were transverselly cut, while the distal part of the trachea was longitudinally cut to enable identification of anatomical parts during histopathological analysis.
Histopathology
The formalin-fixed, paraffin-embedded (FFPE) tissue samples obtained from the total of 86 chickens were cut into 4 µm-thick sections, stained with hematoxylin and eosin (Luna 1968), and evaluated under light microscopy.
Immunohistochemistry
Among 78 chickens from commercial farms in the south of Minas Gerais, 30 chickens with the most classic ILT lesions were selected to perform IHC according to Tadese et al. (2007) with modifications. Briefly, 4µm sections were placed on slides (Starfrost®). The slides were kept for 15 minutes in an incubator at 60°C and placed in xylol for 15 minutes twice for deparaffinization. Sections were then hydrated in increasing ethyl alcohol dilutions for 5 minutes each. Antigen retrieval was done incubating the slides in citrate buffer solution1 (Target retrieval solution concentrated 10X, S1699-1, Dako, Carpinteria, CA, USA) for 20 minutes in water bath at 98°C. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide dilution in methanol for 15 minutes. Non-specific binding was blocked with milk powder diluted in PBS for 30 minutes. A specific monoclonal antibody (Clone IgG2b, Rural Technologies, Inc., Brookings, SD, USA) for the gJ protein of the infectious laryngotracheitis virus was used at 1:25 dilution. Streptavidin-biotin kit Dako® was used as secondary antibody, and development was performed with diaminobenzidine (DAB substrate System, Spring Bioscience). Counterstaining was performed with hematoxylin during four seconds, and then the sections were dehydrated and mounted with coverslips and mounting media for evaluation by light microscopy.
DNA extraction
Single FFPE tissues from individual layer chickens, including larynx/trachea, turbinates and sinuses, lungs, trigeminal ganglia, and conjunctiva, were cut to five micrometers of thickness (20 sections). Tissue sections were then placed into microtubes and deparaffinized with xylene for DNA extraction. In addition, DNA from tracheas and trigeminal ganglia of eight backyard chickens was also recovered as described above. Thinner section were cut to ensure and improve digestion by proteinase K for DNA extraction, according to the manufacturer's instructions using QIAamp® DNA FFPE Tissue Kit (Qiagen, Valencia, California - US).
Polymerase chain reaction
DNA extracted from the paraffinized samples of the 78 layer chickens and of the eight backyard chickens was tested by PCR using primers designed to amplify 237-bp fragments of the GaHV-1 ICP4 gene, according the conditions previously described by Preis et al. (2013).
RESULTS
Clinical signs and gross pathology
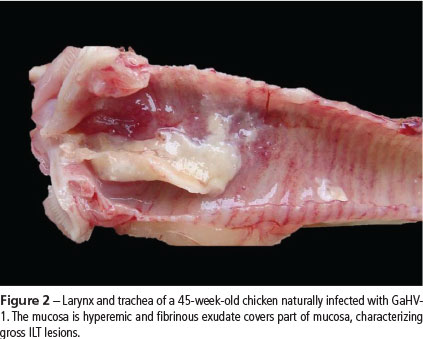
The clinical signs were characterized by apathy and moderate to severe dyspnea. Some chickens presented reddening of the conjunctiva, excessive lacrimation, and swelling of the eyelids and paranasal sinuses (Figure 1). In layers with severe respiratory signs, fibrin and necrotic yellowish-white material covering the laryngeal and tracheal mucosa were observed (Figure 2). Hyperemia and thickening of tracheal mucosa with thin fibrin layer was observed in chickens with mild or moderate respiratory signs.
Histopathology
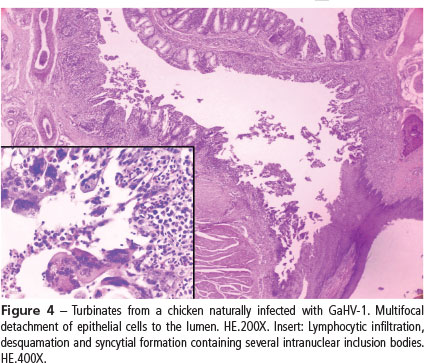
In the present study, the histopathological diagnosis of ILT was achieved in 32 of 78 chickens. Forty six out of the 78 birds necropsied during the months after following outbreak no longer showed any typical lesions. Characteristic lesions of the disease were observed mainly in the larynx and trachea (25/78) (Figure 3), followed by lungs (21/78), turbinates (Figure 4), paranasal sinuses (15/78), and conjunctiva (24/78). The typical lesions were characterized by the formation of syncytial cells with eosinophilic intranuclear inclusion bodies in hyperplastic epithelium, followed by lymphoplasmacytic infiltrate in the lamina propria of the upper respiratory tract, primary and secondary bronchi, and conjunctiva (Preis et al., 2013). No histologic changes were observed in the brain or trigeminal ganglia of either commercial or backyard chickens. The conjunctiva and respiratory tissues of backyard chickens did not present any ILT characteristic lesions, only lymphocytic infiltration in the larynx and trachea distributed diffusely in the lamina propria or forming multifocal lymphoid aggregates, and bronchial associated lymphoid tissue (BALT) hyperplasia in the lungs. In these birds, changes in the tracheal mucosa were suggestive of infection by Mycoplasma gallisepticum (M. gallisepticum).
Immunohistochemistry
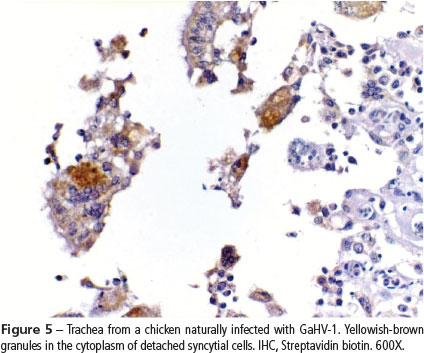
Out of the 30 birds submitted to IHC, 70% (20/30) showed positive signal in the larynx and trachea, characterized by yellowish brown granular staining in the cytoplasm of syncytial cells adhered to the mucosa (Fig. 5) or desquamated (Fig. 6). Positive signal was also observed in the apparently normal epithelial cells. About 53% (14/26) of examined lungs showed positive signal for GaHV-1 in the epithelial cells and syncytia of bronchi. In the turbinates and paranasal sinuses of the 20 chickens analyzed, only 29.6% (8/27) showed positive signal for GaHV-1. The chickens submitted to IHC presented histopathological lesions compatible with the acute stage of the disease.
Polymerase chain reaction
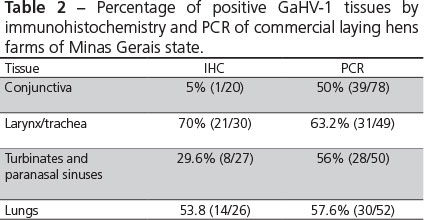
DNA extracted from conjunctiva, turbinates and paranasal sinuses, larynx/trachea and lungs, were 50% (39/78), 56% (28/50), 63.2% (31/49) and, 57% (30/52) GaHV-1 positive by PCR, respectively. Samples of the trigeminal ganglia of laying hens with typical ILT lesions were 90% (9/10) positive by PCR and samples of trigeminal ganglia from the backyard chickens were 16% (1/6) positive. Samples of formalin-fixed trachea of backyard chickens were 25% (2/8) positive. Results of the percentage of positive tissues by IHC and PCR of commercial laying hens are presented in Table 2.
DISCUSSION
The analyzed tissues of 50% of the chickens (with or without typical lesions) were positive for the GaHV-1 by PCR in the present study. Considered only the chickens with typical lesions, PCR results showed 100% positivity. The most frequently affected tissues by GaHV-1, as determined by PCR in the present study were the larynx and trachea, closely followed by lungs, turbinates and paranasal sinuses and conjunctiva. A recent study showed that DNA virus was detected in the larynx, trachea, lungs, cecum, kidney, pancreas, and esophagus of chickens experimentally infected with GaHV-1 (Wang et al., 2013). Another study (Oldoni et al. 2009) showed that GaHV-1 has been more frequently detected in the conjunctiva, sinuses, and trachea, and the authors suggested that these tissues are the site of active replication. Nevertheless, detection of virus in the thymus and cecal tonsil was less frequent and not related to active replication. A hypothesis is that viral particles could be carried by macrophages and/or lymphocytes to these sites and this is supported by the difficulty in recovering the virus from these areas and also by the lack of lesions (Calnek et al., 1986, Oldoni et al. 2009). The present study focused on the respiratory, conjunctiva, and trigeminal ganglia and a comparison with other tissues was not possible.
The conjunctiva and respiratory tissues infected with GaVH-1 in several chickens of this study presented typical ILT lesions. Similar lesions were observed by Purcell (1971) three to four days after experimental infection of chickens with GaHV-1. However, in another experimental study with a low-virulence strain inoculated intranasally, pulmonary and air sac lesions occurred only five days after inoculation (Timurkaan et al. 2003). Similarly, in the turbinates and in the paranasal sinuses, syncytia with intranuclear inclusion bodies were observed four days after infection. Kirkpatrick et al. (2006) mentioned that the GaHV-1 virus may also show tropism for the conjunctiva. In the present study, several of the analyzed chickens presented conjunctivitis, but the formation of syncytia with inclusion bodies was less commonly observed when compared with respiratory epithelium.
In the present study, ILT was diagnosed by histopathology in 32 out of 78 layer chickens. Forty six out of the 78 birds necropsied during the months following the outbreak no longer presented typical lesions, although they were GaVH-1 positive by PCR. These birds may have the virus in latent stage or presumably were necropsied during the subacute phase of the disease. Following the acute stage, complete desquamation of the syncytial cells with intranuclear inclusion bodies occurs, which complicates the diagnosis of ILT using only histopathology (Hayashi et al., 1985). Therefore, the lungs, especially the primary and secondary bronchi, should be collected and analyzed because syncytial cells with intranuclear inclusion bodies are present in the lungs, as observed in 10 birds of this study, allowing histopathological diagnosis. The inclusion bodies are intranuclear accumulations of herpesvirus particles and viral proteins, and may be basophilic (Cowdry type B), consistent with DNA-containing virus particles within the nucleus, and later become eosinophilic (Cowdry type A) due to egress of semi-complete virions from the nucleus (Cowdry, 1934; Reynolds, Watrach & Hanson, 1968). Studies using electron microscopy showed that syncytial cells are formed by fusion of ciliated or/and non-ciliated epithelial cells of the respiratory mucosa (Hayashi et al., 1985). However, the mechanism by which the virus induces the formation of syncytia has not yet been elucidated. Possibly, fusion may be guided by the GaHV-1 virus through the interaction of envelope glycoproteins with cell membrane receptors, similarly to the infection by measles virus, thereby ensuring their replication and spread (Herschke et al., 2007).
The IHC results showed that it is a useful complementary technique for ILT diagnosis. The positive signal for gJ viral protein (Fuchs et al., 2007) was observed in the cytoplasm of syncytial cells and in the cytoplasm of individual cells. One of the advantages of IHC compared with PCR is the possibility of visualizing the lesion associated with the infectious agent, confirming the occurrence of the disease and allowing verifying the distribution of the virus in the tissue. The monoclonal antibody used in this study is specific for the gJ protein, which is a structural glycoprotein encoded by the US5 gene and present in the viral envelope. Monoclonal antibodies specific for the proteins gC and gJ are the most suitable for testing with IHC and immunofluorescence, since experimentally- or naturally-infected birds produce antibodies against these two proteins. An analysis of the GaHV-1 ultrastructure showed that viral capsids are formed in the nucleus and transported to the cytoplasm, where they are enveloped before being released from the cytoplasm (Fuchs et al., 2007). Therefore, when using anti-glycoprotein gJ, which is only present in the viral envelope, the marking will be displayed always in the cytoplasm of infected epithelial cells.
Although the number of samples evaluated by PCR for ICP4 gene and IHC were different in this study, PCR was apparently more sensitive than the IHC technique. The ICP4 gene plays a role in the regulation of early genomic expression in infection, and it is used to differentiate between vaccine strains and field and wild strains in epidemiological studies (Johnson et al., 1995; Chacon & Ferreira, 2009). Tissue samples collected and kept in 10% buffered formalin for no longer than three or four days (then processed or transferred to 70% ethanol) improved DNA recovery and quality after fixation. Tissues fixed in formalin for a long time lead to the formation of crosslinks between the biomolecules present in DNA, impairing PCR results (Gilbert et al., 2007). Furthermore, the process of embedding in paraffin using high temperatures fragments DNA, thereby impairing the quality of the genetic material. It is preferable to use primers with a relatively small amplification product in order to minimize the effect of the problems related with formalin fixation and paraffin embedding (Kleter et al., 1998). The results obtained with conventional PCR, as in this study, allowed detecting the virus at a lower cost than IHC and/or real time PCR.
The GaHV-1 virus, like other herpesviruses, remains latent in the host, and can be reactivated under stress and re-excreted into the environment. Chickens vaccinated with live attenuated vaccines can also develop latent infection, since vaccine virus remains in the host and could also be re-excreted (Hughes, Williams & Gaskell, 1991). The main site of latency is the trigeminal ganglion (Williams, Bennett & Bradbury, 1992). In the present study, the trigeminal ganglia of clinically normal commercial laying chickens were positive for GaHV-1. A sample of the trigeminal ganglion of a healthy backyard chicken was also positive by PCR. However, the larynx and trachea of this chicken were negative by PCR. The results obtained with the backyard chicken suggest that the virus was latent in the trigeminal ganglion, because its trachea was negative by PCR and there were no histological lesions in the analyzed tissues. Similarly, the larynx and trachea of two other backyard chickens were positive for GaHV-1 by PCR in the absence of histological lesions. According to Williams et al. (1992), the tracheal ganglion is another site for GaHV-1 latency. The presence of GaHV-1-positive backyard chickens is an important factor in the epidemiology of the virus, since these chickens may be the source of infection.
The larynx/trachea showed higher incidence of ILT-characteristic lesions by histopathology, but there was also a high incidence of lesions in the lungs and sinuses, followed by conjunctiva. This shows that all these tissues are important for ILT diagnosis and should be collected for histopathology. According to our experience, the availability of all the aforementioned tissues increases the chances for making a histopathological diagnosis. IHC is a useful additional tool for the definitive ILT diagnosis, during in the subacute phase of the disease, when syncytial cells with intranuclear inclusion bodies are no longer observed. However, PCR using specific primers from ICP4 gene, generating a product of 237 base pairs, was more sensitive and it is very useful for the rapid detection of GaHV-1 in chickens. Fixed tissues allow both histopathological examination and detection of GaHV-1 by PCR, and therefore are a good option in areas where farms are located several hundred kilometers away from a diagnostic center, reducing problems with conservation of fresh samples and the risk of virus spread.
ACKNOWLEDGMENTS
This study was funded by "Fundação de Amparo a Pesquisa de Minas Gerais" (FAPEMIG - project number APQ-01938-10). Fellowships were provided by "Conselho Nacional de Desenvolvimento Científico e Tecnológico" (CNPq), and "Coordenação de Aperfeiçoamento de Pessoal de Nível Superior" (CAPES), Brazil. We are grateful to the veterinarians of Instituto Mineiro de Agropecuaria (IMA), Izabella Hergot, Luiz Antonio Torino and Simone G. Palma for assistance on sample collections.
Submitted: November/2013
Approved: June/2014
- Asheg AA, Tarhuni OA, Al-Garib SO, Hamid MA, Kammon AM. An outbreak of infectious laryngotracheitis in layer chickens in Libya. The Indian Veterinary Journal 2011;88(8):135-6.
- Beltrão N, Furian TQ, Leão JA, Pereira RA, Moraes L, Canal CW. 2004. Detecção do vírus da laringotraqueíte das galinhas no Brasil. Pesquisa Veterinária Brasileira 2004;24(2):85-8.
- Calnek BW, Fahey KJ, Bagust TJ. In vitro infection studies with infectious laryngotracheitis virus. Avian Diseases 1986;30(2):327-36.
- Chacón JLV, Ferreira AJP. Differentiation of field isolates and vaccine strains of infectious laryngotracheitis virus by DNA sequencing. Vaccine 2009;27(48):6731-38.
- Chacón JLV, Brandão PEB, Villarreal LYB, Gama NM, Ferreira AJP. Survey of infectious laryngotracheitis outbreak in layer hens and differential diagnosis with other respiratory pathogens. Revista Brasileira de Ciência Avícola 2007;9(1):61-7.
- Cowdry EV. The problem of intranuclear inclusions in virus diseases. Archives of Pathology 1934; 18:527-42.
- Crawshaw GJ, Boycott BR. Infectious laryngotracheitis in peafowl and pheasants. Avian Diseases 1982;26(2):397-401.
- Crespo R, Woolcock PR, Chin RP, Shivaprasad HL, García M. Comparison of diagnostics techniques in an outbreak of infectious laryngotracheitis from meat chickens. Avian Diseases 2007;51(4):858-62.
- Fuchs W, Veits J, Helferich D, Granzow H, Teifke JP, Mettenleiter TC. Molecular biology of avian infectious laryngotracheitis virus. Veterinary Research 2007;38(2):261-79.
- Gilbert MTP, Haselkorn T, Bunce M, Sanchez JJ, Lucas SB, Jewells LD, Marcks EV. The isolation of nucleic acids from fixed, paraffin-embedded tissues-which methods are useful when? PLoS One 2007;2:1-12.
- Guy JS, Garcia M. Laryngotracheitis. In: Saif YM, Barnes HJ, Glisson JR, Fadly AM, McDougald LR, Swayne DE, editors. Diseases of poultry. 12th ed. Ames: Iowa State Press; 2008. p.121-134.
- Hayashi S, Odagiri Y, Kotani T, Horiuchi T. Pathological changes of tracheal mucosa in chickens infected with infectious laryngotracheitis virus. Avian Diseases 1985;29(4):943-50.
- Herschke F, Plumet S, Duhen T, Azocar O, Druelle J, Laine D, Wild TF, Rabourdin-Combe C, Gerlier D, Valentin H. Cell-cell fusion induced by measles virus amplifies the type I interferon response. Journal of Virology 2007;81(23):12859-71.
- Hipólito O, Soares LA, Pereira OAC, Pinto AA, Bottino JA. 1974. Isolamento e identificação do vírus da laringotraqueite infecciosa das galinhas no Brasil. Anais do Congresso Brasileiro de Microbiologia; 1974; Rio de Janeiro, RJ. Brasil. p.16.
- Hughes CS, Williams RA, Gaskell RM. Latency and reactivation of infectious laryngotracheitis vaccine virus. Archives of Virology 1991;121(1-4):213-18.
- Johnson MA, Tyack SG, Prideaux C, Kongsuwan K, Sheppard M. Nucleotide sequence of infectious laryngotracheitis virus (gallid herpesvirus- 1) ICP4 gene. Virus Research 1995;35(2):193-204.
- Kernohan G. Infectious laryngotracheitis in fowls. Journal American of Veterinary Medical Association 1931;78:196-202.
- Kirkpatrick NC, Mahmoudian A, Colson CA, Devlin JM, Noormohammadi AH. Relationship between mortality, clinical signs and tracheal pathology in infectious laryngotracheitis. Avian Pathology 2006;35(6):449-53.
- Kleter B, Doorn LJ, Schegget J, Schrauwen L, Krimpen K, Burger M, Harmsel B, Quint W. Novel Short-Fragment PCR assay for highly sensitive broad-spectrum detection of anogenital human papillomaviruses. American Journal of Pathology 1998;153(6):1731-39.
- Luna LG. Manual of histologic staining methods of the Armed Forces Institute of Pathology. 3rd ed. New York: McGraw-Hill Book; 1968. p.32-46.
- Oldoni I, Rodríguez-Avila A, Riblet SM, Zavala G, García M. Pathogenicity and growth characteristics of selected infectious laryngotracheitis virus strains from the United States. Avian Pathology 2009;38(1):47-53.
- Portz C, Beltrão N, Furian TQ, Macagnan M, Griebeler J, Rosa CAVL, Colodel EM, Driemeier D, Back A, Schatzmayr OMB, Canal CW. Natural infection of turkeys by infectious laryngotracheitis virus. Veterinary Microbiology 2008;131(1-2):57-64.
- Preis IS, Braga JFV, Couto RM, Brasil BSAF., Martins NRS, Ecco R. Outbreak of infectious laryngotracheitis in large multi-age egg layer chicken flocks in Minas Gerais, Brazil. Pesquisa Veterinária Brasileira 2013;33(5):591-96.
- Purcell DA. Histopathology of infectious laryngotracheitis in fowl infected by an aerosol. Journal of Comparative Pathology 1971;81(3):421-31.
- Reynolds HA, Watrach AM, Hanson LE. Development of the nuclear inclusion bodies of infectious laryngotracheitis. Avian Diseases 1968;12(2):332-47.
- Tadese T, Potter E, Fitzgerald S, Reed WM. Concurrent infection in chickens with fowlpox virus and infectious laryngotracheitis virus as detected by immunohistochemistry and a multiplex polymerase chain reaction technique. Avian Diseases 2007;51(3):719-24.
- Timurkaan N, Yilmaz F, Bulut H, Ozer H, Bolat Y. Pathological and immunohistochemical findings in broilers inoculated with a low virulent strain of infectious laryngotracheitis virus. Journal of Veterinary Science 2003;4(2):175-180.
- Wang LG, Ma J, Xue CY, Wang W, Guo C, Chen F, Qin JP, Huang NH, Bi YZ, Cao YC. Dynamic distribution and tissue tropism of infectious laryngotracheitis virus in experimentally infected chickens. Archives of Virology 2013;158(3):659-66.
- Williams RA, Bennett M, Bradbury JM. 1992. Demonstration of sites of latency of infectious laryngotracheitis virus using the polymerase chain reaction. Journal of General Virology 1992; 73(9):2415-20.
- Winterfield RW, So IG. Susceptibility of turkeys to infectious laryngotracheitis. Avian Diseases 1968;12(1):191-202.
Publication Dates
-
Publication in this collection
06 Jan 2015 -
Date of issue
Dec 2014
History
-
Received
Nov 2013 -
Accepted
June 2014