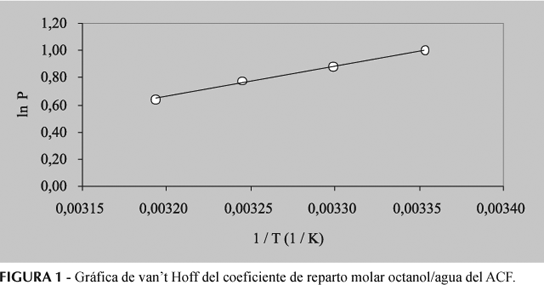
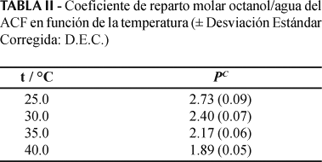
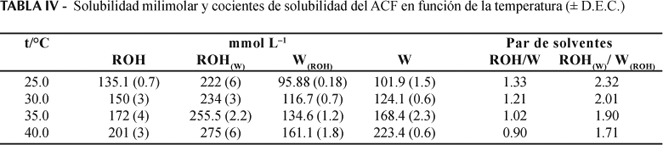
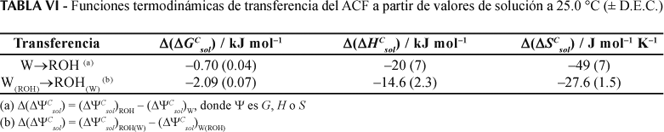
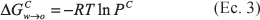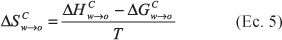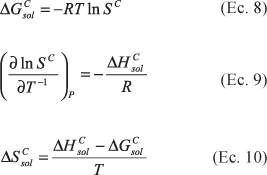The partition coefficients and solubilities in octanol, water and mutually saturated octanol-water phases were determined for acetaminophen at 25.0, 30.0, 35.0, and 40.0 °C. By means of Gibbs and van't Hoff thermodynamic analyses it may observe that the transfer of this drug from water to octanol is spontaneous and mainly driven enthalpically. As in other studies made with guanine derivatives and sulfonamides, it has been shown that the mutual saturation of the octanol and aqueous phases plays an important role in the partitioning and solubility of this drug.
Thermodynamics of transfer; Acetaminophen; Partition coefficient