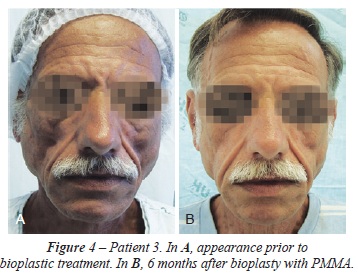
BACKGROUND: When the treatment of acquired immunodeficiency syndrome (AIDS) with highly active antiretroviral therapy (HAART) began in the 1990s, it considerably increased the life expectancy and quality of life of AIDS patients. However, the decrease in morbidity and mortality associated with opportunistic infectious and neoplastic diseases was accompanied by an increase in the prevalence of other diseases, including HIV-associated lipodystrophy. Lipodystrophy is due to the toxicity of drugs used in antiretroviral therapy, including protease inhibitors and nucleoside analog reverse transcriptase inhibitors. This article discusses the treatment of facial lipodystrophy, which confers an appearance of premature aging and brings back the old stigma of the "AIDS face," which negatively impacts the quality of life of HIV carriers. METHODS: Forty-one patients with facial lipoatrophy received filling with polymethylmethacrylate (PMMA) at the Hospital Universitário da Universidade Federal de Juiz de Fora (HU-UFJF) and at the Plastic Center Clinic, Plastic Surgery Clinic in Juiz de Fora between January 2010 and February 2012. RESULTS: Patients received 1 to 4 procedures with a minimum interval of 90 days between procedures. The amount of PMMA used ranged from 3 to 18 mL per procedure according to the degree and region to be corrected. The results were aesthetically favorable in all patients. CONCLUSIONS: The results obtained through bioplasty with PMMA are considered satisfactory by patients. The material used is highly adaptable to the receiving areas, requiring only modeling and an adequate amount in order to obtain good aesthetic results.
HIV-associated lipodystrophy syndrome; Acquired immunodeficiency syndrome; HIV; Lipodystrophy