Abstract
Cercophana frauenfeldii Felder (Lepidoptera: Saturniidae), also known as the “Andean Moon Moth”, is a Neotropical species native to continental Chile whose larvae feed on species of the families Gomortegaceae, Laureaceae and Winteraceae. We describe and document C. frauenfeldii immature stages, namely, egg, its four larval instars, and chaetotaxy of the last instar, pupa and cocoon for the first time. In terms of its phenology, we extend its larval activity, originally described to occur between November and mid-December, to June until the end of January. We report the adult flight period depends on the species’ distributional range following two well-differentiated patterns: February to mid-April in Central-North Chile and April to June in Central-South Chile. Furthermore, we provide a unified view of its current distributional range and host plants (including the endangered tree Gomortega keule) through bibliographic data, field observations and laboratory rearing. Finally, we discuss aspects of the species’ conservation as part of the unique ecosystems found in the temperate forests of southern South-America.
Keywords:
Biology; Camouflage; Cercophana venusta; Chile; Gomortega keule; Temperate forest
Introduction
The Saturniidae family is represented in Chile by two subfamilies, two tribes, seven genera and 19 species distributed from the Coquimbo to the Magallanes regions (Angulo et al., 2004Angulo, A. O., Lemaire, C., Olivares, T. S., 2004. Catálogo crítico e ilustrado de las especies de la familia Saturniidae en Chile (Lepidoptera: saturniidae). Gayana (Concepc.) 68, 20-42. https://doi.org/10.4067/S0717-65382004000100003.
https://doi.org/10.4067/S0717-6538200400...
). Within these groups, the conspicuous Cercophaninae, also known as “Andean Moon Moths” (Heppner, 2002Heppner, J. B., 2002. Mexican Lepidoptera biodiversity. Insecta Mundi 16, 171-190. Available in: http://digitalcommons.unl.edu/insectamundi/550 (accessed 06 November 2020).
http://digitalcommons.unl.edu/insectamun...
), is a subfamily distributed along Chile, whose species resemble the northern hemisphere species of the genus Actias Leach, 1815 or “moon moths”. This subfamily has also been considered to be a basal group of the family Saturniidae (Regier et al., 2008Regier, J. C., Grant, M. C., Mitter, C., Cook, C. P., Peigler, R. S., Rougerie, R., 2008. Phylogenetic relationships of wild silkmoths (Lepidoptera: Saturniidae) inferred from four protein‐coding nuclear genes. Syst. Entomol. 33 (2), 219-228. https://doi.org/10.1111/j.1365-3113.2007.00416.x.
https://doi.org/10.1111/j.1365-3113.2007...
) with a “New World” distribution (Lemaire and Minet, 1999Lemaire, C., Minet, J., 1999. The Bombycoidea and their relatives. In: Kristensen, N.P. (Eds.). Handbuch der Zoologie, Band 4 Arthropods: Insecta, Teilband 35. Lepidoptera, moths and butterflies. Vol. 1: Evolution, systematics, and biogeography. W. de Gruyter, Berlin, pp. 321-353. https://doi.org/10.22215/etd/2007-08316.
https://doi.org/10.22215/etd/2007-08316...
). Within this subfamily, the tribe Cercophanini represents an endemic group that of Chile and Argentina and includes the genera Cercophana Felder, 1862, Microdulia Jordan, 1924 and Neocercophana Izquierdo, 1825 (Kitching et al., 2018Kitching, I. J., Rougerie, R., Zwick, A., Hamilton, C., St Laurent, R., Naumann, S., Mejia, L. B., Kawahara, A. Y., 2018. A global checklist of the Bombycoidea (Insecta: lepidoptera). Biodivers. Data J. (6), https://doi.org/10.3897/BDJ.6.e22236.
https://doi.org/10.3897/BDJ.6.e22236...
).
The genus Cercophana Felder, 1862 is represented in Chile by two species: Cercophana frauenfeldii Felder, 1862 and C. venusta (Walker, 1856; Jordan, 1924Jordan, K., 1924. On the saturnoidean families Oxytenidae and Cercophanidae. Novit. Zool. 31, 135-193.; Artigas, 1994Artigas, J. 1994. Entomología económica, insectos de interés agrícola, forestal, médica y veterinarios (Nativos, introducidos y que pueden ser introducidos). Editorial Universidad de Concepción, Concepción.; Wolfe and Balcázar-Lara, 1994Wolfe, K. L., Balcázar-Lara, M. A., 1994. Chile’s Cercophana venusta and its immature stages (Lepidoptera: cercophanidae). Trop. Lepid. Res. 5, 35-42.; Angulo et al., 2004Angulo, A. O., Lemaire, C., Olivares, T. S., 2004. Catálogo crítico e ilustrado de las especies de la familia Saturniidae en Chile (Lepidoptera: saturniidae). Gayana (Concepc.) 68, 20-42. https://doi.org/10.4067/S0717-65382004000100003.
https://doi.org/10.4067/S0717-6538200400...
), which are also present in Argentina (Núñez-Bustos, 2015Núñez-Bustos, E., 2015. Catálogo preliminar de Saturniidae de Argentina, con veintiún nuevos registros (Lepidoptera: saturniidae). Trop. Lepid. Res. 25, 22-33. Available in: https://journals.flvc.org/troplep/article/view/89749 (accessed 06 November 2020).
https://journals.flvc.org/troplep/articl...
). Both species are medium sized moths with prominent tails in the males and variable colouration (Wolfe and Balcázar-Lara, 1994Wolfe, K. L., Balcázar-Lara, M. A., 1994. Chile’s Cercophana venusta and its immature stages (Lepidoptera: cercophanidae). Trop. Lepid. Res. 5, 35-42.).
Although the latter authors described in detail the immature stages and some aspects of the biology of C. venusta, very little is known regarding the immature stages of C. frauenfeldii, with the available literature restricted only to the descriptions of the last larval instar carried out by Butler (1882)Butler, A., 1882. Additional notes on Bombyces collected in Chili by Mr. Edmonds. T Roy. Ent. Soc. Lond. 30, 101-108. and descriptions of the cocoon made by Jordan (1924)Jordan, K., 1924. On the saturnoidean families Oxytenidae and Cercophanidae. Novit. Zool. 31, 135-193.. Within this context, the aim of this study is to contribute to the knowledge of the immature stages of C. frauenfeldii by describing the external morphology of the egg and the first three instars, to report new aspects of its phenology and to integrate its distributional records and host plants.
Material and methods
Eggs and larvae were collected in Los Queules National Reserve (36 ° 01’S, 72 ° 42’W, 360 m.a.s.l.) and Copiulemu (36 ° 02’S, 72 ° 40’W, 435 m.a.s.l.), in the Maule Region, and Ralbún (36°03’S, 72°38’W, 540 m.a.s.l.), in the Region of Ñuble, Chile. Larvae were collected manually from leaves of the endemic species Cryptocarya alba (peumo) and Gomortega keule (queule), belonging to the families Lauraceae and Gomortegaceae respectively. Larvae were placed in a 36×36×26 cm breeding cage and were fed with fresh shoots of both plant species. Shoots were changed every 3-4 days to avoid dehydration.
Drawings of the samples were obtained by using a stereo microscope MOTIC® TYPE 102 M. Details of the ultrastructure of the fourth instar larva, structures of the head, fourth instar chaetotaxy and details of the pupa were made with the scanning electron microscope Auto scanning ETEC® at the Centre of Spectroscopy and Electronic Microscopy (CESMI), Universidad de Concepción, Chile.
The chaetotaxy was described using the terminology of Hinton (1946)Hinton, H. E., 1946. On the homology and nomenclature of the setae of lepidopterous larvae, with some notes on the phylogeny of the Lepidoptera. T. Roy. Ent. Soc. Lond. Trans. R. Entomol. Soc. Lond. 97, 1-37. https://doi.org/10.1111/j.1365-2311.1946.tb00372.x.
https://doi.org/10.1111/j.1365-2311.1946...
and modifications made by Stehr (1987)Stehr, F. W., 1987. Immature insects. Kendall/Hunt Publishing, Dubuque.. The pupa description was carried out following descriptions made by Wolfe and Balcázar-Lara (1994)Wolfe, K. L., Balcázar-Lara, M. A., 1994. Chile’s Cercophana venusta and its immature stages (Lepidoptera: cercophanidae). Trop. Lepid. Res. 5, 35-42. who described the pupa and cocoon of C. venusta and the directions given by Angulo and Weigert (1974)Angulo, A. O., Weigert, G., 1974. Estados preimaginales de Polythysana cinerascens (Phil.) (Lepidoptera: Saturniidae). Bol. Soc. Biol. Concepción 47, 145-150. Available in: http://www.micra.cl/media/documentos/AnguloWeigert_1974_Polythysana_cinerascens .pdf (accessed 06 November 2020)
http://www.micra.cl/media/documentos/Ang...
who described the pupa of Polythysana cinerascens (Philippi, 1859).
Examined material: one adult male, two females plus their genitalia, one pupa, one larva and eggs (fixed in 70% alcohol) were deposited at the Museo de Zoología Universidad de Concepción (MZUC), Concepción, Chile. Two larvae (fixed in 70% alcohol) were deposited at the Laboratorio de Interacción Invertebrado – Planta (LinvPla), Campus San Isidro Universidad Católica del Maule, Curicó, Chile.
Results
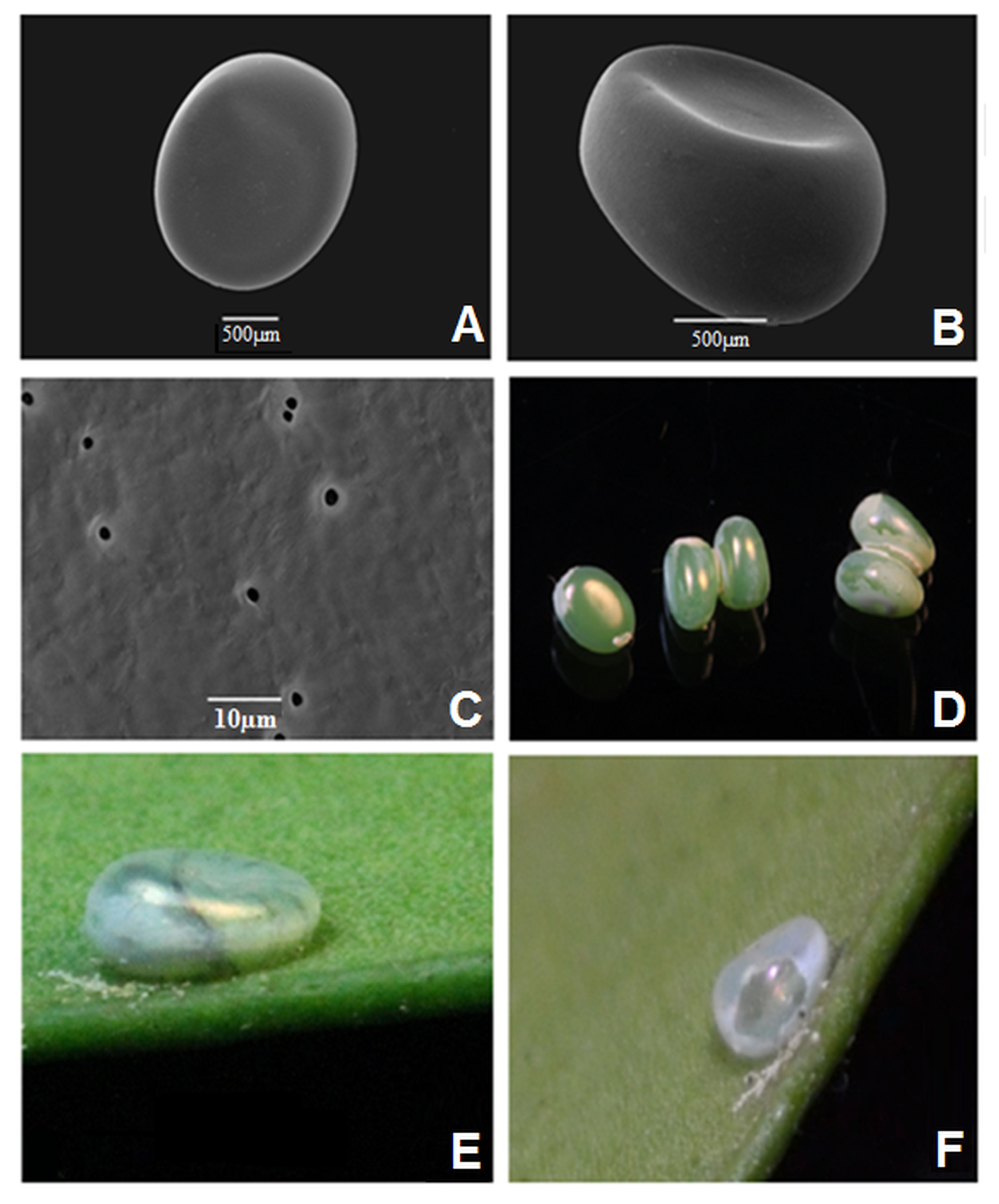
Egg (Figs. 1A-F)
Cercophana frauenfeldii egg details. (A) General view of the egg; (B) Egg side view showing a conspicuous depression; (C) Aeropiles distributed randomly across the chorion surface; (D) Non-fertilized eggs laid by the female inside the rearing cage; (E) Fertilized egg collected from queule leaves and (F) Details of the egg after larval hatching.
Eggs are 1.8 mm long and 1.6 mm in diameter. Elliptically shaped and laterally flattened (Figs. 1A, 1B) as in C. venusta, with aeropiles distributed in a random pattern through the chorion surface (Fig. 1C). Pearly green coloured with smooth and shiny surface (Fig. 1E, 1F). Both, colour and lateral depression, gradually change as a result of the embryonic development turning the egg into an elliptic structure, slightly flattened and of a translucent colour (Fig. 1D).
Larva (Figs. 2A-D)
Cercophana frauenfeldii larva head. (A) Trisegmented Antenna (TrA); (B) Details of the mouthparts, Labrum (Lb) and Mandibles (Man). (C) Hypopharyngeal complex, showing Maxillary Palpi (MaP) and Spinneret (Spn); and (D) Stemmata (Ste) arrangement.
Solitary. All four instars eruciform. Hypognatous head with five stemmata arranged in half moon shape. Stemmata I, II, III close to each other and separated from stemmata IV and V (Fig. 2D), separated from each other by setae (Fig. 2B, 2D). Antennae with three segments, white to greenish coloured when compared to the rest of the head (Fig. 2A). Mouthpieces with well-differentiated labrum, mandibles and hypopharyngeal complex. Hypopharyngeal complex with conical spinneret slightly flattened (Fig. 2C). Oval spots located in the meso and metathorax and in abdominal segments I, II, III, V and VIII, ranging from red to dark reddish in second, third and fourth instars. Their presence, number and colouration change among specimens. Uniordinal crochets arranged in homoidean mesoseries.
Larval instars (Figs. 3A-E)
Larval instars of C. frauenfeldii. (A) First instar showing long setae and lack of coloured spots. (B) Second instar, showing first spots variable in size and number. (C) Third instar, exhibiting the first stages of the scoli on the third thoracic segment and the false eye on the second thoracic segment. (D) Fourth instar showing scoli and false eye fully developed and the yellowish line running from the scoli to the last segment with the hypognathous head hidden within the first and second thoracic segments. (E) Larval camouflage pattern.
First instar: Translucent after hatching from the egg, acquiring a greenish colouring after feeding from the foliage. Head 1 mm wide, yellowish-green coloured after hatching. Head with whitish setae. Body’s maximum length 7-9 mm initially whitish turning into light green in the dorsal area, and turquoise-green from the subdorsal towards the ventral area. Dark reddish spots inconspicuous when present, in meso, metathorax and abdominal segments V to VIII. White elongated setae located in the thoracic and anal shields, pointing forward and backward respectively. Dorsal setae distributed along the entire body. Lateral setae present in groups of 3 with a wart-shape under the spiracles (Fig. 3A).
Second instar: Head 2 mm wide, whitish-green coloured. As in the previous instar, disproportionately large when compared to the rest of the body. The first dark reddish coloured spot becomes conspicuous on the body. Body 16-20 mm long, light green to yellowish green, similar to the first instar but with a variable number of spots on the thoracic segments two and three; and abdominal segments five and eight. The presence and shape of dorsal and lateral setae changes, with small dorsal setae becoming more noticeable and visible only at high magnification. Lateral setae forming a curtain-like structure across the body, below the spiracle line (Fig. 3B).
Third instar: Head, 2.4 mm, whitish green coloured. Morphological changes become evident at this instar. The head changes position, hiding almost entirely within the prothorax. Body 23 mm long when at rest and approximately 25 mm when moving, light green to yellowish green coloured. Dark reddish to violaceus coloured process becomes slightly protuberant on the metathorax. Simultaneously, a yellow line appears laterally immediately below the spiracles and above the lateral setae. This yellow line originates at the scolus and ends at a sharp point by the end of the tenth abdominal segment. The long setae of the thoracic and anal shield become brownish. Lateral setae present in groups of three setae located immediately under the spiracle line. The larva retains the curtain-like setae structure covering the subventral area of the body as the previous instar (Fig. 3C).
Fourth instar: Head approximately 4.3 mm wide, whitish green coloured, without major morphological changes when compared to the previous instar. Body length 50 mm when at rest; 55-60 mm when moving actively. The thoracic dorsal setae of the previous instars are replaced by longer black coloured and club-shaped setae. Setae arranged as a “mane” on the thoracic shield. Scolus densely covered with yellowish warts carrying translucent setae oriented towards the frontal area, with an oval white spot surrounded by a dark red ring, giving the spot the appearance of a “false eye”. Lateral area sprinkled with yellowish warts originates at the peak of the scolus, located below the spiracles on both sides of the body ending in a sharp point by the anal shield. Spiracles are located above this line, each surrounded by dark red coloured spots of irregular shape (Fig. 3D). Larval camouflage pattern exhibited by the last instar (Fig. 3E).
Chaetotaxy of the fourth instar (Fig. 4)
Prothorax: XD1, XD2, SD1, D1 and D2 present on the thoracic shield. All setae arrange as a mane protruding forward. XD1, XD2, D1 and SD1 similar in length. D2 twice the length of D2 present in the rest of the body. L1 unique, located before the spiracle, L2 absent. SV1 and SV2 with one bristle located above the coxa. V group absent.
Mesothorax: D2 located on the anterior margin of the segment followed by D1 located on the opposite margin. SD1 located further down between D1 and D2 on the subdorsal area. L group bisetose. Spiracles absent. SV1 and SV2 following the same location as the one observed in the prothorax. V group absent.
Metathorax: Groups D and SD absent, instead a scolus horn of Vb type with several setae present. Groups L, SV and V as observed in the mesothorax. Spiracles absent.
Abdomen: A1-2, D2 located on the anterior margin of the segment, followed by D1 on the opposite margin. SD1 located further down between D1 and D2 on the subdorsal area. Group L trisetose located below the spiracle. L2 located before the spiracle. L1 immediately below the spiracle and between L2 and L3. Group SV monosetous with a single SV1. V1 present on the ventral line. A3-6: similar pattern for groups D, SD and L as observed in A1-2, except for the presence of SV2 on the proleg and the absence of the V group. A7-8 similar setal pattern to D, SD and L as observed for A1-6. Spiracles larger than in the rest of the body. SV group absent. A single seta V1 present at the same level as the proleg. A9: just four setae present: D1, SD1, L1 and V1. Spiracle absent. A10: D1, D2 and SD1 present on the anal shield. L1 single, thrice the length of the rest of the L1 present on the rest of the body: located at the same level as the spiracles. SV1 and SV2 on the anal proleg. Group V absent.
Pupa (Figs. 5A-D, 6A-C)
Pupae of C. frauenfeldii. (A) Frontal view. (B) Detail of the cremaster. (C) Yellow-orange membranes of the fourth, fifth and sixth abdominal segments; and (D) pupa inside the cocoon.
(A) Ventral, (B) Lateral and (C) Dorsal view of the female pupa of C. frauenfeldii. Modified from Angulo and Weigert (1974)Angulo, A. O., Weigert, G., 1974. Estados preimaginales de Polythysana cinerascens (Phil.) (Lepidoptera: Saturniidae). Bol. Soc. Biol. Concepción 47, 145-150. Available in: http://www.micra.cl/media/documentos/AnguloWeigert_1974_Polythysana_cinerascens .pdf (accessed 06 November 2020)
http://www.micra.cl/media/documentos/Ang... .
Pupa obtecta, 18 mm long and 7 mm wide. Brownish-red tegument (Fig. 5A-D). Pectinated antennae half of the body length. Semicircular eyes separated by the sub-quadrangular shaped clypeus-labrum, two thirds the length of the antennae. Seven spiracles present from the second to the eighth abdominal segment (Fig. 6B). Fourth, fifth and sixth abdominal segments divided by a dark brownish-red band, located below the spiracles crossing the whole segment. An orange-yellow pleural membrane, which gives mobility to these segments, located immediately under the brownish-red band. Opening of the ovipositor, whose anterior border projects medially towards the eighth segment, located in the ninth-tenth segments. The anal opening is located towards the centre of the tenth abdominal segment (Fig. 6A). Cremaster with a large number of hook-shaped spines located towards the end of the abdomen (Figs. 6A-C).
Cocoon (Figs. 7A-F).
Cercophana frauenfeldii cocoon. (A) Collected from rocky substrate at Laguna Torca National Reserve, Vichuquén, Chile. (B-D) Cocoons from larvae reared at the laboratory. (E-F) Details of the silk thread arrangement in the cocoon.
Cocoon oval shaped, 23 mm long and 11 mm wide. Light to brownish-yellow ivory coloured, compactly built through the interlacing of silk threads (Figs. 7E,8F). Sealed from the outside by both poles, since it is possible to find remains of the last exuvia when opening the cocoon. The larva weaves the cocoon around a tree branch (Fig. 7D), sheltering it by neighbouring leaves (Figs. 7B, 7C) or even between rocks (Fig. 7A).
Phenology of C. frauenfeldii. Striped area of each phenology bar shows previous records. Grey coloured area of the bars represents the findings of this study.
Phenology (Fig. 8)
The eggs were collected between the last week of June (in Ralbún) and early September (in Los Queules National Reserve). On the other hand, first instar larvae (recently hatched) were found at the end of June. Second instar at the beginning of August and third instar larvae between September and the beginning of November (observations made in the locality of Ralbún). Fourth instar larvae occur from the beginning of November to the beginning of January (observations made in the laboratory from third instar larvae collected in the field). In the final instar, before the ecdysis, the larva stops feeding and moving around for a period ranging from three to seven days, when it also produces a silk shelter where moulting occurs. The pupation period extends from the beginning of November to March (Figure 8). Field observations showed that from April onwards all cocoons found showed evidence of imago emergence (Fig. 9A-D). Adults fly from February to June.
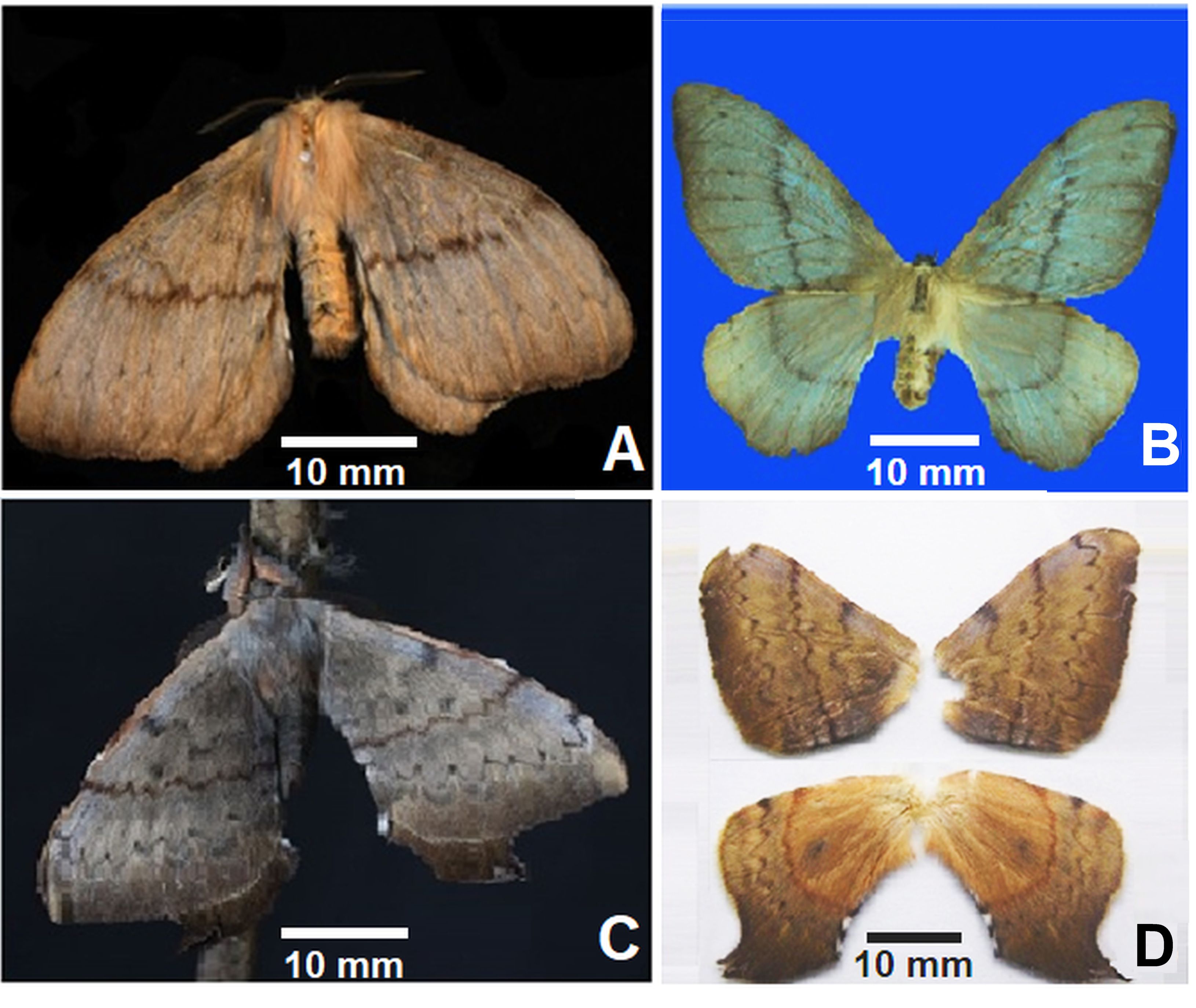
Cercophana frauenfeldii adults emerged from laboratory rearing. (A) Recently emerged female perching on a twig and (B) Details of the wing design. (C) Recently emerged male perching on a twig and (D) details of its wing design.
Distributional range (Fig. 10)
Cercophana frauenfeldii distributional map of continental Chile showing Central-North and Central-South areas (Roman numbers = Administrative regions, MA=metropolitan area).
Cercophana frauenfeldii shows a wide distribution along continental Chile, occurring from the V to the XII regions (Fig. 10). Based on our observations and previous studies, we determined that, depending on the geographical distribution, the flight period of C. frauenfeldii exhibits two different time patterns. In Central-North Chile (between the V and VII regions, including the Metropolitan Area), adults can be found from February to mid-April. In Central – South Chile (between the VIII and XII regions), adults can be found from April to June.
Host plants recorded in this study (Fig. 11)
Larvae of C. frauenfeldii occurring on two host plants. (A) Last instar larva feeding on Cryptocarya alba (peumo). (B) Second instar larva feeding on Gomortega keule (queule) leaves.
Larvae of C. frahuenfeldii were recorded feeding on leaves of two host plant species: Cryptocarya alba (Mol) (peumo) (Lauraceae) (Fig. 11A) and Gomortega keule (Mol) Baillon (queule) (Gomortegaceae) (Fig. 11B).
Discussion
In this study, we described all immature stages, from egg to pupa, of Cercophana frauenfeldii. We found that eggs of C. frauenfeldii were individually laid on the abaxial surface of a single leaf in the host plant. Although Lemaire and Minet (1999)Lemaire, C., Minet, J., 1999. The Bombycoidea and their relatives. In: Kristensen, N.P. (Eds.). Handbuch der Zoologie, Band 4 Arthropods: Insecta, Teilband 35. Lepidoptera, moths and butterflies. Vol. 1: Evolution, systematics, and biogeography. W. de Gruyter, Berlin, pp. 321-353. https://doi.org/10.22215/etd/2007-08316.
https://doi.org/10.22215/etd/2007-08316...
indicated that in several subfamilies of Saturniidae larvae have a gregarious behaviour, for C. frauenfeldii we observed a solitary behaviour similar to the larvae of the species C. venusta described by Wolfe and Balcázar-Lara (1994)Wolfe, K. L., Balcázar-Lara, M. A., 1994. Chile’s Cercophana venusta and its immature stages (Lepidoptera: cercophanidae). Trop. Lepid. Res. 5, 35-42.. Morphologically, C. frauenfeldii larvae exhibit some remarkable morphological differences among its four instars, such as a prominence present from the second instar, which looks as the metanotal protuberance mentioned by Wolfe and Balcázar-Lara (1994)Wolfe, K. L., Balcázar-Lara, M. A., 1994. Chile’s Cercophana venusta and its immature stages (Lepidoptera: cercophanidae). Trop. Lepid. Res. 5, 35-42. for C. venusta. In C. frauenfeldii, however, such structure is present only in the first instar and evolving into a type Vb scolus, derived from warts (Deml and Dettner, 2002Deml, R., Dettner, K., 2002. Morphology and classification of larval scoli of Saturniinae and Hemileucinae (Lepidoptera: saturniidae). J. Zool. Syst. Evol. Res. 40, 82-91. https://doi.org/10.1046/j.1439-0469.2002.00181.x.
https://doi.org/10.1046/j.1439-0469.2002...
), in the second instar showing a conspicuous structure in the third instar, and acquiring a “horn” shaped structure in the fourth instar. The pupa of C. frauenfeldii, on the other hand, shows many morphological similarities to those described for the pupa of C. venusta and pupae of species of the genus Polythysana (Wolfe and Balcázar-Lara, 1994Wolfe, K. L., Balcázar-Lara, M. A., 1994. Chile’s Cercophana venusta and its immature stages (Lepidoptera: cercophanidae). Trop. Lepid. Res. 5, 35-42.; Angulo et al., 2004Angulo, A. O., Lemaire, C., Olivares, T. S., 2004. Catálogo crítico e ilustrado de las especies de la familia Saturniidae en Chile (Lepidoptera: saturniidae). Gayana (Concepc.) 68, 20-42. https://doi.org/10.4067/S0717-65382004000100003.
https://doi.org/10.4067/S0717-6538200400...
).
Regarding its phenology, we extended the presence of larvae of all instars, which were originally described to occur from November to mid-December (Butler, 1882Butler, A., 1882. Additional notes on Bombyces collected in Chili by Mr. Edmonds. T Roy. Ent. Soc. Lond. 30, 101-108.; Barlett-Calvert, 1894Barlett-Calvert, W., 1894. Nuevos Lepidópteros de Chile. Anales Univ Chile. Mem. Cient. 87, 133-182.; Ureta, 1944Ureta, E., 1944. La familia Saturniidae (Heterocera) en Chile. III Parte. Bol. Mus. Nac. Chil. 22, 49-64.), to June until the end of January. We also reported for the first time the pupation period to occur from November to the end of March. Furthermore, we found that the flight period depends on its geographical location, spanning from February to mid-April in Central-North Chile, and mid-April (as reported in Butler, 1882Butler, A., 1882. Additional notes on Bombyces collected in Chili by Mr. Edmonds. T Roy. Ent. Soc. Lond. 30, 101-108.; Barlett-Calvert, 1894Barlett-Calvert, W., 1894. Nuevos Lepidópteros de Chile. Anales Univ Chile. Mem. Cient. 87, 133-182.; Ureta, 1944Ureta, E., 1944. La familia Saturniidae (Heterocera) en Chile. III Parte. Bol. Mus. Nac. Chil. 22, 49-64.; Artigas, 1994Artigas, J. 1994. Entomología económica, insectos de interés agrícola, forestal, médica y veterinarios (Nativos, introducidos y que pueden ser introducidos). Editorial Universidad de Concepción, Concepción.; Angulo et al., 2004Angulo, A. O., Lemaire, C., Olivares, T. S., 2004. Catálogo crítico e ilustrado de las especies de la familia Saturniidae en Chile (Lepidoptera: saturniidae). Gayana (Concepc.) 68, 20-42. https://doi.org/10.4067/S0717-65382004000100003.
https://doi.org/10.4067/S0717-6538200400...
; Rebolledo et al., 2006Rebolledo, R. R., Rojas, R. P., Parra, B. L., Angulo, O. A., 2006. Vuelo y abundancia estacional de los satúrnidos (Lepidoptera) del llano central de la novena Región de La Araucanía, Chile. Rev. Chil. Entomol. 32, 31. Available in: http://www.insectachile.cl/rchen/pdfs/2006v32/Rebolledo_et_al_2006.pdf (accessed 06 November 2020).
http://www.insectachile.cl/rchen/pdfs/20...
) to mid-June (as reported in this study) in Central-South Chile.
In relation to its distributional range, we summarise in this article all the information available so far about the species distribution. We indicate, for example, that the report made by Zúñiga-Reinoso and Sepúlveda (2016)Zúñiga-Reinoso, Á., Sepúlveda, J., 2016. Sobre la planta hospedera de Cercophana frauenfeldii (Felder) (Lepidoptera: Saturniidae) en Magallanes. An. Inst. Patagon. 44, 81-83. https://doi.org/10.4067/s0718-686x2016000200009.
https://doi.org/10.4067/s0718-686x201600...
of a parasitized larva of C. frauenfeldii occurring at the Omora Ethnobotanical Park in Magallanes, confirms previous reports made by Ureta (1944)Ureta, E., 1944. La familia Saturniidae (Heterocera) en Chile. III Parte. Bol. Mus. Nac. Chil. 22, 49-64., validated by Artigas (1994)Artigas, J. 1994. Entomología económica, insectos de interés agrícola, forestal, médica y veterinarios (Nativos, introducidos y que pueden ser introducidos). Editorial Universidad de Concepción, Concepción. and Angulo et al. (2004)Angulo, A. O., Lemaire, C., Olivares, T. S., 2004. Catálogo crítico e ilustrado de las especies de la familia Saturniidae en Chile (Lepidoptera: saturniidae). Gayana (Concepc.) 68, 20-42. https://doi.org/10.4067/S0717-65382004000100003.
https://doi.org/10.4067/S0717-6538200400...
, who proposed a distributional range between Valparaíso and Tierra del Fuego (in the XII Region).
In regards to its host plants, we add G. keule as a new host plant for the larval stage of C. frauenfeldii, which has also been recorded in Cryptocarya alba (Mol.) (peumo) by Butler (1882)Butler, A., 1882. Additional notes on Bombyces collected in Chili by Mr. Edmonds. T Roy. Ent. Soc. Lond. 30, 101-108. and on buds of Beilschmiedia miersii (Gay) (the northern acorn) by Barlett-Calvert (1894)Barlett-Calvert, W., 1894. Nuevos Lepidópteros de Chile. Anales Univ Chile. Mem. Cient. 87, 133-182.. Furthermore, Artigas (1994)Artigas, J. 1994. Entomología económica, insectos de interés agrícola, forestal, médica y veterinarios (Nativos, introducidos y que pueden ser introducidos). Editorial Universidad de Concepción, Concepción. mentions that larvae of C. frauenfeldii can defoliate Persea americana (Mill.) (common avocado tree), which was in turn also confirmed by Angulo et al. (2004)Angulo, A. O., Lemaire, C., Olivares, T. S., 2004. Catálogo crítico e ilustrado de las especies de la familia Saturniidae en Chile (Lepidoptera: saturniidae). Gayana (Concepc.) 68, 20-42. https://doi.org/10.4067/S0717-65382004000100003.
https://doi.org/10.4067/S0717-6538200400...
, although in the case of Beilschmiedia miersii this should be carefully considered as no other observations have been made so far. Also, the record of C. frauenfeldii feeding on Drymis winteri J.R. Forst. and G. Forst (winter’s bark or canelo) made by Zúñiga-Reinoso and Sepúlveda (2016)Zúñiga-Reinoso, Á., Sepúlveda, J., 2016. Sobre la planta hospedera de Cercophana frauenfeldii (Felder) (Lepidoptera: Saturniidae) en Magallanes. An. Inst. Patagon. 44, 81-83. https://doi.org/10.4067/s0718-686x2016000200009.
https://doi.org/10.4067/s0718-686x201600...
makes it possible to consider D. winteri as the first non-Laurales species of host plant ever recorded for C. frauenfeldii. In this regard, all five recorded host plant species are aromatic trees phylogenetically placed in the Magnoliid clade, a basal lineage of the angiosperms (Endress and Igersheim, 2000Endress, P. K., Igersheim, A., 2000. Gynoecium structure and evolution in basal Angiosperms. Int. J. Plant Sci. 161, 211-223. https://doi.org/10.1086/317572.
https://doi.org/10.1086/317572...
). Three hosts belong to the Lauraceae family: C. alba, B. miersii and P. americana, the latter being the only non-native host recorded so far. These three species, along with G. keule (Gomortegaceae), belong to Laurales.
Another remarkable feature of C. frauenfeldii is the colouration pattern observed on the dorsal area, which resembles that of a damaged leaf of G. keule (Fig. 3E). This interesting feature should be carefully explored in the future as it could represent a case of, for instance, the ‘reduction in camouflage’ hypothesis proposed by Koski et al. (2017)Koski, T., Lindstedt, C., Klemola, T., Troscianko, J., Mäntylä, E., Tyystjärvi, E., Stevens, M., Helander, M., Laaksonen, T., 2017. Insect herbivory may cause changes in the visual properties of leaves and affect the camouflage of herbivores to avian predators. Behav. Ecol. Sociobiol. 71, 97. https://doi.org/10.1007/s00265-017-2326-0.
https://doi.org/10.1007/s00265-017-2326-...
, which states that changes in leaf colour caused by the herbivore can increase its conspicuousness and the risk of being detected by predators. In the case of this species, this could be crucial to understand its life cycle and survival strategies (Gunnarsson et al., 2018Gunnarsson, B., Wallin, J., Klingberg, J., 2018. Predation by avian insectivores on caterpillars is linked to leaf damage on oak (Quercus robur). Oecologia 188, 733-741. https://doi.org/10.1007/s00442-018-4234-z.
https://doi.org/10.1007/s00442-018-4234-...
).
Conclusions
This study contributes to advancing the knowledge of the immature stages of C. frauenfeldii. Furthermore, the paper also unifies scattered knowledge about its phenology, distributional range and host plants, which becomes crucial to understanding the relationship of the species with its environment. Of particular interest is the presence of this saturnid on the host G. keule, since few insects have been consistently observed to feed on this endangered tree that is endemic to the Central-South coast of Chile. Future research should investigate the relation of C. frauenfeldii with its special set of primitive angiosperm host trees. The conservation of these organisms becomes particularly relevant since their habitat occurs in a global biodiversity hotspot (Myers et al., 2000Myers, N., Mittermeier, R. A., Mittermeier, C. G., Da Fonseca, G. A. B., Kent, J., 2000. Biodiversity hotspots for conservation priorities. Nature 403, 853-858. https://doi.org/10.1093/acrefore/9780199389414.013.95.
https://doi.org/10.1093/acrefore/9780199...
). Hence, the temperate forests of southern South America in Chile and Argentina are part of insufficiently known ecosystems, where species such as the one studied here face severe threats posed by forestry and agricultural activities, urban expansion and climatic change.
Acknowledgments
The authors would like to thank the Government of Chile - ANID (Agencia Nacional de Investigación y Desarrollo) [FONDECYT Iniciación 11110375] for funding our fieldwork, Forestal CELCO S.A. and the Corporación Nacional Forestal (CONAF) for providing field permissions. We would also like to thank the staff of Facultad de Ciencias Naturales y Oceanográficas de la Universidad de Concepción: Einer, María José and Francisca for making us feel part of the team and for the support provided during the laboratory work. Thanks also to Mr. Marcos Beéche of the Servicio Agrícola y Ganadero (SAG) de Chile, who kindly provided us with information about the species genitalia. Finally, we would like to thank Dr. Mariana Lazzaro-Salazar for proofreading the manuscript.
References
- Angulo, A. O., Lemaire, C., Olivares, T. S., 2004. Catálogo crítico e ilustrado de las especies de la familia Saturniidae en Chile (Lepidoptera: saturniidae). Gayana (Concepc.) 68, 20-42. https://doi.org/10.4067/S0717-65382004000100003
» https://doi.org/10.4067/S0717-65382004000100003 - Angulo, A. O., Weigert, G., 1974. Estados preimaginales de Polythysana cinerascens (Phil.) (Lepidoptera: Saturniidae). Bol. Soc. Biol. Concepción 47, 145-150. Available in: http://www.micra.cl/media/documentos/AnguloWeigert_1974_Polythysana_cinerascens pdf (accessed 06 November 2020)
» http://www.micra.cl/media/documentos/AnguloWeigert_1974_Polythysana_cinerascens - Artigas, J. 1994. Entomología económica, insectos de interés agrícola, forestal, médica y veterinarios (Nativos, introducidos y que pueden ser introducidos). Editorial Universidad de Concepción, Concepción.
- Barlett-Calvert, W., 1894. Nuevos Lepidópteros de Chile. Anales Univ Chile. Mem. Cient. 87, 133-182.
- Butler, A., 1882. Additional notes on Bombyces collected in Chili by Mr. Edmonds. T Roy. Ent. Soc. Lond. 30, 101-108.
- Deml, R., Dettner, K., 2002. Morphology and classification of larval scoli of Saturniinae and Hemileucinae (Lepidoptera: saturniidae). J. Zool. Syst. Evol. Res. 40, 82-91. https://doi.org/10.1046/j.1439-0469.2002.00181.x
» https://doi.org/10.1046/j.1439-0469.2002.00181.x - Endress, P. K., Igersheim, A., 2000. Gynoecium structure and evolution in basal Angiosperms. Int. J. Plant Sci. 161, 211-223. https://doi.org/10.1086/317572
» https://doi.org/10.1086/317572 - Gunnarsson, B., Wallin, J., Klingberg, J., 2018. Predation by avian insectivores on caterpillars is linked to leaf damage on oak (Quercus robur). Oecologia 188, 733-741. https://doi.org/10.1007/s00442-018-4234-z
» https://doi.org/10.1007/s00442-018-4234-z - Heppner, J. B., 2002. Mexican Lepidoptera biodiversity. Insecta Mundi 16, 171-190. Available in: http://digitalcommons.unl.edu/insectamundi/550 (accessed 06 November 2020).
» http://digitalcommons.unl.edu/insectamundi/550 - Hinton, H. E., 1946. On the homology and nomenclature of the setae of lepidopterous larvae, with some notes on the phylogeny of the Lepidoptera. T. Roy. Ent. Soc. Lond. Trans. R. Entomol. Soc. Lond. 97, 1-37. https://doi.org/10.1111/j.1365-2311.1946.tb00372.x
» https://doi.org/10.1111/j.1365-2311.1946.tb00372.x - Jordan, K., 1924. On the saturnoidean families Oxytenidae and Cercophanidae. Novit. Zool. 31, 135-193.
- Kitching, I. J., Rougerie, R., Zwick, A., Hamilton, C., St Laurent, R., Naumann, S., Mejia, L. B., Kawahara, A. Y., 2018. A global checklist of the Bombycoidea (Insecta: lepidoptera). Biodivers. Data J. (6), https://doi.org/10.3897/BDJ.6.e22236
» https://doi.org/10.3897/BDJ.6.e22236 - Koski, T., Lindstedt, C., Klemola, T., Troscianko, J., Mäntylä, E., Tyystjärvi, E., Stevens, M., Helander, M., Laaksonen, T., 2017. Insect herbivory may cause changes in the visual properties of leaves and affect the camouflage of herbivores to avian predators. Behav. Ecol. Sociobiol. 71, 97. https://doi.org/10.1007/s00265-017-2326-0
» https://doi.org/10.1007/s00265-017-2326-0 - Lemaire, C., Minet, J., 1999. The Bombycoidea and their relatives. In: Kristensen, N.P. (Eds.). Handbuch der Zoologie, Band 4 Arthropods: Insecta, Teilband 35. Lepidoptera, moths and butterflies. Vol. 1: Evolution, systematics, and biogeography. W. de Gruyter, Berlin, pp. 321-353. https://doi.org/10.22215/etd/2007-08316
» https://doi.org/10.22215/etd/2007-08316 - Myers, N., Mittermeier, R. A., Mittermeier, C. G., Da Fonseca, G. A. B., Kent, J., 2000. Biodiversity hotspots for conservation priorities. Nature 403, 853-858. https://doi.org/10.1093/acrefore/9780199389414.013.95
» https://doi.org/10.1093/acrefore/9780199389414.013.95 - Núñez-Bustos, E., 2015. Catálogo preliminar de Saturniidae de Argentina, con veintiún nuevos registros (Lepidoptera: saturniidae). Trop. Lepid. Res. 25, 22-33. Available in: https://journals.flvc.org/troplep/article/view/89749 (accessed 06 November 2020).
» https://journals.flvc.org/troplep/article/view/89749 - Rebolledo, R. R., Rojas, R. P., Parra, B. L., Angulo, O. A., 2006. Vuelo y abundancia estacional de los satúrnidos (Lepidoptera) del llano central de la novena Región de La Araucanía, Chile. Rev. Chil. Entomol. 32, 31. Available in: http://www.insectachile.cl/rchen/pdfs/2006v32/Rebolledo_et_al_2006.pdf (accessed 06 November 2020).
» http://www.insectachile.cl/rchen/pdfs/2006v32/Rebolledo_et_al_2006.pdf - Regier, J. C., Grant, M. C., Mitter, C., Cook, C. P., Peigler, R. S., Rougerie, R., 2008. Phylogenetic relationships of wild silkmoths (Lepidoptera: Saturniidae) inferred from four protein‐coding nuclear genes. Syst. Entomol. 33 (2), 219-228. https://doi.org/10.1111/j.1365-3113.2007.00416.x
» https://doi.org/10.1111/j.1365-3113.2007.00416.x - Stehr, F. W., 1987. Immature insects. Kendall/Hunt Publishing, Dubuque.
- Ureta, E., 1944. La familia Saturniidae (Heterocera) en Chile. III Parte. Bol. Mus. Nac. Chil. 22, 49-64.
- Wolfe, K. L., Balcázar-Lara, M. A., 1994. Chile’s Cercophana venusta and its immature stages (Lepidoptera: cercophanidae). Trop. Lepid. Res. 5, 35-42.
- Zúñiga-Reinoso, Á., Sepúlveda, J., 2016. Sobre la planta hospedera de Cercophana frauenfeldii (Felder) (Lepidoptera: Saturniidae) en Magallanes. An. Inst. Patagon. 44, 81-83. https://doi.org/10.4067/s0718-686x2016000200009
» https://doi.org/10.4067/s0718-686x2016000200009
Edited by
Associate Editor:
Publication Dates
-
Publication in this collection
28 May 2021 -
Date of issue
2021
History
-
Received
06 Nov 2019 -
Accepted
09 May 2021