Abstract
Interaction between Musca domestica L. and its predator Muscina stabulans (Fallén) (Diptera, Muscidae): Effects of prey density and food source abundance. The objective of this work was to evaluate the influence of prey density and food source abundance on the predatory behavior of Muscina stabulans over M. domestica. Three predator/prey proportions were evaluated (1:1, 1:3 and 1:6), using 100 third instar predator larvae against second instar prey larvae. Each proportion was maintained using three different levels of food substrate (25, 50 and 100 g). The experiments were carried out in triplicate in BOD incubators (25ºC, UR 70% ± 10% and 12 h photoperiod). The mortality of the M. domestica larvae was 100% under all conditions, except in the 1:6 predator/prey proportion, at the 50g and 100g food substrate levels, where it was 99.99% and 99.22%, respectively. There was a significant increase in the development period of M. stabulans in relation to the increase in prey density and decrease in quantity of food substrate. An increase in the proportion of individuals and a reduction in the amount of resource slowed down larval development. Muscina stabulans pupal weight was proportional to the increase in prey density and the amount of food substrate. The proportion or the density influenced the survival of M. stabulans, with no difference in relation to the amount of food source and consequently in the interaction of the factors. There was no difference between the 1:1 and 1:3 predator-prey densities, with both differing from the 1:6 density.
Insecta; intraguild predation; larval predation; predatory behavior
BIOLOGY, ECOLOGY AND DIVERSITY
Interaction between Musca domestica L. and its predator Muscina stabulans (Fallén) (Diptera, Muscidae): effects of prey density and food source abundance
Juliano Lessa Pinto DuarteI,II; Rodrigo Ferreira KrügerI,III; Paulo Bretanha RibeiroI,IV
IDepartamento de Microbiologia e Parasitologia, Instituto de Biologia, Universidade Federal de Pelotas, Caixa postal 354, 96010900, Pelotas, RS, Brazil
ABSTRACT
Interaction between Musca domestica L. and its predator Muscina stabulans (Fallén) (Diptera, Muscidae): Effects of prey density and food source abundance. The objective of this work was to evaluate the influence of prey density and food source abundance on the predatory behavior of Muscina stabulans over M. domestica. Three predator/prey proportions were evaluated (1:1, 1:3 and 1:6), using 100 third instar predator larvae against second instar prey larvae. Each proportion was maintained using three different levels of food substrate (25, 50 and 100 g). The experiments were carried out in triplicate in BOD incubators (25ºC, UR 70% ± 10% and 12 h photoperiod). The mortality of the M. domestica larvae was 100% under all conditions, except in the 1:6 predator/prey proportion, at the 50g and 100g food substrate levels, where it was 99.99% and 99.22%, respectively. There was a significant increase in the development period of M. stabulans in relation to the increase in prey density and decrease in quantity of food substrate. An increase in the proportion of individuals and a reduction in the amount of resource slowed down larval development. Muscina stabulans pupal weight was proportional to the increase in prey density and the amount of food substrate. The proportion or the density influenced the survival of M. stabulans, with no difference in relation to the amount of food source and consequently in the interaction of the factors. There was no difference between the 1:1 and 1:3 predator-prey densities, with both differing from the 1:6 density.
Keywords: Insecta; intraguild predation; larval predation; predatory behavior.
Due to their importance in livestock, Muscina stabulans (Fallén, 1817) and Musca domestica L. are among the muscid species with the best known biology and ecology (Axtell & Arends 1990; Mascarini & Prado 2002; Krüger & Erthal 2006; Krüger et al. 2010).
These species develop on a variety of discrete and ephemeral food substrates, such as feces and carcasses, which provide a limited amount of food. Therefore, they often face several levels of intra- and interespecific competition, with individuals of various species trying to acquire the maximum amount of food in the shortest period of time before the complete exhaustion of the resource (Zimmer et al. 2006).
The interaction between the insect and the food resource is extremely important because adult size will be determined by the amount of food consumed in the larval stage, with implications in their fecundity and survival that reflects directly on the population dynamics (Reis et al. 1994; Zimmer et al. 2006).
Muscina stabulans is a third instar facultative predator and, in this case, high levels of local competition can turn the competitor into an active predator. This kind of facultative predation was classified by Polis et al. (1989) as intraguild predation (IGP), which consists of a combined interaction of competition and predation. Whereby individuals from one species kill and consume individuals from another species that use similar limiting resources. Therefore, this interaction may result in drastic changes to the population dynamics of those involved and, depending on the intensity of this interaction, may even eliminate the prey population (Holt & Polis 1997).
For these reasons, previous research has suggested that M. stabulans could be used for the biological control of M. domestica in livestock farms (Skidmore 1985; Legner & Dietrich 1989). The use of predators to control fly larvae requires an evaluation of the predator-prey relationship, as their densities are the two basic components necessary for understanding the population dynamics of these species (Holling 1961).
Little is known on how this IGP acts in situations that involve different amounts of available food sources and different prey densities. These aspects must be investigated in order to understand the dynamics of the interaction between the prey and the predator species, as well as the factors that trigger the switch from a competitor-type behavior to a predator-type (Rosa et al. 2006). Thus, the objective of this work was to evaluate how prey availability and the amount of food resource influences the predatory behavior of M. stabulans on M. domestica larvae.
MATERIAL AND METHODS
Colony maintenance. The M. domestica and M. stabulans colonies were kept in an acclimatized room, at 25ºC ± 2ºC, relative air humidity of 80% ± 10% and a 12 h photo phase. The adults were maintained in 30x30x30 cm cages and fed on a diet composed of one part meat meal and two parts of sugar; water was supplied ad libitum. To obtain eggs, plates with culture medium (two parts of meat meal, one part of sawdust and water) were placed inside the cages. The eggs were transferred to containers with the same diet and placed inside larvae rearing funnels. The larvae were fed until they reached the third instar stage, when they left the funnel and fell into another container of humid sawdust. The post-feeding larvae (Fraenkel & Bhaskaran 1973) were transferred to glass containers with humid sawdust and kept until adult emergence for the replacement of the cages.
Experimental design. The larvae used in the experiments were obtained from the stock colonies. The culture medium was exposed to the adults for one hour, the deposited eggs were removed and incubated in BOD chamber at 25ºC until the larvae reached the desired stage. The M. stabulans larvae were kept in excess culture medium until they reached the third larval instar, when they were considered to be predators (Skidmore 1985). The M. domestica larvae were kept under the same conditions as the M. stabulans larvae, until they reached the second larval instar when they were considered to be prey. The larval instars were based on previous studies of the biology of the species (Krüger & Erthal 2006; Mascarini & Prado 2002; Zimmer et al. 2006), and the confirmation based in morphological characters (Skidmore 1985).
Using 100 predator larvae, three predator-prey proportions were established: 1:1, 1:3, 1:6. Each proportion was maintained under three food substrate levels of 25, 50 and 100g and each experiment was carried out in triplicate. The larvae were kept in plastic recipients in B.O.D. chambers at 25ºC, relative air humidity of 70 ± 10% and a 12 hour photoperiod and were observed daily. Forty-eight hours after pupation thirty M. stabulans pupae were randomly collected and weighed. The development period of the third instar M. stabulans larvae was determined from the beginning of the experiment until the pupation of the larvae. Musca domestica and M. stabulans survival levels were determined from the number of adults that emerged by the end of the experiment.
The influence of prey density and food abundance in the development period and survival of M. stabulans were evaluated by an analysis of covariance (ANCOVA), while their influence on the pupal weight was evaluated by an analysis of variance (ANOVA). All the data were analyzed using the software R (R Development Team 2010), and differences were considered significant when the P value was < 0.05.
RESULTS AND DISCUSSION
The mortality of the M. domestica larvae was 100% under all conditions, except at the 1:6 predator-prey proportion with 50g and 100g of food source. Under these conditions, only four and 14 larvae survived, respectively, resulting in a mortality of 99.99% and 99.22%. This high predatory rate was already shown by other azeliins, such as Ophyra aenescens (Geden et al. 1988) and O. capensis (Olckers & Hulley 1984). The high rate, even with a high abundance of food source and a low predator-prey proportion, could suggest that the M. stabulans larvae may have a preference for feeding on the M. domestica larvae instead of the food substrate. This behavior was also observed with O. capensis (Olckers & Hulley 1984) and Ophyra leucostoma (Anderson & Poorbaugh 1964).
Holt & Polis (1997) stated that in a general model of IGP, an optimally foraging predator would be expected to drop the prey from its diet when the food resource is abundant, as represented by the 1:1 predator-prey proportion/100g experiment, thus avoiding the additional energetic loss involved in the search, capture and handling of the prey. However, for these insects, the larval stage is the main period of resource limitation (Price 1997), and therefore, in addition to the nutritional gain, predation eliminates future or present competitors, thereby ensuring the maintenance of the resource so that the predators can complete their development. This concept was also treated by Holt & Polis (1997) while considering an IGP model that incorporates the aspects of exploitative competition (Tilman 1982) where the inferior competitor (predator) must gain sufficient from predation to offset competitive inferiority in order to co-exist. Faria et al. (1999) and Rosa et al. (2006), also showed that predation offered more advantages to Chrysomya albiceps (Diptera, Calliphoridae) than competition for food in limiting resources.
The development period of M. stabulans increased in direct proportion to the prey density and was inversely proportional to the quantity of food substrate (F5,21 = 30.38; P < 0.001). An increase in the prey proportion (F2,21 = 61.55; P < 0.001) and a reduction in the amount of resources (F1,21 = 19.34; P < 0.001) slowed down larvae development (Fig. 1). There were distinct patterns of interaction between these factors (F2,21 = 4.73; P = 0.02) ranging from 5.7 ± 0.59 days at the 1:1 proportion with 100g substrate to 9.17 ± 0.65 days at the 1:6 proportion with 25g substrate.
According to Roper et al. (1996) and Zimmer et al. (2006), an increase in the development period can be explained by a delay in obtaining the minimum weight required for the pupation process, since the immature larvae stay longer in the substrate in order to acquire sufficient mass. The increase in the development period of M. stabulans was probably due to the difficulty in obtaining food in the low resource experiments.
Similarly, the observed increase in the development period in the experiments with higher prey density could be explained by an increase in the interespecific competition from the first moment of the interaction, where the M. domestica larvae could deplete the substrate significantly before they were preyed upon by the M. stabulans larvae. Moreover, the increase in the development period of M. stabulans related to the increase in the M. domestica density could be explained by an additional energetic expenditure involved in the predatory act, since with a greater number of available preys, there is a greater time spent handling them.
Muscina stabulans pupal weight tended to rise with increasing prey density and food (Fig. 2), in contrast to what was reported by Zimmer et al. (2006) on intraspecific competition in M. stabulans. However, at the 1:6 proportion the weight increase seemed to show a tendency to stabilize at the higher prey proportion. An experiment with higher prey proportions should be performed in order to confirm this trend.
The increase in the pupal weight due to the increased supply of prey suggests that predation provides nutritional benefits to the M. stabulans larvae over the saprophagic behavior. Anderson & Poorbaugh (1964) observed this behavior in O. leucostoma and noted that even M. domestica larvae preferred to feed on previously killed and not consumed conspecifics, suggesting that the nutritional content of dead larvae is higher than the food substrate in question.
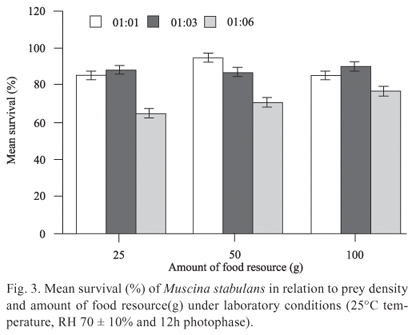
The average survival of M. stabulans was inversely proportional to the increase in prey density, showing a relatively low average survival at the 1:6 proportion (Fig. 3). The predator-prey proportion influenced M. stabulans survival (F2,21 = 19.34; P < 0.001), while the amount of food resources had no impact on survival (F1,21 = 1.60; P = 0.22) and consequently in the interaction of the factors (F2,21 = 1.49; P = 0.24). There was no significant difference between the 1:1 and 1:3 proportions (P = 0.78), with both differing significantly from the 1:6 proportion (P < 0.001).
It is known that individuals within a population have different genetically determined biological parameters, where the fittest individuals have a competitive advantage over their conspecifics (Slansky & Rodrigues 1987). Thus, the increase in energy expenditure may have influenced the survival of the predators, as in an early stage of interaction, those individuals with lower predation rates, and consequently lower rates of food conversion, are overcome by the more efficient predators, failing to reach the minimum weight for pupation.
Mueller et al. (2005) stated that the best competitors on limited resources are those who feed in a shorter period of time. Thus, this decrease in the average survival of M. stabulans in the proportions with higher prey density was followed by an increase in the average pupal weight, since the death of less fit individuals should have produced a decrease in intraspecific competition.
Several studies with various species of flies showed that pupal weight is directly related to the size and fecundity of the adult (Goodbrod & Goff 1990; Reis et al. 1994; Tardelli et al. 2004; Zimmer et al. 2006; Pires et al. 2009), showing that bigger adults have higher fecundity. Thus, considering these results at a population level, the reduced survival of M. stabulans would not have a significant impact on the population, since the higher fecundity of the adults generates a compensatory effect in the next generation.
Classic IGP is thought to occur when food sources are scarce, providing an alternative resource (Polis et al. 1989; Hanski 1987). However, our results suggest that for M. stabulans, IGP happens even when food is available. Perhaps the quality of the food source may play an important role in changing the behavior of the predator. Therefore, further studies with different diets are needed in order to better understand the predation behavior of M. stabulans.
ACKNOWLEDGEMENTS
We thank the two anonymous reviewers for valuable suggestions. This study was supported by a CNPq grant (process number 136323/20081).
Received 5 July 2012; accepted 7 November 2012
Associate Editor: Maurício O. Moura
- Anderson, J.R. & Poorbaugh, J.H. 1964. Biological control possibility for house flies. California Agriculture 18: 2-4.
- Axtell, R.C & Arends, J.J. 1990. Ecology and management of arthropod pests of poultry. Annual Review of Entomology 35: 101-126.
- Faria, L.D.B., Orsi, L., Trinca, L.A. & Godoy, W.A.C. 1999. Larval predation by Chrysomya albiceps on Cochliomyia macellaria, Chrysomya megacephala and Chrysomya putoria Entomologia experimentalis et applicata 90: 149-155.
- Fraenkel, G. & Bhaskaran, G. 1973. Pupariation and pupation in cyclorrhaphous flies (Diptera): terminology and interpretation. Annals of the Entomological Society of America 66: 418-422.
- Geden, C.J., Stinner, R.E. & Axtell, R. C. 1988. Predation by predators of the house fly in poultry manure: effects of predator density, feeding history, interspecific interference, and field conditions. Environmental Entomology 17: 320-329.
- Goodbrod, J.R. & Goff, M.L. 1990. Effects of larval population density on rates of development and interactions between two species of Chrysomya (Diptera: Calliphoridae) in laboratory culture. Journal of Medical Entomology 27: 338-343.
- Hanski, I. 1987. Carrion fly community dynamics: patchiness, seasonality and coexistence. Ecological Entomology 12: 257-266.
- Holling, C. 1961. Principles of insect predation. Annual Review of Entomology 6: 163-182.
- Holt, R.D. & Polis, G.A. 1997. A theoretical framework for intraguild predation. Oikos 37: 306-312.
- Krüger, R.F. & Erthal, S.G. 2006. Estimativa de entropia de Muscina stabulans (Fallén) (Diptera, Muscidae) em condições artificiais. Revista Brasileira de Entomologia 50: 275-279.
- Krüger, R.F., Ribeiro, P.B., Erthal, S.G. & DeSouza, O. 2010. Reproduction and survival of Muscina stabulans under laboratory conditions. Ciência Rural 40: 674-677.
- Legner, E.F. & Dietrich, E.J. 1989. Coexistance of predatory Muscina stabulans and Ophyra aenescens (Diptera: Muscidae) with dipterous preys in poultry manure. Entomophaga 34: 453-461.
- Mascarini, L.M. & Prado, A.P.P. 2002. Thermal constant of an experimental population of Muscina stabulans (Fallén 1817) (Diptera: Muscidae) in the laboratory. Memórias do Instituto Oswaldo Cruz 97: 281-283.
- Mueller, L.D., Folk, D.G., Nguyen, N., Nguyen, P., Lam, P., Rose, M.R. & Bradley, T. 2005. Evolution of larval foraging behavior in Drosophila and its effects on growth and metabolic rates. Physiological Entomology 30: 262-269.
- Olckers, T. & Hulley, P.E. 1984. Facultative predation of house fly larvae by larvae of Ophyra capensis (Wiedemann) (Diptera: Muscidae). Journal of the Entomological Society of South Africa 47: 231-237.
- Pires, S.M., Cárcamo, M.C., Zimmer, C.R. & Ribeiro, P.B. 2009. Influência da dieta no desenvolvimento e investimento reprodutivo de Chrysomya megacephala (Fabricius, 1794) (Diptera: Calliphoridae). Arquivos do Instituto Biológico 76: 1-47.
- Polis, G.A.; Myers, C.A. & Holt, R.D. 1989. The ecology and evolution of intraguild predation: potential competitors that eat each other. Annual Review of Ecology and Systematics 20: 297-330.
- Price, P.W. 1997. Insect Ecology. New York, John Wiley & Sons, 874 p.
- R Development Core Team. 2010. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Version 2.9.0. Available at http://www.R-project.org (accessed 19 May 2011).
- Reis, S.F., Stangenhaus, G., Godoy, W.A.C., Von Zuben, C.J. & Ribeiro, O.B. 1994. Variação em caracteres bionômicos em função de densidade larval em Chrysomya megacephala e Chrysomya putoria (Diptera: Calliphoridae). Revista Brasileira de Entomologia 38: 33-34.
- Roper, C., Pignatelli, P. & Partridge, L. 1996. Evolutionary responses of Drosophila melanogaster life history to differences in larval density. Journal of Evolutionary Biology 9: 609-622.
- Rosa, G.S., Carvalho, L.R., Reis, S.F. & Godoy, W.A.C. 2006. The dynamics of intraguild predation in Chrysomya albiceps Wied. (Diptera: Calliphoridae): Interactions between instars and species under different abundances of food. Neotropical Entomology 35: 775-780.
- Skidmore, P. 1985. The Biology of the Muscidae of the World. Dordecht, Kunk Publishers, 550 p.
- Slansky, F., Jr. & Rodriguez, J.G. 1987. Nutritional ecology of insects, mites, spiders and related invertebrates: an overview, p.1-69. In: Slansky, F., Jr. & Rodriguez, J.G. (eds.). Nutritional ecology of insects, mites, spiders and related invertebrates. New York, John Wiley, 1016 p.
- Tardelli, C.A., Godoy, W.A.C. & Mancera, P.F.A. 2004. Population dynamics of Musca domestica (Diptera: Muscidae): Experimental and theoretical studies at different temperatures. Brazilian Archives of Biology and Technology 47: 775-783.
- Tilman, D. 1982. Resource Competition and Community Structure. Princeton, Princeton University Press, 296 p.
- Zimmer, C.R., Pires, S.M., Cárcamo, M.C. & Ribeiro, P.B. 2006. Efeitos da competição larval intra-específica sobre caracteres biométricos de Muscina stabulans (Fallén, 1817). Arquivos do Instituto Biológico 73: 203-209.
Publication Dates
-
Publication in this collection
01 Apr 2013 -
Date of issue
Mar 2013
History
-
Received
05 July 2012 -
Accepted
07 Nov 2012