Abstracts
Human cases of American cutaneous leishmaniasis (ACL) have been recorded in Serra da Cantareira, in the Greater São Paulo Metropolitan Region, where two conservation units are situated, the Parque Estadual da Cantareira and the Parque Estadual Alberto Löfgren. The present study aimed to identify the sandfly fauna and some of its ecological aspects in these two parks and their surrounding area to investigate Leishmania sp. vectors. The captures were undertaken monthly from January to December 2009, from 6:00 p.m. to 6:00 a.m., with automatic light traps installed in forests and peridomicile areas and with modified black/white Shannon traps in the peridomicile. A total of 12 species and 5,436 sandflies were captured: with automatic light traps (141), Shannon traps (5,219) and attempting to bite the researchers while they were conducting the collection in Shannon traps (76). Pintomyia fischeri and Migonemyia migonei were the most abundant species. Pi. fischeri predominated in all three kinds of captures (49%, 88.8% and 65.8%, respectively). Mg. migonei was the second most prevalent in Shannon traps (10.0%) and attempting to bite the researchers (22.4%). Pi. fischeri females were significantly more attracted to black and those of Mg. migonei to white Shannon traps. A positive and significant correlation was observed between the numbers of Pi. fischeri and the mean of minimum relative humidity values on the fifteen days prior to capture, while there was a negative and significant correlation between the relative humidity on the capture day and the two most abundant species. The anthropophilia and high frequencies of Pi. fischeri and Mg. migonei suggest that both species may be transmitting ACL agents in this region.
Cutaneous leishmaniasis; Ecology; Vectors; Pintomyia fischeri; Migonemyia migonei
Casos humanos de leishmaniose tegumentar americana (LTA) têm sido registrados na Serra da Cantareira, região da Grande São Paulo, onde se situam o Parque Estadual da Cantareira e o Parque Estadual Alberto Löfgren. O estudo teve como objetivo identificar a fauna flebotomínea e alguns de seus aspectos ecológicos nos dois parques e área adjacente, para investigar vetores de Leishmania sp. As capturas ocorreram de janeiro a dezembro de 2009, das 18 às 06 horas, com armadilhas automáticas luminosas instaladas em matas e peridomicílios, e com armadilhas de Shannon modificadas, nas cores branca e preta, em peridomicílio. Foram capturadas 12 espécies e 5.436 flebotomíneos por armadilhas automáticas luminosas (141), armadilhas de Shannon (5.219) e tentando picar os pesquisadores enquanto coletavam na armadilha de Shannon (76). Pintomyia fischeri e Migonemyia migonei foram as mais abundantes. Pi. fischeri predominou nos três métodos de coleta com 49,0%, 88,8% e 65,8%, respectivamente, e Mg. migonei foi a segunda mais prevalente na Shannon (10,0%) e tentando picar os pesquisadores (22,4%). Fêmeas de Pi. fischeri foram significativamente mais atraídas à Shannon preta e Mg. migonei à branca. Houve correlação positiva e significante entre o número de Pi. fischeri e a média das mínimas da umidade relativa nos 15 dias anteriores ao dia da coleta, e negativa e significante para as duas espécies mais abundantes em relação a umidade relativa no dia da coleta. Altas frequências e antropofilia de Pi. fischeri e de Mg. migonei sugerem que ambas podem estar atuando na transmissão da LTA na área.
Leishmaniose tegumentar americana; Ecologia; Vetores; Pintomyia fischeri; Migonemyia migonei
Introduction
Leishmaniases are significant zoonoses in Public Health, occurring in 88 countries, with 350 million people at risk of contracting the infections, with an annual incidence of 1.5 million new cases of the cutaneous form. In addition, Brazil is to be found among the five countries with the highest incidences1,2. The American cutaneous leishmaniasis (ACL) has various species of Leishmania as their etiological agents, several orders of mammals as their reservoir and diverse species of phlebotomines as vectors, depending on environmental factors1.
In the state of São Paulo, ACL has represented a serious Public Health problem in the northeastern region with the felling of forests in preparation for the construction of railways. In that environment, Nyssomyia whitmani and Pintomyia pessoai were suspected of being vectors of Leishmania. In the 1970s, isolated outbreaks of this disease were identified in rural areas of the state and in more extensive areas of the coastal strip, where the disease had not previously been recorded3. More recently, the disease has been found to occur practically throughout the entire state, with Ny. intermedia s. lat as its principal vector and Migonemyia migonei playing a secondary role4. However, it is worth noting that Nyssomyia intermedia s. lat. constitutes a complex of two species, Ny. intermedia s. str. and Ny. neivai5 . The former occurs on the coast, the latter on the high plains and both are found sympatrically in the Ribeira and Paraíba valley regions6,7.
As from this period, depending on the inter-relations of the particular biological, ecological and biogeographical factors of each region, ACL has acquired new epidemiological characteristics8. It occurs mainly in the proximity of forests in both rural and periurban areas, with an epidemiological pattern characterized by isolated micro-events interspersed with outbreaks8,9. In the Greater São Paulo metropolitan region, ACL cases have been recorded in fragmentary areas of the Atlantic forest, suggesting that Leishmania may remain in small forest ecosystems and human beings inhabiting the adjacent areas could be affected sporadically10. Recent reports of autochthonous ACL cases in this region in populated areas close to residual forests of Serra da Cantareira and the absence of recent data due to the scarcity of entomological studies on this region led to the investigation of the ecological aspects of the sandfly fauna. The following three areas of this range of hills were included to obtain data to identify potential vectors of Leishmania spp.: Parque Estadual da Cantareira (PEC - Cantareira State Park), Parque Estadual Alberto Löfgren (PEAL - Alberto Löfgren State Park) and an adjacent area outside the PEC.
Material and methods
Study area
Parque Estadual da Cantareira (PEC), situated at 23°22'S and 46°36'W at an altitude between 760 and 1,095 m above sea level, is considered by UNESCO (1994) as the largest native urban forest in the world. It covers an area of 7,916.52 ha and includes part of the cities of São Paulo, Caieiras, Mairiporã and Guarulhos. As part of the Atlantic Forest biome, it is characterized by dense ombrophilous and heterogeneous vegetation, in addition to many exotic species. The fauna of medium and large-sized mammals includes opossums, sloths, monkeys, deers, capybaras, coatis, armadillos, ocelots and pumas11. The climate is considered mesothermal, humid and without dry spells, the mean temperature in the warmest month being 22 °C and in the coldest, 14 ºC. The rainy season occurs from October to March, with a monthly average precipitation of 186 mm; and the dry season from April to September, when the monthly average precipitation is 51 mm. The total annual average rainfall is 1,570 mm12. Parque Estadual Alberto Löfgren (PEAL), popularly known as the "Horto Florestal", is a fully protected conservation unit and the largest green area for recreation for the population of the city of São Paulo. It is situated in the northern area of this city and occupies an area adjacent to the PEC., There is a farmstead located on the Roseiras road, in an area next to and northeast of this park, in the city of Mairiporã. A family used to live on the ground floor of the farm dwelling, while there was a real estate agency on the upper floor, about 50 m from a remnant of forest. In the region where this property is located, there have been reports of ACL cases.
Collections of sandflies
Sandflies were captured monthly from January to December 2009 (except in February, when there were two collections), using two techniques simultaneously: automatic light traps (CDC modified type)13, and modified black and white Shannon traps14, from 6:00 p.m. to 6:00 a.m., irrespective of summer time.
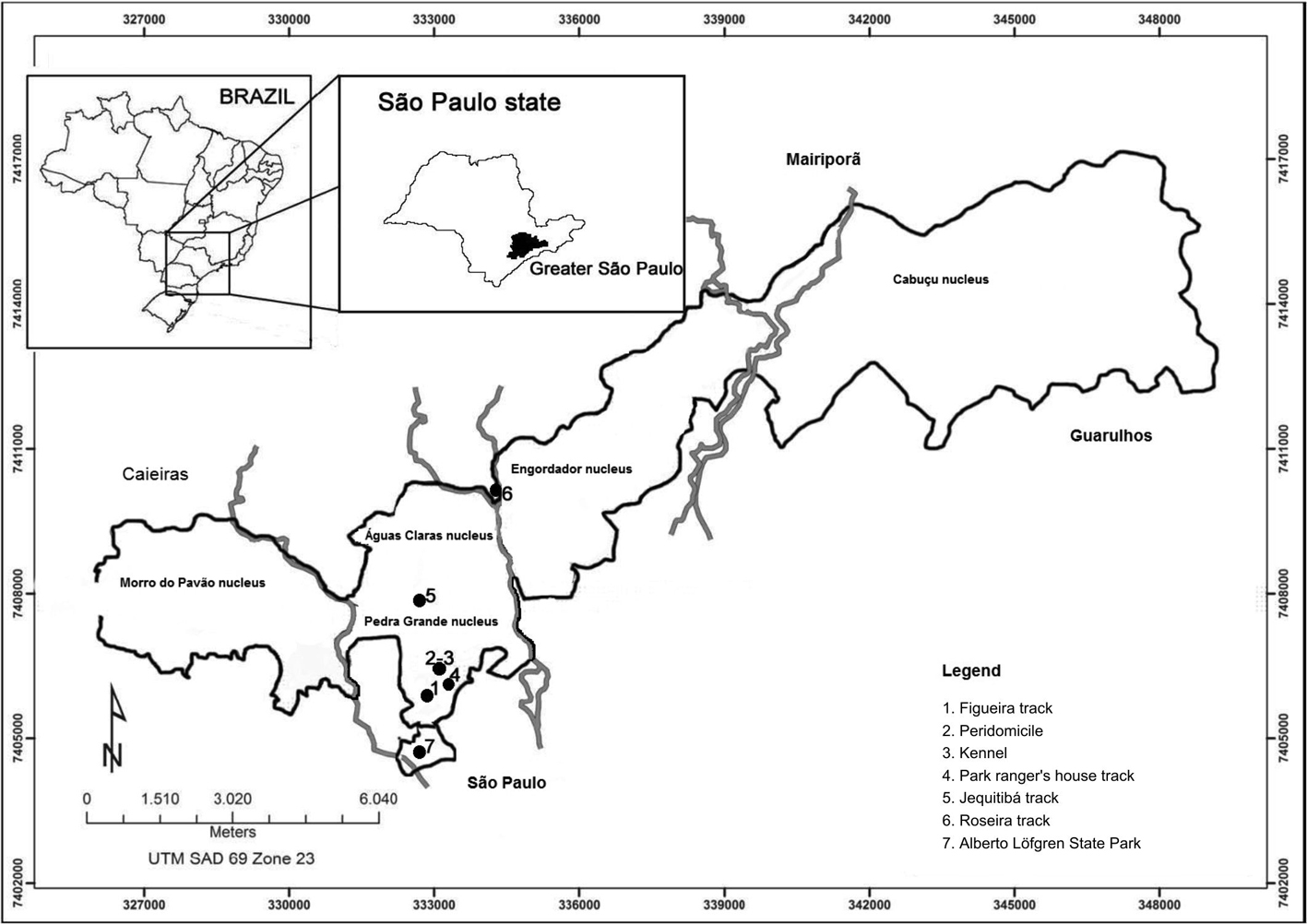
The automatic light traps were installed 1.5 m above the ground in seven sites, five in forests and two in the peridomicile, with the following distribution: PEC - 4 sites: 1) patch of forest in the Figueira track (23º 26' 59" S, 46º 38' 10" W); 2) path of forest in the Jequitibá track (23º 25' 50" S, 46º 38' 12" W); 3) patch of forest in the Park ranger's house track (23º 26' 48" S, 46º 37' 58" W); 4) a kennel in the peridomicile of an inhabited house (23º 26" 53" S, 46º 38' 13" W); PEAL - one site: 1) patch of forest on the lakeside in the capybaras' refuge (23º 27' 31" S, 46º 38' 08" W); and an area outside the parks on the Roseira road - two sites: 1) the porch of a residence (23º 24' 47" S, 46º 37' 20" W) and 2) patch of forest 100 m away from this residence (Figure 1).
Localization of the sites (1 a 7) where the captures of sandflies were undertaken: the two parks of Serra da Cantareira: Cantareira State Park (PEC) with its nuclei - Morro do Pavão, ÁguasClaras, Pedra Grande, Engordador and Cabuçu- and the sites (1 to 5), which are situated in the Pedra Grande Nucleus; and Alberto Löfgren State Park (PEAL) (7); and on the Roseira road (6). Figura 1 - Localização dos pontos(1 a 7)onde as capturas de flebotomíneos foram desenvolvidas: dois parques na Serra da Cantareira: Parque Estadual da Serra da Cantareira (PEC) com seus núcleos – Morro do Pavão, Águas Claras, Pedra Grande, Engordador e Cabuçu, e os pontos 1 a 5, todos localizados no núcleo Pedra Grande; e Parque Estadual Alberto Löfgren (PEAL) (7); e na estrada da Roseira (6).
Captures with black and white Shannon traps were carried out in the PEC, about 20 meters away from the kennel where the automatic light trap was installed.
To observe the attractiveness of the black and white colors, traps were installed side by side and had their position inverted for each successive capture. One gas lantern (350 watts) and two manual lanterns (15 amperes, 6 volts) were used as light sources. Each trap was continuously observed by one person and the sandflies that landed on their walls were captured with a 6-volt electric aspirator and separated into flasks at hourly intervals.
In certain collections with Shannon traps, the insects attempting to bite researchers were also captured.
Insects were kept in flasks, which were stored in Styrofoam boxes with a thin humid lining of plaster of Paris until the dissection of a sample of females for the investigation of natural infection by flagellates could be performed in the Laboratory of Entomology of the School of Public Health at the University of São Paulo. For this investigation, the females were dissected under a stereoscopic microscope (60x magnification) in sterile saline solution with the gut and spermathecae exposed. The identification15 of sandfly species and the search for flagellates was made under a microscope (400x magnification).
The undissected females and males were macerated according to the technique proposed by Forattini16 and identified in accordance with Galati's keys15. The abbreviation of species names follows Marcondes17.
Climatic data
Climatic data on rainfall, temperature and relative humidity were obtained from the Estação Meteorológica do Instituto Florestal do Estado de São Paulo (State of São Paulo Forest Institute Meteorology Agency).
Statistical analysis
The abundance, diversity and evenness of the species were calculated on the basis of the data on the captures carried out with automatic light traps. The Standardized Index of Species Abundance (SISA) was calculated in accordance with Roberts & Hsi18 and the Shannon's Diversity Index (H) and Pielou Evenness Index (J) were in accordance with Hayek and Buzas19. For the sandflies captured with black/white Shannon traps, the seasonal trend and the hourly rhythm of the most abundant species in four concurrent seasonal periods were obtained by Williams' average20. To compare the attractiveness of the black and white colors to sandfly species, the Chi square (c2) or binomial (n < 20) tests were used.
To investigate the correlation between the frequencies of both sexes of the two most abundant species and rainfall, the means of the minimum, maximum and mean temperatures on the 7th, 15th and 30th days prior to the capture day and also between the minimum, maximum and mean relative humidity on the capture day, Pearson's and Spearman correlation coefficients (PASW V.18) were used.
Results
A total of 5,436 sandflies of 12 species were captured using the three techniques of capture (Tables 1-3).
Numbers of sandflies captured with automatic light traps by sex, ecotopes, and Standardized index of species abundance (SISA), in the Cantareira State Park, from January to December 2009. Tabela 1 - Números de flebotomíneos capturados com armadilhas automáticas luminosas, por sexo, ecótopo e índice de abundância das espécies padronizado (IAEP), no Parque Estadual da Serra da Cantareira, de janeiro a dezembro de 2009.
Percentages of sandflies captured with automatic light traps, arithmetical average of specimens per trap, Shannon's diversity species (H) and Pielou evenness species (J) indices by site and locality. Cantareira State Park, JanuarytoDecember 2009. Tabela 2 - Porcentagens de flebotomíneos capturados com armadilhas automáticas luminosas, média aritmética de espécimes por armadilha, índices de diversidade de Shannon (H) e de equitabilidade de Pielou (J) por ponto e por localidade. Parque Estadual da Canteira (PEC). Janeiro a Dezembro de 2009.
Number of sandflies captured in the black and white Shannon traps by species, sex, female/male rate and black/white rate. Cantareira State Park (PEC). January to December 2009. Tabela 3- Número de flebotomíneos capturados nas armadilhas de Shannon branca ou preta, por espécie, sexo, razão fêmea/macho erazão preta/branca.Parque Estadual da Serra da Cantareira (PEC). Janeiro a Dezembro de 2009.
The percentages of both sexes captured with light traps, by species and site, with their respective arithmetical average per trap and the Shannon diversity (H) and Pielou evenness (J) indices, as well as the frequencies of species by locality are shown in Table 2. Only two species were captured in the PEAL and Psychodopygus lloydi was found in this locality exclusively. In the PEC, among the eleven species captured, Pi. fischeri predominated, followed by Mg. migonei and Pa. pascalei in equal percentages. On the Roseira road, among the four species captured, Psychodopygus ayrozai was the most frequent, followed by Pi. fischeri. The highest average per trap was obtained in the PEC, specifically on the Figueira track. The highest Shannon's diversity and low Pielou evenness indices were observed on the Figueira track, reflecting the highest richness of species and great predominance of one such species, whereas the opposite occurred at the site sampled in the PEAL.
The sandflies captured on the black and white modified Shannon traps and the attractiveness of these colors to sandfly species can be seen in Table 3. Pi. fischeri predominated greatly over all species. Mg. migonei was the second most frequent. Females of Pi. fischeri predominated significantly over males in both traps, while the females of Mg. migonei were significantly less attracted to black and more attracted to white than males. Females of Pi. fischeri were significantly more attracted to black and those of Mg. migonei to white.
The respective hourly rhythm of males and females of Pi. fischeri and Mg. migonei in the black and white Shannon traps is shown in Figure 1.
When comparing the hourly rhythm of the two species in both traps, among females in the white traps, those of Pi. fischeri showed three peaks of activity, two before and one after midnight, while those of Mg. migonei showed three; however, the latter were much smaller, none of which coincided with those of Pi. fischeri. In the black traps, the females of Pi. fischeri showed two peaks, one before and the other after midnight, while Mg. migonei showed very small peaks, none of which coincided with those of Pi. fischeri. Among males, on the white trap, both Pi. fischeri and Mg. migonei showed three peaks and the lowest and highest ones of both species coincided with each other; in the black trap, Pi. fischeri and Mg. migonei showed two peaks, but only that before midnight coincided.
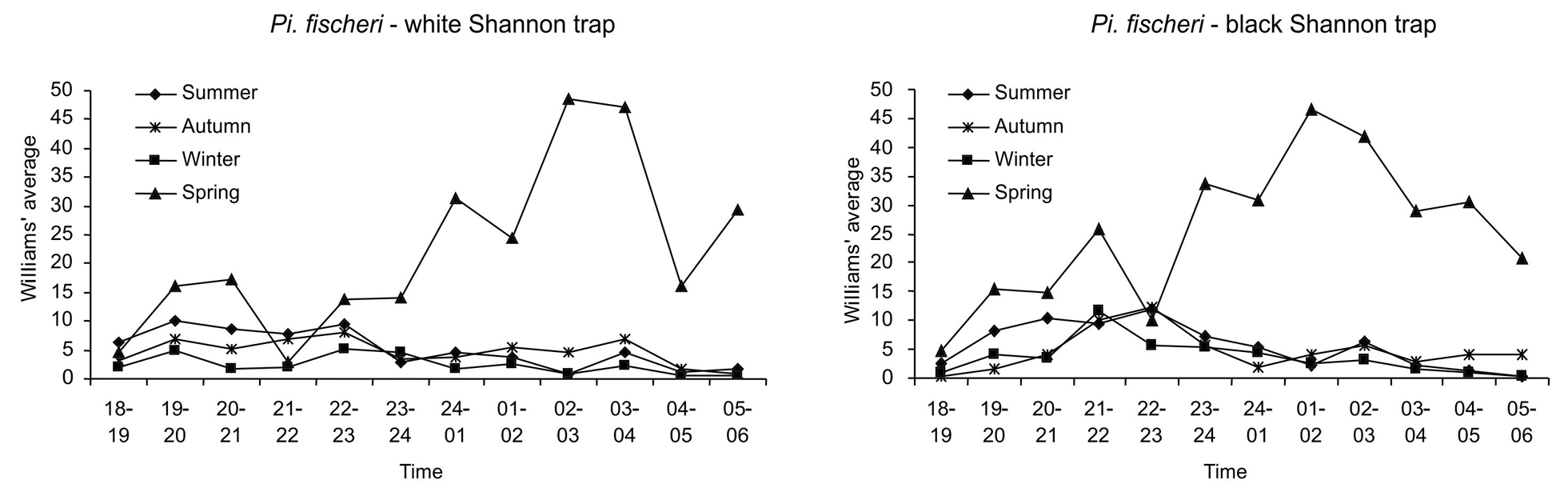
The respective hourly rhythms of both sexes of Pi. fischeri by season in the white and black Shannon traps are plotted in Figure 2. In spring, both traps showed clearly higher averages during almost all the hourly intervals, mainly in the second half of the night, as compared to those of other seasons. In all seasons, the white trap had a first peak occurring between 7:00 p.m. and 8:00 p.m., a second between 10:00 p.m. and 11:00 p.m. and a third between 3:00 a.m. and 4:00 a.m.. The black trap had a first peak between 9:00 p.m. and 10:00 p.m., which is common to all seasons; however, the highest peak in the summer and autumn (10:00 p.m.-11:00 p.m.) does not coincide with those of winter (9:00 p.m.-10:00 p.m.) and spring (1:00 a.m.-2:00 a.m.).
Nocturnal hourly rhythm (18.00-06.00h) of females and males of Pi.fischeri and Mg. migonei captured with black and white Shannon traps. ParqueEstadual da Cantareira, January to December 2009. Figura 2Ritmo horário noturno (18h00-06h00) de fêmeas e machos de Pi. fischeri e Mg. migonei capturados com armadilhas de Shannon branca e preta. ParqueEstadual da Cantareira, janeiro a dezembro de 2009.
Nocturnal hourly rhythm (6:00 p.m. – 6:00 a.m.) of both sexes of Pi.fischeri by season and color of trap (black or white Shannon trap). Cantareira State Park, January to December 2009. Figura 3Ritmo horário noturno (18h00-06h00) de ambos os sexos de Pi. fischeri por estação do ano e cor (branca ou preta) da armadilha de Shannon. ParqueEstadual da Cantareira, janeiro a dezembro de 2009.
The analysis of correlation between the numbers of both sexes of the two most abundant species captured in Shannon traps and the climatic parameters investigated showed a positive and significant correlation of Pi. fischeri with the mean minimum humidity on the fifteen days prior to the capture day (Pearson's coefficient: r = 0.584, p = 0.035) and a negative and significant correlation with the mean minimum humidity on the capture day (Spearman's coefficient: r = -0.757 p = 0.003 and r = -0.663 p = 0.014, respectively). For Mg. migonei, the correlation was negative and significant only for the mean and minimum humidity on the capture day (Spearman's coefficient: r = -0.903 p = 0.000 and r = -0.884 p = 0.000, respectively).
During the initial sandfly collections with Shannon traps, six species were also collected while attempting to bite the researchers. The numbers of females captured in Shannon traps during these collections and those attempting to bite the researchers are shown in Table 4. Pi. fischeri and Mg. migonei had the highest frequency both in Shannon traps and attempting to bite the researchers; however, the latter species was more attracted to humans than the former.
Numbers of female sandflies by species attempting to bite researchers during captures with Shannon traps and those collected in these traps. January to March 2009. Tabela 4Números de fêmeas de flebotomíneos, segundo espécie, tentando picar os pesquisadores durante coletas com armadilhas de Shannon e aquelas coletadas nessas armadilhas. Janeiro a março de 2009.
A total of 61 females were dissected: Pi. fischeri (52), Mg. migonei (3), Pa. pestanai (2), Pa. pascalei (2), Pi. monticola (1) and Ps. ayrozai (1), with negative results for flagellates.
Discussion
In the area studied, Pi. fischeri and Mg. migonei were the most abundant species. Both species were attracted to humans, as directly demonstrated by their attempting to bite researchers in the collections made in Shannon traps and as indirectly demonstrated by their captures in high numbers in Shannon traps; a method known to attract mainly anthropophilic species14.
On the other hand, in none of the three areas sampled in the city of Mairiporã, PEC, PEAL and the Roseira road, were the known vectors (Ny. whitmani, Ny. intermedia and Ny. neivai) of the principal ACL agent in the Brazilian Southeastern region, Leishmania braziliensis21-23 , found. However, of the two most predominant species in this study, Pi. fischeri was found with an experimental infection by Leishmania sp.24 and naturally infected by L. braziliensis25 . Furthermore, due to its high degree of anthropophilia and abundance in ACL endemic foci, a secondary role in the transmission of this Leishmania species has been attributed21. Mg. migonei was found naturally infected by this parasite22 and has been considered a secondary vector in several Brazilian ACL endemic regions21,26,27.
Pintomyia fischeri, highly predominant in the captures using black and white Shannon traps, also predominated in the pioneer studies conducted in the late 1930s and early 1940s in the city of São Paulo28.At that time, Pi. fischeri (68.5%), Psychodopygus arthuri (22.7%), Psychodopygus lloydi (5.3%) and Ny. intermedia s. lat. (2.0%) were the most prevalent species in captures with Shannon traps. On the other hand, the presence of Mg. migonei had not been recorded.
In the present study, it is noteworthy that only two specimens of Ps. lloydi were captured and none of Ps. arthuri, Ny. intermedia or Ny. neivai were investigated in the three study areas. The frequency of Ny. intermedia and Ny. neivai in preserved forests is low, compared with that found in anthropic environments, especially if domestic animals are present, as observed in the Ribeira Valley, where they occur in sympatry29. However, the absence of any such species in the three localities investigated and also the low prevalence of Ps. lloydi seem to reflect changes in the population structure of the sandfly fauna of this region, when compared with that found in the 1930s and 1940s, perhaps as a result of the high level of urbanization around the park and air and soil pollution.
The predominance of Pi. fischeri in the peridomicile in the PEC and that of Ps. ayrozai on the Roseira road differ from the situation in other areas with remnants of forest in rural or transitional (between wild and rural) environments, where ACL cases have been reported. In these areas, Ny. intermedia s. lat. has been considered the main vector, while Mg. migonei plays a secondary role4. On the other hand, Ps. ayrozai greatly predominated in preserved forests in the Ribeira Valley, but it was also captured in peridomicile, however less frequently than Ny. intermedia s. lat.30.
Pintomyia fischeri, although widely dispersed throughout the state of São Paulo31, is scarce or absent in the northeastern part of the state, significantly warmer than other areas and with a dry winter. However, it is in the forests of Greater São Paulo where this species has the highest frequencies28,32. This fact may be related to the altitude of the Greater São Paulo area (? 800 m) and its vegetation cover, offering the special conditions required for the development of its immature stages, with temperature and humidity of about 20ºC and 70-80%, respectively28,33.
The captures with automatic light traps were little effective and the Figueira track was the most productive site (42 specimens), with the highest richness (8) and diversity index score (1.40). However, it showed the lowest Pielou evenness index (0.20) due to the great predominance of Pi. fischeri. In spite of this, such environment appears to show the highest degree of preservation, offering better conditions for breeding and blood sources for females in the forests. Both Shannon and Pielou indices were similar on the only site sampled in the PEAL. This implies the maximum equitability for a specific site, where all the species were captured in equal numbers.
At the site in the forest on the edge of the lake, which serves as a resting place for capybaras, only two sandflies were captured. This low yield may reflect a lower attractiveness of these animals to sandfly females and the high degree of degradation of the forest. As the PEAL receives about 5,000 visitors per week11, the result is an unfavorable environment for breeding sites.
The low densities of sandflies collected with automatic light traps in forests and peridomicile were also recorded in the high Ribeira Valley, with altitudes close to the sampled area; however, in this region, the densities found in Shannon traps were also very low34, which contrasts with the study area for the two most abundant species. This may be related to the anthropophilic populations of these species in Serra da Cantareira.
In the comparison between the black and white Shannon traps, the females of Pi. fischeri and Mg. migonei were more attracted to the black and the white ones, respectively. This greater attractiveness of Pi. fischeri to black traps may be related to its preference for shaded and humid environments, while Mg. migonei is more attracted to white because it is more adapted to human-altered environments. This same contrast has been noted, respectively, for Nyssomyia intermedia and Ny. neivai in the Ribeira Valley region35.
The females of Pi. fischeri were considerably more attracted to both traps than males, while those of Mg. migonei were slightly more attracted to the white traps. These artificial attractive environments produced by the traps, added to the kairomones (CO2 and odors of the individuals making the collections) and the lantern's light and heat, may have contributed to the attractiveness of traps to these anthropophilic species. The low attractiveness of automatic light traps to Ps. ayrozai, a known anthropophilic species36 and the most prevalent in the peridomicile of Roseiras' road and on the Park ranger's house track, may be due to its dependence on a particular environment and climatic condition, not present where the captures with Shannon traps were undertaken.
The hourly rhythm is one the factors which help to understand when the highest interaction vector-host occurs. The competition for the same food source may have led to the different peak activities of Pi. fischeri and Mg. migonei, as observed in Figure 1.
Bearing in mind that the median pupal period of Pi. fischeri is 14 days and that of Mg. migonei is 10-11 days28,33, the significant, positive correlation between the mean minimum humidity on the fifteen days prior to the capture day and the numbers of specimens of Pi. fischeri seems to indicate that the change from larval to pupal stage of Mg. migonei shows greater tolerance to variations in humidity than Pi. fischeri. For the two species, the significant negative correlation with low mean humidity on the capture days may be associated with the absence of rain on these days, since rain prevents sandflies from leaving their natural shelters.
High temperatures and humidity ranging between 50 and 60% (data not given) could accelerate the larval and pupal periods, resulting in high frequencies of adults, as observed during spring. The significant positive correlation of Pi. fischeri with the mean minimum humidity on the 15 days prior to capture and the significant negative correlation of Pi. fischeri and Mg. migonei with the mean and minimum humidity on the capture day seem to corroborate this aspect. Similar behavior was observed in forested areas for Ny. whitmani in the state of Mato Grosso do Sul37. Thus, in warmer periods with less humidity, the risk of transmission of Leishmania may be increased.
The negative results for flagellate investigation were expected due to the small sample (61 females) dissected, since the infection rate found in several studies using this technique is close to 0.2%, in endemic ACL areas37,38.
Although Pi. fischeri was the prevalent species captured while attempting to bite researchers, the proportion of Mg. migonei was greater when the total number of females attracted to that microenvironment (traps, light sources and humans) was considered. Thus, it seems that the latter species are more attracted to humans. However, its low density is a limiting factor for its vectorial capacity33. Evidences of anthropophilia28,34,36,39 have been reported among the other four species, Ex. firmatoi, Pa. pestanai, Ps. ayrozai and Pi. monticola, caught while attempting to bite researchers.
Conclusion
Based on the high frequencies of Pi. fischeri and Mg. migonei and their anthropophilia, it can be inferred that these two species may be transmitting the ACL agent in the area studied. Since Pi. fischeri is the most abundant species, with higher frequencies in the spring and activity throughout the night, especially at dawn, this season and the second half of the night are the periods of greatest risk of transmission by Leishmania sp. in the area.
Despite the high frequencies of Pi. fischeri and Mg. migonei captured in Shannon traps, their densities and those of the other species collected in the seven sampled areas were low in the automatic light traps.
The capture of females of Pi. fischeri in significantly greater numbers in the black Shannon traps than white traps, while the opposite occurred for Mg. migonei, seems to indicate that the former is more dependent on forests than the latter.
Authors would like to thank the residents of the dwellings where the collections were performed.
Process SMA number 260108-000/231/0 2008 (503/2008 D77 08M) approved by the Comissão Técnico-Científica do Instituto Florestal (Forest Institute Technical-Scientific Committee) state of São Paulo.
References
-
1Lainson R, Shaw JJ. New World leishmaniasis. In: Cox FEG, Wakelin D. Gillespie SH, Despommier DD (eds.). Topley & Wilson's Microbiology and microbial infections. (Vol. 2) London: Hodder Arnold; 2005. p. 313-49.
-
2World Health Organization. Leishmaniasis. Available in: http://who.int/leishmaniasis/en/index.html (Accessed 30 March 2009)
» http://who.int/leishmaniasis/en/index.html -
3Forattini OP, Rabello EX, Serra OP, Cotrim MD, Galati EAB, Barata JMS. Observações sobre a transmissão da leishmaniose tegumentar no Estado de São Paulo, Brasil. Rev Saúde Pública 1976; 10: 1-43.
-
4Camargo-Neves VLF, Gomes AC, Antunes JLF. Correlação da presença de espécies de flebotomíneos (Diptera: Psychodidae) com registros de casos da leishmaniose tegumentar americana no Estado de São Paulo, Brasil. Rev Soc Bras Med Trop 2002: 35(4): 299-306.
-
5Marcondes CB. A redescription of Lutzomyia (Nyssomyia) intermedia (Lutz & Neiva, 1912), and resurrection of L. neivai (Pinto, 1926) (Diptera, Psychodidae, Phlebotominae). Mem Inst Oswaldo Cruz 1996; 91: 457-62.
-
6Marcondes CB, Lozovel AL, Vilela JH. Distribuição geográfica de flebotomíneos do complexo Lu. intermedia (Lutz & Neiva, 1912) (Diptera, Psychodidae). Rev Soc Bras Med Trop 1998; 31: 51-8.
-
7Andrade Filho JD, Galati EAB, Falcão AL. Nyssomyia intermedia (Lutz & Neiva, 1912) and Nyssomyia neivai (Pinto, 1926) (Diptera: Psychodidae: Phlebotominae) geographical distribution and epidemiological significance. Mem Inst Oswaldo Cruz 2007; 102: 481-7.
-
8Gomes AC. Perfil epidemiológico da leishmaniose tegumentar no Brasil. An Bras Dermatol 1992; 67: 55-60.
-
9Tolezano JE, Taniguchi HH, Elias CR, Larosa R. Epidemiologia da leishmaniose tegumentar americana (LTA) no Estado de São Paulo. III. Influência da ação antrópica na sucessão vetorial da LTA. Rev Inst Adolfo Lutz 2001; 60: 47-51.
-
10Proença NG, Muller H. Nota sobre a ocorrência de leismaniose tegumentar na Serra da Cantareira, São Paulo, Rev de Saúde Pública 1979; 13: 60-2.
-
11Dias AR, Descio F, Leite MO, Pereira A, Moimo EO. Parque Estadual da Cantareira. Maior Floresta Urbana Nativa do Mundo, São Paulo. Available in: http://www.iflorestsp.br/cantareira (Accessed 25 May 2009).
» http://www.iflorestsp.br/cantareira -
12Montes J. Fauna de Culicidae da Serra da Cantareira, São Paulo, Brasil. Rev Saúde Pública 2005; 39: 578-84.
-
13Natal D, Marucci D, Reis IM, Galati EAB. Modificação da armadilha CDC com testes para coletas de flebotomíneos (Diptera) Rev Bras Entomol 1991; 35; 697-700.
-
14Galati EAB, Nunes VLB, Dorval MEC, Cristaldo G, Rocha HC, Gonçalves-Andrade RM, Naufel G. Attractiveness of black Shannon trap for plebotomines. Mem Inst Oswaldo Cruz 2001; 96: 641-7.
-
15Galati EAB. Morfologia e Taxonomia: morfologia, terminologia de adultos e identificação dos táxons da América In: Rangel EF, Lainson R. Flebotomíneos do Brasil. Rio de Janeiro: Ed. Fiocruz; 2003. p. 53-175.
-
16Forattini OP. Entomologia Médica. Psychodidae. Phlebotominae. Leishmaniose. Bartonelose. São Paulo: Editora Edgard Blücher Ltda; 1973.
-
17Marcondes CB. A proposal of generic and subgeneric abbreviations for phlebotomine sandflies (Diptera: Psychodidae: Phlebotominae) of the world. Entomol News 2007; 118: 351-6.
-
18Robert DR, Hsi BP. An index of species abundance for use with mosquito surveillance data. Environ Entomol 1979; 8(6): 1007-13.
-
19Hayek LAC, Buzas MA. Surveying Natural Populations. New York: Columbia Univerty Press; 1997.
-
20Haddow AJ. 1960. Studies on the biting-habits and medical importance of East African mosquitos in the genus Aedes. I. Subgenera Aedimorphus, Banksinella and Nunnius. Bull Entomol Res 1960; 50: 759-79.
-
21Rangel EF, Lainson R. Transmissores de leishmaniose tegumentar americana, In Rangel EF, Lainson R (orgs.). Flebotomíneos do Brasil. Rio de Janeiro: Fiocruz; 2003; 291-309.
-
22Pita-Pereira D, Alves CR, Souza MB, Brazil RP, Bertho AL, Barbosa AF et al. Identification of naturally infected Lutzomyia intermedia and Lutzomyia migonei with Leishmania (Viannia) braziliensis in Rio de Janeiro (Brazil) revealed by PCR multiplex non-isotopic hybridisation assay. Trans Roy Soc Trop Med Hyg 2005; 99: 905-13.
-
23Pita-Pereira D, Souza MB, Zuewtsch A, Alves CR, Britto C, Rangel EF. Short communication: First report of Lutzomyia (Nyssomyia) neivai (Diptera: Psychodidae: Phlebotominae) naturally infected by Leishmania (Viannia) braziliensis in a periurban area of South Brazil using multiplex polymerase chain reaction assay. Amer J Med Hyg 2009; 80:5 93-5.
-
24Pessôa SB, Coutinho JO. Infecção natural e experimental dos flebótomos pela Leishmania braziliensis no Estado de São Paulo. Hospital 1941; 20: 25-35.
-
25Rocha LS, Santos CB, Falqueto A, Grimaldi Jr G, Cupolillo E. Molecular biological identification of monoxenous trypanosomatids and Leishmania from antropophilic sandflies (Diptera: Psychodidae) in Southeast Brazil. Parasitol Res 2010; 107: 465-8.
-
26Azevedo ACR, Rangel EF. A study of sand fly species (Diptera:Psychodidae, Phlebotominae) In focus of cutaneous leishmaniasis in the municipality of Baturité, Ceará, Brazil. Mem Inst Oswaldo Cruz 1991; 86: 405-10.
-
27Mayo RC, Casanova C, Mascarini LM, Pignatti MG, Rangel O, Galati EAB, Wanderley DM, Corrêa FM.. Flebotomíneos (Diptera, Psychodidae) de área de transmissão de leishmaniose americana, no município de Itupeva, região sudeste do estado de São Paulo, Brasil. Rev Soc Bras Med Trop 1998; 31: 339-45.
-
28Barretto MP. Observações sobre a biologia em condições naturais, dos flebótomos do Estado de São Paulo [Tese de concurso à Livre-Docência da Cadeira de Parasitologia]. São Paulo: Faculdade de Medicina da USP; 1943.
-
29Galati EAB, Marassá, AM, Fonseca MB, Gonçalves-Andrade, Consales CA, Bueno EFA. Phlebotomines (Diptera, Psychodidae) in the Speleological Province of the Ribeira Valley: 3. Serra district - area of hostels for tourists who visit the Parque Estadual do Alto Ribeira (PETAR), state of São Paulo, Brazil. Rev Brasil Entomol 2010;5 4(4): 665 - 76.
-
30Gomes AC, Galati EAB. Aspectos ecológicos da leishmaniose tegumentar americana. Capacidade vetorial flebotomínea em ambiente florestal primário do Sistema da Serra do Mar, região do vale do Ribeira, estado de São Paulo. Rev Saúde Pública 1989; 23: 136-42.
-
31Shimabukuro PHF, Silva TRR, Ribeiro FOF, Baton LA, Galati EAB. Geographical distribution of American cutaneous leishmaniasis and its phlebotomine vectors (Diptera: Psychodidae) in the state of São Paulo, Brazil. Parasites & Vectors 2010, 3: 121-32.
-
32Silva DA. Aspectos ecológicos da fauna flebotomínea (Diptera, Psychodidae) e suas implicações na epidemiologia das leishmanioses em Cotia, Estado de São Paulo, Brasil. São Paulo. [dissertação de mestrado ]. São Paulo: Faculdade de Saúde Pública da USP; 2005.
-
33Galvis FO. Estudo da capacidade vetorial de Migonemyia migonei (França) e de Pintomyia fischeri (Pinto) (Diptera: Psychodidae) para Leishmania (Leishmania) infantum chagasi Cunha & Chagas. [dissertação de mestrado ]. São Paulo: Faculdade de Saúde Pública da USP; 2011.
-
34Galati EAB, Marassá AM, Gonçalves-Andrade RM, Consales CA, Bueno EA. Phlebotomines (Diptera, Psychodidae) in the Ribeira Valley Speleological Province - 1. Parque Estadual Intervales, state of São Paulo, Brazi. Rev Bras Entomol 2010; 54(2): 311-21.
-
35Galati EAB, Marassá AM, Gonçalves-Andrade RM, Bueno EMF, Paiva BR, Malafronte RS. Nyssomyia intermedia (Lutz & Neiva) and Nyssomyia neivai (Pinto) (Diptera, Psychodidae, Phlebotominae) in a sympatric area: seasonal and nocturnal hourly rhythm in black and white modified Shannon traps. Rev Bras Entomol 2010; 54: 677-86.
-
36Galati EAB, Marassá AM, Gonçalves- Andrade RM, Consales, CA, Bueno EMF. Phlebotomines (Diptera, Psychodidae) in the Speleological Province of the Ribeira Valley: 2. Parque Estadual do Alto Ribeira (PETAR), São Paulo State, Brazil. Rev Bras Entomol 2010; 54: 477-87.
-
37Galati EAB, Nunes VLB, Dorval MEC, Oshiro ET, Cristaldo G, Rocha H et al. Estudo dos flebotomíneos (Diptera, Psychodidae), em área de leishmaniose tegumentar no Estado de Mato Grosso do Sul, Brasil. Rev Saúde Pública 1996; 30:115-28.
-
38Casanova C, Mayo RC, Rangel O, Mascarini LM, Pignatti MG, Galati EAB et al. Natural Lutzomyia intermedia (Lutz & Neiva) infection in the Valley of the Mogi Guaçu River, State of são Paulo, Brazil. Bol Dir Malariol San Amb1 995; 35(S1): 77-84.
-
39Gomes AC, Barata JMS, Silva EOR, Galati EAB. Aspectos ecológicos da leishmaniose tegumentar americana. 6. Fauna flebotomínea de matas residuais situadas na região centro-nordeste do Estado de São Paulo, Brasil. Rev Inst Med Trop S Paulo 1989;1: 2-39.
Publication Dates
-
Publication in this collection
Mar 2013
History
-
Received
18 Aug 2011 -
Reviewed
10 Apr 2012 -
Accepted
23 May 2012