ABSTRACT
Parkinson's disease is a neurodegenerative disorder characterized by motor impairment, cognitive decline and psychiatric symptoms. Schinus terebinthifolius Raddi, Anacardiaceae, had been studied for its anti-inflammatory and antioxidant properties, and in this study, the stem bark was evaluated for the neuroprotective effects on behavioral and biochemical alterations induced by administrations of rotenone in rats. Behavioral evaluations were performed using open-field and rotarod. The in vitro and in vivo antioxidant activities were determined by the DPPH radical scavenging activity and lipid peroxidation method respectively. The administration of rotenone (3 mg/kg, s.c.) produced hypolocomotion, increase of immobility and muscle incoordination, while the treatment with S. terebinthifolius stem bark extract (150, 300 and 600 mg/kg p.o.) for seven days prevented rotenone-induced dysfunctional behavior. Biochemical analysis of the substantia nigra, striatum and cortex revealed that rotenone administration significantly increased lipid peroxidation, which was inhibited by treatment with all doses of S. terebinthifolius. The results suggested neuroprotective effect of S. terebinthifolius possibly mediated through its antioxidant activity, indicating a potential therapeutic benefit of this species in the treatment of Parkinson's disease.
Keywords:
Antioxidant effects; Lipidic peroxidation; Parkinson's disease; Rotenone; Schinus terebinthifolius
Introduction
The increase of elderly population has led to an increasing incidence of neurodegenerative diseases worldwide, leading, thus, to the interest in studies for prevention and treatment of these pathologies. Natural constituents derived from plants are important to investigate, since studies have shown that these compounds exhibited a range of biological activities, with therapeutic potential, for instance antioxidant and anti-inflammatory effects.
Parkinson's disease (PD) is one if the major neurodegenerative disorder in the entire world and is characterized by progressive degeneration of dopamine-containing neurons that project from substantia nigra pars compacta to the striatum. Besides, motor impairment (bradykinesia, rigidity, tremor at rest and disturbances in balance), cognitive decline and psychiatric symptoms (like depression) are the cardinal symptoms of the pathology (Dutta and Mohanakumar, 2015Dutta, D., Mohanakumar, K.P., 2015. Tea and Parkinson's disease: constituents of tea synergize with antiparkinsonian drugs to provide better therapeutic benefits. Neurochem. Int. 89, 181-190.; Fernandez, 2015Fernandez, H.H., 2015. Update on Parkinson disease. Clev. Clin. J. Med. 82, 563-568.). Main mechanisms have been proposed to explain the events culminating in neuronal death in PD, which are associated with oxidative stress, mitochondrial dysfunction, neuroinflammation and environmental exposures, like pesticides, contributing for the appearance of behavioral and biochemical alterations (Dauer and Przedborski, 2003Dauer, W., Przedborski, S., 2003. Parkinson's disease: mechanisms and models. Neuron 39, 889-909.; Renaud et al., 2015Renaud, J., Nabavi, S.F., Daglia, M., Nabavi, S.M., Martinoli, M.G., 2015. Epigallocatechin-3-gallate, a promising molecule for Parkinson's disease?. Rejuvenation Res. 18, 257-269.). Several animal models have been used to mimetize and elucidate the pathogenesis of PD, in special rotenone, MPTP and 6-hydroxydopamine (Dauer and Przedborski, 2003Dauer, W., Przedborski, S., 2003. Parkinson's disease: mechanisms and models. Neuron 39, 889-909.; Blesa et al., 2012Blesa, J., Phani, S., Jackson-Lewis, V., Przedborski, S., 2012. Classic and new animal models of Parkinson's disease. J. Biomed. Biotechnol. 2012(ID), 845618.).
Rotenone (Derris sp., Fabaceae) has been a potent hydrophobic pesticide and its environmental exposure mimics the clinical and pathological features of PD, such as behavioral, biochemical and pathological changes, providing a reproducible animal model for PD (Betarbet et al., 2000Betarbet, R., Sherer, T.B., Mackenzie, G., Garcia-Osuma, M., Panov, A.V., Greenamyre, J.T., 2000. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat. Neurosci. 3, 1301-1306.). It provoked neurodegeneration via multiple mechanisms, mainly by inhibition of complex I, increase of oxidative stress and induction of apoptosis (Zaitone et al., 2012Zaitone, S.A., Abo-Elmatty, D.M., Shaalan, A.A., 2012. Acetyl-L-carnitine and α-lipoic acid affect rotenone-induced damage in nigral dopaminergic neurons of rat brain; implication for Parkinson's disease therapy. Pharmacol. Biochem. Behav. 100, 347-360.; von Wrangel et al., 2015von Wrangel, C., Schwabe, K., John, N., Krauss, J.K., Alam, M., 2015. The rotenone-induced rat model of Parkinson's disease: behavioral and electrophysiological findings. Behav. Brain Res. 279, 52-61.).
Schinus terebinthifolius Raddi, Anacardiaceae, is a native plant of South America (Corrêa, 1974Corrêa, M.P., 1974. Dicionário de plantas úteis do Brasil e das plantas exóticas cultivadas, vol. 3. Imprensa Nacional, Rio de Janeiro, Brazil, pp. 125–126.). In Brazil it is popularly called Aroeira or Cabuí. In folk medicine, it has been used for the treatment of ulcers, respiratory problems, wounds and arthritis (Morton, 1978Morton, J.F., 1978. Brazilian pepper: its impact on people, animals and the environment. Econ. Bot. 32, 353-359.) and as an antiseptic, antiinflammatory and haemostatic (Medeiros et al., 2007Medeiros, K.C.P., Monteiro, J.C., Diniz, M.F.F.M., Medeiros, I.A., Silva, B.A., Piuvezam, M.R., 2007. Effect of the activity of the Brazilian polyherbal formulation: Eucalyptus globulus Labill, Peltodon radicans Pohl and Schinus terebinthifolius Raddi in inflammatory models. Rev. Bras. Farmacogn. 7, 23-28.). Many of its properties or healing effects attributed by folk medicine are associated to the presence of polyphenols in the plant, which give the plant its antioxidant properties (Medeiros et al., 2007Medeiros, K.C.P., Monteiro, J.C., Diniz, M.F.F.M., Medeiros, I.A., Silva, B.A., Piuvezam, M.R., 2007. Effect of the activity of the Brazilian polyherbal formulation: Eucalyptus globulus Labill, Peltodon radicans Pohl and Schinus terebinthifolius Raddi in inflammatory models. Rev. Bras. Farmacogn. 7, 23-28.; Abdou et al., 2015Abdou, R.H., Saleh, S.Y., Khalil, W.F., 2015. Toxicological and biochemical studies on Schinus terebinthifolius concerning its curative and hepatoprotective effects against carbon tetrachloride-induced liver injury. Pharmacogn. Mag. 11, S93-S101.). The aim of this study was to investigate the possible neuroprotective effects of S. terebinthifolius in the behavior activity and on the oxidative stress induced by rotenone as experimental model of Parkinson's disease in rats.
Materials and methods
Plant material
Stem bark of Schinus terebinthifolius Raddi, Anacardiaceae, were collected in the remains of the Atlantic rainforest located in the municipality of Cabo de Santo Agostinho, Pernambuco, Brazil between February and April 2013 (8º20′33″ S and 34º56′59″ W). A voucher specimen was authenticated in the Department of Botany of the Federal University of Pernambuco by the curator M. Barbosa and was deposited at Geraldo Mariz Herbarium under record nº. 8758. Extraction was performed by maceration and air dried, and 500 g of pulverized S. terebinthifolius bark was added to 1.0 l of ethanol 70% at room temperature, for 7 days, and was occasionally shaken. In the laboratory, the crude ethanolic extract was evaporated to dryness under reduced pressure for the total elimination of alcohol, followed by lyophilization, yielding approximately 35 g of dry residue. The lyophilized extract of S. terebinthifolius was kept at room temperature until use and suspended in distilled water.
High performance liquid chromatography (HPLC) analysis
The main phytochemical markers (gallic acid, ellagic acid, catechin and epicatechin) in S. terebinthifolius samples were analyzed by way by way of liquid chromatography-diode array detection (LC-DAD) analysis using a Shimadzu system (LC-20AT) equipped with a photo diode array detector (SPD-M20A). The chromatographic separation was performed using a Gemini RP-18 column 5 µm particle size and 250 mm × 4.60 mm i.d. (Phenomenex), protected by a guard column of the same material. A gradient elution was performed by varying the proportion of Solvent A (0.5% acetic acid in distilled water, v/v) and solvent B (methanol) at a flow rate of 0.8 ml/min following gradient program: 20–40% B (10 min), 40–60% B (10 min), 60% B (10 min), 60–40% B (10 min), and 40–20% B (10 min). The dried extracts and standards were dissolved in methanol:water (20/80, v/v) and filtered through a membrane of 0.45 µm (Millipore®, USA) prior to injection of 20 µl. The peaks of each marking on dry substance were identified by comparing retention times and UV spectra of DAD.
Animals
Adult male Wistar rats (Rattus norvegicus var. albinus), age 3 months and weighing between 250 and 300 g, were obtained from the Department of Physiology and Pharmacology at the Federal University of Pernambuco, Brazil. The animals were maintained in standard environmental conditions (22 ± 2 ºC, 12:12 h dark/light cycle). Commercial food (Presence®, Purina, Brazil) and water were available ad libitum. All protocols were approved by the Animal Experimentation Ethics Committee of the Federal University of Pernambuco, under license nº. 23076.050131/2013-82 in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Drugs
Butylated hydroxytoluene (BHT), 2,2-diphenyl-1-picrylhydrazyl (DPPH), malondialdehyde (MDA), thiobarbituric acid (TBA), epinephrine, 1,1,3,3-tetrametoxipropane (TMP), Tween 80, DMSO and rotenone, were purchased from Sigma–Aldrich® (St. Louis, MO, USA). Rotenone was dissolved in sunflower oil one day before beginning treatment. On subsequent days, solutions remained in the refrigerator (4 ºC). Sunflower oil was obtained in local market.
Experimental groups
The animals were randomly divided into five experimental groups (n = 10). Rats from Group 1 received water by gavage (1 ml/kg) and 1 h later a subcutaneous (s.c.) administration of the vehicle (90%, w/v sunflower oil, +10% w/v DMSO) for seven days. Group 2 also received water by gavage and rotenone (3 mg/kg, s.c.) diluted in vehicle after 1 h during seven days. Groups 3, 4 and 5 received by gavage 150, 300 or 600 mg/kg of S. terebinthifolius, respectively, suspended in water, and 1 h later were given them rotenone (3 mg/kg, s.c.) also during seven days. 24 h after the last day of treatment, the rats were evaluated with behavioral tests and biochemical assays (Linard-Medeiros et al., 2015Linard-Medeiros, C.F.B., Sales, V.A.W., Ramos, A.C., Sereniki, A., Trevisan, M.T.S., Wanderley, A.G., Lafayette, S.S., 2015. Neuroprotective effect of extract of Anacardium occidentale Linn on a rotenone model of Parkinson's disease. Int. J. Pharm. Sci. Res. 6, 123-129.).
Open field test
The open field test was carried out to Broadhurst (1957)Broadhurst, P.L., 1957. Determination of emotionality in the rat. I. Situational factors. Br. J. Psychol. 48, 1-12. and Bernardi and Palermo-Neto (1979)Bernardi, M.M., Palermo-Neto, J., 1979. Effects of abrupt and gradual withdrawal from long-term haloperidol treatment on open field behavior of rats. Psychopharmacology 65, 247-250.. To quantify exploratory and general locomotor activity, each rat was placed into the center of an open-field arena (a circular wooden box with a diameter of 100 cm and 40 cm high, with floor divided into 19 regions). Rats were assessed individually for 5 min, while four parameters were analyzed: (i) latency to start the movement (time to leave the inner circle, in s), (ii) locomotion frequency (number of square crossed with four paws), (iii) rearing frequency (number of times the animal stood on their hind paws) and (iv) immobility time (lack of movement during testing, in s). The apparatus was cleaned with a 5% ethanol solution before behavioral testing to eliminate possible bias due to odors left by previous rat.
Rotarod activity test
In order to quantify motor deficiency, the rotarod test was used at a fixed speed, according to Monville et al. (2006)Monville, C., Torres, E.M., Dunnett, S.B., 2006. Comparison of incremental and accelerating protocols of the rotarod test for the assessment of motor deficits in the 6-OHDA model. J. Neurosci. Methods 158, 219-223.. To perform this test, the animal was placed with all four paws on a bar with a diameter of 7 cm and set 25 cm above the floor. The bar rotated at a speed of 25 rpm. Before being submitted to the different treatments, the rats were trained in two sessions of 180 s each for habituation. Animals were placed on the rotating bar and the time spent on the rotating bar was recorded. A cut-off time of 180 s was maintained throughout the experiment. The average results were recorded as time of fall.
Biochemical analysis
After behavioral observations, animals were immediately anesthetized, euthanized by decapitation and their brains removed. Bilateral substantia nigra, striatum and cortex were dissected, weighed and homogenized in a Potter–Elvehjem type homogenizer with a tissue homogenate 1× phosphate buffered saline (10% w/v, PBS) to which was added butylated hydroxytoluene (0.004% w/v, BHT) for preventing oxidation of the samples. The homogenate was centrifuged at 10,000 × g for 30 min at 4 ºC and an aliquot of supernatant was separated for biochemical analysis. Each individual experiment was carried out in triplicate and repeated two or three times.
DPPH radical scavenging activity in vitro
Antioxidant radical scavenging activity was evaluated using DPPH (2,2-diphenyl-1-picrylhydrazyl) radicals (Brand-Williams et al., 1995Brand-Williams, W., Cuvelier, M.E., Berset, C., 1995. Use of free radical method to evaluate antioxidant activity. Lebensm-Wiss. U. Technol. 28, 25-30.). 1 ml of DPPH solution (30 mM, in 95% ethanol) was incubated with 2.5 ml of varying concentrations of S. terebinthifolius extract (2.5–250 µg/ml). The reaction mixture was shaken well and incubated for 30 min at room temperature, and the absorbance of the resulting solution was read at 515 nm against a blank. The radical scavenging activity was measured as an absorbance decrease of DPPH and was calculated using the following equation: (Absorbance of control − Absorbance of test) × 100/Absorbance of control. The synthetic antioxidant butylated hydroxytoluene (BHT) was included in experiments as a positive control. The EC50 value for each sample (tree replicates), was calculated from a calibration curve obtained for the percentage of radical scavenging activity versus concentration of the sample required to reduce the absorbance of the negative control (DPPH solution) by 50%. The BHT curve antioxidant was y = 15.695 ln(x) + 16.477 (R 2 = 0.9544). The S. terebinthifolius extract curve was y = 18.322 ln(x) − 24.936 (R2 = 0.9026).
Measurement of lipid peroxidation in vivo
The quantitative measurement of lipid peroxidation in the substantia nigra, striatum and cortex was performed according to the method described by Buege and Aust (1978)Buege, J.A., Aust, S.D., 1978. Microssomal lipid peroxidation. Methods Enzymol. 52, 302-310.. The amount of malondialdehyde (MDA) of lipid peroxidation was measured by reaction with thiobarbituric acid. Briefly, aliquots (500 µl) of supernatant were placed in test tubes and added to 1 ml of TBA reagent: 0.38% (w/w) TBA, 250 ml of 1 N hydrochloric acid, 15% (w/w) of trichloroacetic acid and 20 ml of 2% (w/v) ethanolic BHT. The solution was shaken and heated to 100 ºC for 15 min, followed by cooling in an ice bath. Then, 1.5 ml of n-butanol was added. The mixture was shaken and centrifuged to 3000 × g for 20 min. After centrifugation, the upper layer was collected and assessed with a spectrophotometer (CARY 3E UV–Visible Spectrophotometer Varian, Inc. Brazil) at 532 nm. Results of TBARS of substantia nigra, striatum and cortex were expressed as nmol MDA/mg protein using a standard curve generated with different concentrations of 1,1,3,3-tetramethoxypropane solution.
Statistical analysis
Data were expressed as mean ± standard error of mean (SEM). The difference between groups was analyzed by one-way analysis of variance (ANOVA) followed by post hoc Newman–Keuls test. The comparisons were carried out by GraphPad Prism® 5.0 and significant at p ≤ 0.05.
Results
High performance liquid chromatography (HPLC) analysis
From the comparison of retention times with extract standards previously injected into the HPLC, it was possible to confirm that, S. terebinthifolius sample demonstrated the presence of phenolic acids (gallic acid, 4.66 min; ellagic acid, 7.51 min) and also flavonoids (catechin, 10.24 min; epicatechin 19.05 min) (Fig. 1).
Chromatogram of Schinus terebinthifolius stem bark detected at 278 nm. Peaks: gallic acid (Rt = 4.66), catechin (7.51), epicatechin (10.24) and ellagic acid (Rt = 19.05) obtained by HPLC method.
Effects of S. terebinthifolius on behavioral tests
Rotenone administration (3 mg/kg, s.c.) during 7 days significantly reduced the frequencies of locomotion and rearing and increased the time of immobility and latency to start the movement in the open field test. Pre-treatment with the stem bark (150, 300 and 600 mg/kg) significantly improved locomotor activity and rearing and decreased the time of immobility and latency to start the movement when compared to rotenone animals. However, the lower dose of the extract (150 mg/kg) was unable to prevent the rotenone-induced locomotor deficits in the open field test (Fig. 2a–d).
Effect of Schinus terebinthifolius (St, 150, 300 and 600 mg/kg, p.o.) on open field test in rats before rotenone (3 mg/kg, s.c.) administration. (a) Total ambulatory activity, (b) rearing frequency, (c) immobility time and (d) latency to start de movement. The values are expressed as mean ± S.E.M. (n = 10/group). One-way ANOVA followed by Newman–Keuls test (*p ≤ 0.05 vs. control group and # p ≤ 0.05 vs. rotenone group).
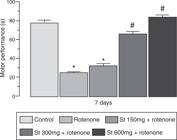
On rotarod test, rotenone administration (3 mg/kg, s.c.) induced a significant decrease in the time of permanency of the animals compared with control rats during the observation in the apparatus. Daily pre-treatment of 300 and 600 mg/kg doses was able to increase the time of permanency in comparison to rotenone group. However, the lower dose of the extract was unable to prevent the rotentone-induced locomotor deficits in the open field test (Fig. 3).
Effect of Schinus terebinthifolius (St, 150, 300 and 600 mg/kg, p.o.) on rotarod test in rats before rotenone (3 mg/kg, s.c.) administration. The values are expressed as mean ± S.E.M. (n = 10/group). One-way ANOVA followed by Newman–Keuls test (*p ≤ 0.05 vs. control group and # p ≤ 0.05 vs. rotenone group).
Reduction of 2,2-diphenyl-1-picrylhydrazyl radical in vitro
Significant DPPH radical scavenging activity was evident in all concentrations tested of S. terebinthifolius stem bark (1–250 µ/ml). The extract was able to reduce the stable free radical DPPH to the yellow-colored 1,1-diphenyl-2-picrylhydrazyl with an EC50 12.176 ± 0.077 µg/ml. Under similar conditions, the synthetic antioxidant BHT showed EC50 25.768 ± 0.147 µg/ml. This activity was about 2.1 times smaller when compared to S. terebinthifolius.
Measurement of lipid peroxidation in vivo (TBARS)
Rotenone administration (3 mg/kg) during 7 days provoked an increase on oxidative stress as indicated by the significant increase of MDA concentrations in substantia nigra, striatum and cortex when compared to control group. However, the pre-treatment with all doses of S. terebinthifolius was able to inhibit the lipid peroxidation in comparison to rotenone animals (Fig. 4a–c).
Effect of Schinus terebinthifolius (St, 150, 300 and 600 mg/kg, p.o.) on lipid peroxidation levels in rats before rotenone (3 mg/kg, s.c.) administration. (a) Substantia nigra, (b) striatum and (c) cortex of brain rats. The values are expressed as mean ± S.E.M. (n = 10/group). One-way ANOVA followed by Newman–Keuls test (*p ≤ 0.05 vs. control group and #p ≤ 0.05 vs. rotenone group).
Discussion
This report showed the neuroprotective effect of S. terebinthifolius in Parkinson's disease using the model of rotenone subcutaneous administration during 7 days. All doses of S. terebinthifolius (150, 300 and 600 mg/kg) significantly prevented motor deficits in the open field and rotarod tests, and mainly, was able to inhibit the oxidative stress induced by systemic rotenone administration observed in in vivo TBARS method. It was also demonstrated the in vitro antioxidant effect of S. terebinthifolius, as well its mainly compounds in HPLC method. The antioxidant activity of the extract was also measured in the DPPH radical assay, which primarily evaluates proton radical-scavenging ability.
Rotenone is a potent inhibitor of complex I (NADH: ubiquinoneoxidoreductase) of the mitochondrial electron transport chain. In rats, this toxin causes a syndrome that replicates both neuropathological findings and behavioral symptoms of Parkinson's disease. Application of low doses of rotenone in vitro and in vivo have been shown to affect many of the mechanisms involved in the pathogenesis of Parkinson's disease, such as altered calcium signaling, induction of oxidative stress and apoptosis, loss of tyrosine hydroxlase, proteasomal dysfunction, nigral iron accumulation and the formation of fibrillar cytoplasmic inclusions that contain ubiquitin and α-synuclein (von Wrangel et al., 2015von Wrangel, C., Schwabe, K., John, N., Krauss, J.K., Alam, M., 2015. The rotenone-induced rat model of Parkinson's disease: behavioral and electrophysiological findings. Behav. Brain Res. 279, 52-61.). Sherer et al. (2003)Sherer, T.B., Kim, J.H., Betarbet, R., Greenamyre, J.T., 2003. Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and alpha-synuclein aggregation. Exp. Neurol. 179, 9-16. also showed that the exposure to low doses of rotenone (2–3 mg/kg/day) caused highly selective nigrostriatal dopaminergic lesion. A common feature of all toxin-induced models of Parkinson's disease is their ability to produce an oxidative stress and to cause cell death in dopamine neuronal populations that reflect what is seen in Parkinson's disease (Blesa et al., 2012Blesa, J., Phani, S., Jackson-Lewis, V., Przedborski, S., 2012. Classic and new animal models of Parkinson's disease. J. Biomed. Biotechnol. 2012(ID), 845618.). Markers of oxidative stress are typically found in brain biopsies, peripheral cells, and biological fluids derived from patients with Parkinson's disease, indicating that indeed oxidative stress is a key factor in pathogenesis. Malondialdehyde (MDA) is a specific marker of lipid peroxidation, which is largely a result of the peroxidation of polyunsaturated fatty acids with more than two double bonds (Sanders and Greenamyre, 2013Sanders, L.H., Greenamyre, J.T., 2013. Oxidative damage to macromolecules in human Parkinson disease and the rotenone model. Free Radic. Biol. Med. 62, 111-120.).
In our study, administration of rotenone promoted increased levels of MDA in substantia nigra, striatum and cortex, suggesting an increased lipid peroxidation and, thus, oxidative stress. In this way, there was an increase in brain MDA levels (an indicator of lipid peroxidation due to free radicals) which was inhibited by the administration of stem bark of all doses of S. terebinthifolius, suggesting its antioxidant action. Indeed, recently Costa et al. (2015)Costa, C.O.D'S., Ribeiro, P.R., Loureiro, M.B., Simões, R.C., Castro, R.D., Fernandez, L.G., 2015. Phytochemical screening, antioxidant and antibacterial activities of extracts prepared from different tissues of Schinus terebinthifolius Raddi that occurs in the coast of Bahia, Brazil. Phcog. Mag. 11, 607-614. shown that extract from different tissues of S. terebinthifolius present antioxidant activity by DPPH radical assay. Abdou et al. (2015)Abdou, R.H., Saleh, S.Y., Khalil, W.F., 2015. Toxicological and biochemical studies on Schinus terebinthifolius concerning its curative and hepatoprotective effects against carbon tetrachloride-induced liver injury. Pharmacogn. Mag. 11, S93-S101. investigated the curative and protective effects of S. terbenthifolius ethanolic extract (350 mg/kg, p.o.) against CCl4-induced acute hepatotoxicity in rats and shown that the administration significantly reinstated the antioxidant capacity. Oxidative stress due to an increase in free radical generation or an impaired endogenous antioxidant mechanism is an important factor that had been implicated in various neurodegenerative diseases (Sanders and Greenamyre, 2013Sanders, L.H., Greenamyre, J.T., 2013. Oxidative damage to macromolecules in human Parkinson disease and the rotenone model. Free Radic. Biol. Med. 62, 111-120.; Solanki et al., 2015Solanki, I., Parihar, P., Mansuri, M.L., Parihar, M.S., 2015. Flavonoid-based therapies in the early management of neurodegenerative diseases. Adv. Nutr. 6, 64-72.).
The brain is highly susceptible to free radical damage because of its high utilization of oxygen and the presence of a relatively low concentration of antioxidant enzymes and free radical scavengers. There is a growing interest in establishing therapeutic and dietary strategies to combat oxidative stress induced damage to the central nervous system (Gao et al., 2012Gao, X1, Cassidy, A., Schwarzschild, M.A., Rimm, E.B., Ascherio, A., 2012. Habitual intake of dietary flavonoids and risk of Parkinson disease. Neurology 78, 1138-1145.; Virmani et al., 2013Virmani, A., Pinto, L., Binienda, Z., Ali, S., 2013. Food, nutrigenomics, and neurodegeneration–neuroprotection by what you eat!. Mol. Neurobiol. 48, 353-362.). Studies in humans and animals have suggested that flavonoids, a large group of polyphenolic compounds found in fruits and vegetables are capable of counteracting neuronal injury, thereby delaying the progression of neurodegenerative diseases, motivating researches efforts to identify the mechanisms of neuronal death as well to discover new compounds to control them (Dutta and Mohanakumar, 2015Dutta, D., Mohanakumar, K.P., 2015. Tea and Parkinson's disease: constituents of tea synergize with antiparkinsonian drugs to provide better therapeutic benefits. Neurochem. Int. 89, 181-190.; Chen et al., 2015Chen, M., Wang, T., Yue, F., Li, X., Wang, P., Li, Y., Chan, P., Yu, S., 2015. Tea polyphenols alleviate motor impairments, dopaminergic neuronal injury, and cerebral α-synuclein aggregation in MPTP-intoxicated Parkinsonian monkeys. Neuroscience 286, 383-392.; Solanki et al., 2015Solanki, I., Parihar, P., Mansuri, M.L., Parihar, M.S., 2015. Flavonoid-based therapies in the early management of neurodegenerative diseases. Adv. Nutr. 6, 64-72.; Renaud et al., 2015Renaud, J., Nabavi, S.F., Daglia, M., Nabavi, S.M., Martinoli, M.G., 2015. Epigallocatechin-3-gallate, a promising molecule for Parkinson's disease?. Rejuvenation Res. 18, 257-269.).
The major compounds of stem bark of S. terebinthifolius Raddi are flavonoids (cathechin and epicatechin) and phenolics acids (gallic acid and ellagic acid). Catechins are powerful antioxidants and free radical scavengers in vitro assays and in vivo models (Srividhya et al., 2009Srividhya, R., Zarkovic, K., Stroser, M., Waeg, G., Zarkovic, N., Kalaiselvi, P., 2009. Mitochondrial alterations in aging rat brain: effective role of (-)-epigallo catechin gallate. Int. J. Dev. Neurosci. 27, 223-231.; Solanki et al., 2015Solanki, I., Parihar, P., Mansuri, M.L., Parihar, M.S., 2015. Flavonoid-based therapies in the early management of neurodegenerative diseases. Adv. Nutr. 6, 64-72.). It has been found that catechins have much higher antioxidant activity in comparison to vitamins C and E in vitro. The chemical structures have been contributed to this effect to chelate metal ions, prevent the generation of free radicals, allow the displacement of electrons and provide high reactivity to eliminate the free radicals (Mandel et al., 2006Mandel, S., Amit, T., Reznichenko, L., Weinreb, O., Youdim, M.B., 2006. Green tea catechins as brain-permeable, natural iron chelators-antioxidants for the treatment of neurodegenerative disorders. Mol. Nutr. Food Res. 50, 229-234.; Khan and Mukhtar, 2007Khan, N., Mukhtar, H., 2007. Tea polyphenols for health promotion. Life Sci. 81, 519-533.; Senthil, 2008Senthil, K., 2008. Repletion of antioxidant status by EGCG and retardation of oxidative damage induced macromolecular anomalies in aged rats. Exp. Gerontol. 43, 143-183.). Furthermore, the catechins and epicatechins were also able to indirectly modulate expression of some protective enzymes antioxidants (Hidgon and Frei, 2003Hidgon, J.V., Frei, B., 2003. Tea catechins and polyphenols: health effects, metabolism and antioxidant functions. Crit. Rev. Food Sci. Nutr. 43, 89-143.; Weinreb et al., 2004Weinreb, O., Mandel, S., Amit, T., Youdim, M.B., 2004. Neurological mechanisms of green tea catechin polyphenols in Alzheimer's and Parkinson's diseases. J. Nutr. Biochem. 15, 506-516.). The chronic treatment with catechins prevented the decline in the activity of SOD and GSH enzymes in the serum of old mice, preventing also significantly increased TBARS in the hippocampus of these animals (Abd El Mohsen et al., 2002Abd El Mohsen, M.M., Kuhnle, G., Rechner, A.R., Schroeter, H., Rose, S., Jenner, P., Rice-Evans, C.A., 2002. Uptake and metabolism of epitatechin and its access to the brain after oral ingestion. Free Radic. Biol. Med. 33, 1693-1702.; Mandel et al., 2006Mandel, S., Amit, T., Reznichenko, L., Weinreb, O., Youdim, M.B., 2006. Green tea catechins as brain-permeable, natural iron chelators-antioxidants for the treatment of neurodegenerative disorders. Mol. Nutr. Food Res. 50, 229-234.). Otherwise, gallic acid and ellagic acid are hydrolyzed tannins of natural products found in abundance in grapes, tea, fruit and wine (Singh et al., 2004Singh, R.P., Sharad, S., Kapur, S., 2004. Free radicals and oxidative stress in neurodegenerative diseases: relevance of dietary antioxidants. J. Indian Acad. Clin. Med. 5, 218-225.). These acids were able to reduce the production of ROS and superoxide, such as hydrogen peroxide, hydroxyl radicals and hypochlorous acid; also due to their antimutagenic and anticarcinogenic activities (Kim, 2007Kim, Y.J., 2007. Antimelanogenic and antioxidant properties of gallic acid. Biol. Pharm. Bull. 30, 1052-1055.). In the cognitive impairment induced by another animal model of Parkinson's disease in animals, 6-OHDA, the treatment with gallic acid significantly reduced the level of TBARS in the rat brains (Kim, 2007Kim, Y.J., 2007. Antimelanogenic and antioxidant properties of gallic acid. Biol. Pharm. Bull. 30, 1052-1055.).
In conclusion, the results of this study confirm that the subcutaneous administration of rotenone in rats induces neurobehavioral and biochemical changes mimicking those observed in Parkinson's disease. Treatment of rats with S. terebinthifolius produced significant neuroprotection probably mediated through its antioxidant activity. Taken together, our data suggested neuroprotective effects of S. terebinthifolius Raddi, probably mediated through its antioxidant activity. Thus, the present work brought out new insights for the treatment of neurodegenerative diseases and highlighted the pharmacological properties for Parkinson's disease and other neurodegenerative pathologies.
Acknowledgements
The authors would like to thank CAPES for financial support. The authors also would like to dedicate this paper to Simone Sette Lopes Lafayette.
References
- Abd El Mohsen, M.M., Kuhnle, G., Rechner, A.R., Schroeter, H., Rose, S., Jenner, P., Rice-Evans, C.A., 2002. Uptake and metabolism of epitatechin and its access to the brain after oral ingestion. Free Radic. Biol. Med. 33, 1693-1702.
- Abdou, R.H., Saleh, S.Y., Khalil, W.F., 2015. Toxicological and biochemical studies on Schinus terebinthifolius concerning its curative and hepatoprotective effects against carbon tetrachloride-induced liver injury. Pharmacogn. Mag. 11, S93-S101.
- Bernardi, M.M., Palermo-Neto, J., 1979. Effects of abrupt and gradual withdrawal from long-term haloperidol treatment on open field behavior of rats. Psychopharmacology 65, 247-250.
- Betarbet, R., Sherer, T.B., Mackenzie, G., Garcia-Osuma, M., Panov, A.V., Greenamyre, J.T., 2000. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat. Neurosci. 3, 1301-1306.
- Blesa, J., Phani, S., Jackson-Lewis, V., Przedborski, S., 2012. Classic and new animal models of Parkinson's disease. J. Biomed. Biotechnol. 2012(ID), 845618.
- Brand-Williams, W., Cuvelier, M.E., Berset, C., 1995. Use of free radical method to evaluate antioxidant activity. Lebensm-Wiss. U. Technol. 28, 25-30.
- Broadhurst, P.L., 1957. Determination of emotionality in the rat. I. Situational factors. Br. J. Psychol. 48, 1-12.
- Buege, J.A., Aust, S.D., 1978. Microssomal lipid peroxidation. Methods Enzymol. 52, 302-310.
- Chen, M., Wang, T., Yue, F., Li, X., Wang, P., Li, Y., Chan, P., Yu, S., 2015. Tea polyphenols alleviate motor impairments, dopaminergic neuronal injury, and cerebral α-synuclein aggregation in MPTP-intoxicated Parkinsonian monkeys. Neuroscience 286, 383-392.
- Corrêa, M.P., 1974. Dicionário de plantas úteis do Brasil e das plantas exóticas cultivadas, vol. 3. Imprensa Nacional, Rio de Janeiro, Brazil, pp. 125–126.
- Costa, C.O.D'S., Ribeiro, P.R., Loureiro, M.B., Simões, R.C., Castro, R.D., Fernandez, L.G., 2015. Phytochemical screening, antioxidant and antibacterial activities of extracts prepared from different tissues of Schinus terebinthifolius Raddi that occurs in the coast of Bahia, Brazil. Phcog. Mag. 11, 607-614.
- Dauer, W., Przedborski, S., 2003. Parkinson's disease: mechanisms and models. Neuron 39, 889-909.
- Dutta, D., Mohanakumar, K.P., 2015. Tea and Parkinson's disease: constituents of tea synergize with antiparkinsonian drugs to provide better therapeutic benefits. Neurochem. Int. 89, 181-190.
- Fernandez, H.H., 2015. Update on Parkinson disease. Clev. Clin. J. Med. 82, 563-568.
- Gao, X1, Cassidy, A., Schwarzschild, M.A., Rimm, E.B., Ascherio, A., 2012. Habitual intake of dietary flavonoids and risk of Parkinson disease. Neurology 78, 1138-1145.
- Hidgon, J.V., Frei, B., 2003. Tea catechins and polyphenols: health effects, metabolism and antioxidant functions. Crit. Rev. Food Sci. Nutr. 43, 89-143.
- Khan, N., Mukhtar, H., 2007. Tea polyphenols for health promotion. Life Sci. 81, 519-533.
- Kim, Y.J., 2007. Antimelanogenic and antioxidant properties of gallic acid. Biol. Pharm. Bull. 30, 1052-1055.
- Linard-Medeiros, C.F.B., Sales, V.A.W., Ramos, A.C., Sereniki, A., Trevisan, M.T.S., Wanderley, A.G., Lafayette, S.S., 2015. Neuroprotective effect of extract of Anacardium occidentale Linn on a rotenone model of Parkinson's disease. Int. J. Pharm. Sci. Res. 6, 123-129.
- Mandel, S., Amit, T., Reznichenko, L., Weinreb, O., Youdim, M.B., 2006. Green tea catechins as brain-permeable, natural iron chelators-antioxidants for the treatment of neurodegenerative disorders. Mol. Nutr. Food Res. 50, 229-234.
- Medeiros, K.C.P., Monteiro, J.C., Diniz, M.F.F.M., Medeiros, I.A., Silva, B.A., Piuvezam, M.R., 2007. Effect of the activity of the Brazilian polyherbal formulation: Eucalyptus globulus Labill, Peltodon radicans Pohl and Schinus terebinthifolius Raddi in inflammatory models. Rev. Bras. Farmacogn. 7, 23-28.
- Monville, C., Torres, E.M., Dunnett, S.B., 2006. Comparison of incremental and accelerating protocols of the rotarod test for the assessment of motor deficits in the 6-OHDA model. J. Neurosci. Methods 158, 219-223.
- Morton, J.F., 1978. Brazilian pepper: its impact on people, animals and the environment. Econ. Bot. 32, 353-359.
- Renaud, J., Nabavi, S.F., Daglia, M., Nabavi, S.M., Martinoli, M.G., 2015. Epigallocatechin-3-gallate, a promising molecule for Parkinson's disease?. Rejuvenation Res. 18, 257-269.
- Sanders, L.H., Greenamyre, J.T., 2013. Oxidative damage to macromolecules in human Parkinson disease and the rotenone model. Free Radic. Biol. Med. 62, 111-120.
- Senthil, K., 2008. Repletion of antioxidant status by EGCG and retardation of oxidative damage induced macromolecular anomalies in aged rats. Exp. Gerontol. 43, 143-183.
- Sherer, T.B., Kim, J.H., Betarbet, R., Greenamyre, J.T., 2003. Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and alpha-synuclein aggregation. Exp. Neurol. 179, 9-16.
- Singh, R.P., Sharad, S., Kapur, S., 2004. Free radicals and oxidative stress in neurodegenerative diseases: relevance of dietary antioxidants. J. Indian Acad. Clin. Med. 5, 218-225.
- Solanki, I., Parihar, P., Mansuri, M.L., Parihar, M.S., 2015. Flavonoid-based therapies in the early management of neurodegenerative diseases. Adv. Nutr. 6, 64-72.
- Srividhya, R., Zarkovic, K., Stroser, M., Waeg, G., Zarkovic, N., Kalaiselvi, P., 2009. Mitochondrial alterations in aging rat brain: effective role of (-)-epigallo catechin gallate. Int. J. Dev. Neurosci. 27, 223-231.
- Virmani, A., Pinto, L., Binienda, Z., Ali, S., 2013. Food, nutrigenomics, and neurodegeneration–neuroprotection by what you eat!. Mol. Neurobiol. 48, 353-362.
- von Wrangel, C., Schwabe, K., John, N., Krauss, J.K., Alam, M., 2015. The rotenone-induced rat model of Parkinson's disease: behavioral and electrophysiological findings. Behav. Brain Res. 279, 52-61.
- Weinreb, O., Mandel, S., Amit, T., Youdim, M.B., 2004. Neurological mechanisms of green tea catechin polyphenols in Alzheimer's and Parkinson's diseases. J. Nutr. Biochem. 15, 506-516.
- Zaitone, S.A., Abo-Elmatty, D.M., Shaalan, A.A., 2012. Acetyl-L-carnitine and α-lipoic acid affect rotenone-induced damage in nigral dopaminergic neurons of rat brain; implication for Parkinson's disease therapy. Pharmacol. Biochem. Behav. 100, 347-360.
Publication Dates
-
Publication in this collection
Mar-Apr 2016
History
-
Received
08 July 2015 -
Accepted
30 Nov 2015