Abstract
Chitosan is largely known for its activity against a wide range of microorganisms, in which the most acceptable antimicrobial mechanism is found to include the presence of charged groups in the polymer backbone and their ionic interactions with bacteria wall constituents. This interaction suggests the occurrence of a hydrolysis of the peptidoglycans in the microorganism wall, provoking the leakage of intracellular electrolytes, leading the microorganism to death. The charges present in chitosan chains are generated by protonation of amino groups when in acid medium or they may be introduced via structural modification. This latter can be achieved by a methylation reaction resulting in a quaternized derivative with a higher polymeric charge density. Since the charges in this derivative are permanents, it is expected a most efficient antimicrobial activity. Hence, in the present study, commercial chitosan underwent quaternization processes and both (mother polymer and derivative) were evaluated, in gel form, against Staphylococcus aureus (Gram-positive) and Escherichia coli (Gram-negative), as model bacteria. The results, as acquired from turbidity measurements, differ between materials with an expressive reduction on the Gram-positive microorganism (S. aureus) growth, while E. coli (Gram-negative) strain was less sensitive to both polymers. Additionally, the antibacterial effectiveness of chitosan was strongly dependent on the concentration, what is discussed in terms of spatial polymer conformation.
Keywords
Biopolymer; Chitosan; N,N,N-trimethylchitosan; Antimicrobial activity; Turbidity measurements; Quaternization process
Introduction
Chitosan is a natural unbranched homopolymer obtained from chitin, an abundant by-product of seafood processing, via a deacetylation reaction (removal of acetyl groups COCH3 from the chitin original structure) with alkali (Kurita, 2006Kurita, K., 2006. Chitin and chitosan: functional biopolymers from marine crustaceans. Mar. Biotechnol. 89, 2203-2226). The final chitosan structure has one primary amine and two free hydroxyl groups for each monomer and can be expressed by the general formula C6H11O4N. Chitosan has good film-forming ability and due to its high versatility, this polymer has been extensively evaluated for uses in food conservation (Britto and Assis, 2007aBritto, D., Assis, O.B.G., 2007. Synthesis and mechanical properties of quaternary salts of chitosan-based films for food application. Int. J. Biol. Macromol. 41, 198-203), in biomedical applications (Singh and Ray, 2000Singh, D.K., Ray, A.R., 2000. Biomedical applications of chitin, chitosan, and their derivatives. JMS Rev. Macromol. Chem. Phys. C40, 69-83), as material for chemicals encapsulation and controlled release (Patel and Jivani, 2009Patel, J.K., Jivani, N.P., 2009. Chitosan based nanoparticles in drug delivery. Int. J. Pharm. Sci. Nanotech. 2, 517-522) and in environmental remediation (Assis and Britto, 2008Assis, O.B.G., Britto, D., 2008. Formed-in-place polyelectrolyte complex membranes for atrazine recovery from aqueous media. J. Polym. Environ. 16, 192-197).
Commercially, chitosan is found from a variety of sources such as crabs, shrimp, lobster etc., usually sold in powder or as flakes form. The molecular weight and the degree of deacetylation are the main parameters which defines solubility and physic-chemical properties of this polymer. To be transformed in films or pieces, the chitosan should be first solubilized into gel by appropriate solvent dissolution. Crude chitosan however, is only soluble in acid medium, in pH below its pKa (around 6.4). Such means a drawback for broader applications of chitosan, as the pH plays an important role on its biocompatibility (Kurita, 2006Kurita, K., 2006. Chitin and chitosan: functional biopolymers from marine crustaceans. Mar. Biotechnol. 89, 2203-2226) and on film mechanical properties (Britto et al., 2005Britto, D., Campana-Filho, S.P., Assis, O.B.G., 2005. Mechanical properties of N,N,N-trimethylchitosan chloride films. Polímeros. 5, 129-132).
When chitosan molecules are submitted to an intensive methylation process, a derivative salt with permanent positive charges is generated as consequence of the quaternization of the amino groups (identified as trimethylchitosan – TMC) (Britto and Assis, 2007bBritto, D., Assis, O.B.G., 2007. A novel method for obtaining quaternary salt of chitosan. Carbohydr. Polym. 69, 305-310). The presence of these charges in the polymer backbone gives to chitosan a cationic characteristic independent of the solvent pH. TMC can be prepared into gel in neutral medium and therefore, better suited, mainly for food and medical applications (Singh and Ray, 2000Singh, D.K., Ray, A.R., 2000. Biomedical applications of chitin, chitosan, and their derivatives. JMS Rev. Macromol. Chem. Phys. C40, 69-83; Ji et al., 2009Ji, Q.X., Chen, X.G., Zhao, Q.S., Liu, C.S., Cheng, X.J., Wang, L.C., 2009. Injectable thermosensitive hydrogel based on chitosan and quaternized chitosan and the biomedical properties. J. Mater. Sci. Mater. Med. 20, 1603-1610).
Additionally several models suggested that the antimicrobial activity of chitosan is a result from its cationic nature (Rabea et al., 2003Rabea, E.I., Badawy, M.E.-T., Stevens, C.V., Smagghe, G., Steurbaut, W., 2003. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules. 4, 1457-1465; Goy et al., 2009Goy, R.C., Britto, D., Assis, O.B.G., 2009. A review of the antimicrobial activity of chitosan. Polímeros. 19, 241-247). The electrostatic interaction between positively charged R-N(CH3)3+ sites and negatively charged microbial cell membranes, is predicted to be responsible for cellular lysis and assumed as the main antimicrobial mechanism (Rabea et al., 2003Rabea, E.I., Badawy, M.E.-T., Stevens, C.V., Smagghe, G., Steurbaut, W., 2003. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules. 4, 1457-1465; Tripathi et al., 2008Tripathi, S., Mehrotra, G.K., Dutta, P.K., 2008. Chitosan based antimicrobial films for food packaging applications. e-Polymers. 93, 1-7). Charged chitosan can also interact with essential nutrients therefore interfering on microbial growth (Jia et al., 2001Jia, Z., Shen, D., Xu, W., 2001. Synthesis and antibacterial activities of quaternary ammonium salt of chitosan. Carbohydr. Res. 333, 1-6). Consequently is expected that polymers with higher charge densities resulted in an improved antimicrobial activity.
In the present study, commercial chitosan was used as precursor for transformation into charged derivative TMC and both materials, in gel form, was evaluated as antimicrobial agent against the Gram-negative bacterium E. coli and the Gram-positive S. aureus (common foodborne and hospital-acquired pathogen) as a function of polymer concentration.
Materials and methods
Methylation process
The starting chitosan was of medium molecular weight (400,000 g/mol, 75–85% unities deacetylated – shrimp origin) purchased from Sigma-Aldrich Co. (St. Louis, MO, USA) and used as supplied. For the methylation reaction, a developed methodology patented by Embrapa (Empresa Brasileira de Pesquisa Agropecuária) (Britto and Assis, 2007cBritto, D., Assis, O.B.G., 2007. Obtenção de N,N,N-Trimetilquitosana por método simplificado empregando dimetilsulfato como agente metilante – Patent deposition at 16/Mar/2007. Protocol number 0120700262 INPI/DE-DF. Brasília, 2007.), was used. In brief the reaction consists in the addition of 1.2 g of NaOH (0.015 mol) plus 0.88 g of NaCl (0.015 mol) in a suspension of 1 g of chitosan (0.005 mol) in 16 ml of dimethylsulfate (Synth, R. Janeiro, Brazil) and 4 ml of deionized water. The mixture was stirred and the derivative obtained by precipitation with acetone was rinsed and vacuum dried.
The methylation process results in the quaternized derivative TMC (N,N,N-trimethylchitosan), by inserting methyl functionality onto chitosan amino groups at the C-2 position (Fig. 1). Methylation details and a full characterization of TMC structure can be found elsewhere (Britto and Assis, 2007aBritto, D., Assis, O.B.G., 2007. Synthesis and mechanical properties of quaternary salts of chitosan-based films for food application. Int. J. Biol. Macromol. 41, 198-203, bBritto, D., Assis, O.B.G., 2007. A novel method for obtaining quaternary salt of chitosan. Carbohydr. Polym. 69, 305-310).
Schematic representation of the reaction leading to the quaternization of the amino groups of chitosan resulting in N,N,N-trimethylchitosan (TMC). Quaternization experimental details and TMC characterization and can be found in Britto and Assis (2007a,b).
Gel forming
Gels were prepared by dissolving the chitosan in 1% acetic acid (pH 4.0) in deionized water and the TMC solubilized directly in distillated water (pH 6.6). The gels were homogenized for 2 h under moderated magnetic stirring. Polymer concentrations of 0.5, 1.0, 1.5 and 2.0 g/l were prepared for each material.
Inoculums preparation
Escherichia coli (ATCC 8739) and Staphylococcus aureus (ATCC 25923) both provided by Fundação Tropical André Tosello, Campinas, Brazil, were used as bacteria models to evaluate the activity of parent and derivate polymer. The bacteria pre-culture was incubated under aerobiosis and moderate shaking for 24 h. The E. coli was kept at 37 °C and the S. aureus at 32 °C, considering the ideal temperature for each colony growth (Aneja et al., 2009Aneja, K.R., Jain, P., Aneja, R., 2009. A Textbook of Basic and Applied Microbiology. New Age International Publishers Ltd., New Delhi, India.). The bacterial kinetic was determined by measuring the absorbance at 620 nm wavelength hourly, following Bohinc et al. (2015)Bohinc, K., Mojca, J., Rok, F., Goran, D., Peter, R., 2015. Surface characteristics dictate microbial adhesion ability. In: Prokopovich, P. (Ed.), Biological and Pharmaceutical Applications of Nanomaterials. , 1st ed. CRC Press, Boca Raton, p. p208. procedure, using a Shimadzu UVPC 2000 (Shimadzu Co. Kyoto, Japan) spectrophotometer. The stationary phase was taken as a reference time for comparing the polymeric effect on bacterial growth.
Antibacterial assay
The inhibitory effects of chitosan and TMC on the bacterial growth were first estimated by means of turbidity measurements. We took 2 × 108 bacteria/ml as a reference for initial colony quantification (Koch, 1994Ngamviriyavong, P., Thananuson, A., Pankongadisak, P., Tanjak, P., Janvikul, W., 2010. Antibacterial hydrogels from chitosan derivatives. J. Met. Mater. Miner. 20, 113-117). To attain this figure, sequential dilution was necessary (six for E. coli and four for S. aureus) according to simultaneous counting of plate colonies (CFU). For antimicrobial analysis, aliquots of 1 ml of bacterial broth were added to 9 ml of chitosan diluted suspensions and kept under moderate shaking at room temperature. The turbidity was measured in each polymeric sample solutions by adding to the mixture of the cultured bacteria medium and PBS (Phosphate-buffered saline), pH 7.4.
The plate well diffusion method (Rayn et al., 1996Rayn, M.P., Rea, M.C., Hill, C., Ross, R.P., 1996. An application in cheddar cheese manufacture for a strain of Lactococcus lactis producing a novel broad-spectrum bacteriocin, lacticin 3147. Appl. Environ. Microbiol. 62, 612-619), was also used to visualize the formation of a zone of inhibition in a TSB (tryptic soy broth) solid culture medium. The procedure carried used in this analysis follows the agar diffusion method according to Dutta et al. (2009)Dutta, P.K., Tripathi, S., Mehrotra, G.K., Dutta, J., 2009. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 114, 1173-1182 procedure, in which small circular cavities are punctured in the culture medium and filled with approximately 0.25 ml of gels for each polymer concentration. 50 µl of bacterial suspension were spread and the plates stored for 24 h at 32–37 °C to allow microorganism growth. Inhibition zones were measured on bases of the average diameter of the clear area, directly on the dishes. Three replicate plates were used for each concentration and data were subjected to statistical evaluation by one-way analysis of variance (ANOVA). The significance p ≤ 0.05 was considered using a Microcal Origin 9.0 software (OriginLab Co., Northampton, MA, USA).
Results and discussion
The growth kinetics curves of E. coli and S. aureus, as measured by turbidity at 620 nm, is presented in Fig. 2, where OD stands for Optical Density. Both bacteria grow in a similar way but with differences in turbidity. It is recorded an exponential increasing (log phase) during the first 6–8 h, followed by the stable stationary phase. The log phase for E. coli appears to be longer than that measured for S. aureus. The kinetics curves for both bacteria are in complete agreement to several examples found in the literature (Duffy et al., 1999Duffy, G., Whiting, R.C., Sheridan, J.J., 1999. The effect of a competitive microflora, pH and temperature on the growth kinetics of Escherichia coli O157:H7. Food Microbiol. 16, 299-307; Fujikawa and Morozumi, 2006Fujikawa, H., Morozumi, S., 2006. Modeling Staphylococcus aureus growth and enterotoxin production in milk. Food Microbiol. 23, 260-267). The turbidity readings with the polymer addition were then carried out after 12 h incubation, assuring the attainment of maximum microorganisms per volume (plateau).
Growth profile for E. coli (Gram-negative) and S. aureus (Gram-positive) as assayed by turbidity method at 620 nm.
When the polymeric medium is mixed with the referential broth, the growth rate is temporarily affected, causing a reduction on the maximum absorbance. For the Gram-negative bacteria E. coli, the effect of commercial chitosan and TMC addition as a function of polymer concentration is shown in Fig. 3, as measured at 620 nm wavelength.
Absorbance, as measured at 620 nm, as a function of chitosan and TMC concentration added in the medium with E. coli, according to turbidity method after 12 h interaction. Zero polymeric concentration means the absorbance measured in bacterial growth in neat TSB medium. Bars with different letters are statistically different at p < 0.05.
The results indicate that both polymers act positively in reducing bacteria proliferation but within a short range of statistical significance between materials. For all tested polymer concentrations in the gel (0.5–2.0 g/l), chitosan and its charged derivative showed a similar effect against E. coli with an apparent linear tendency in reducing bacterial population as the concentration increases, as can be followed by the dashed line in Fig. 3.
A different behavior however was observed when these polymers were tested against the Gram-positive bacteria S. aureus (Fig. 4). For this strain, both materials show a reduction on the growth colonies, where the chitosan concentration is confirmed to play important role in the antimicrobial activity. For solutions at a concentration of 1 g/l of commercial chitosan, the absorbance is significantly reduced indicating this as an efficient concentration for inhibiting the S. aureus growth in liquid medium. The TMC, although efficient too in reducing number of colonies, does not show a quantitative dependence on the concentration.
Absorbance, as measured at 620 nm as a function of chitosan and TMC concentration in contaminated medium with S. aureus, according to turbidity method after 12 h interaction. Zero polymeric concentration means the absorbance measured in bacterial growth in neat TSB medium. Bars with different letters are statistically different at p < 0.05.
To better quantify the reduction of the bacterial growth as a function of polymer concentration, the antimicrobial efficiency can be mathematically deduced from the turbidity data by considering the time derivative at the maximum absorbance measured in each sample. In other words, an inhibitory “efficiency” can be set as a relation between the maximum bacterial colonies (Nc) growth in the culture medium without polymer (control) and the proportional maximum (Npi) as measured in the presence of each polymer. In the stationary phase the absorbance attained a plateau (Silva et al., 2010Silva, L.P., Britto, D., Seleghim, M.H.R., Assis, O.B.G., 2010. In vitro activity of water-soluble quaternary chitosan chloride salt against E coli . World J. Microbiol. Biotechnol. 26, 2089-2092), so the absorbance intensity can be considered as OD at dN/dt = 0, then:
where Ic and Ipi are the maximum absorbance, for control (c) and for the medium with added polymer (pi), when the bacterial growth reach the maximum population (maximum absorbance).
Such a derivative relation is useful in defining the “Inhibitory Proportional Factor” (If), which can be considered as the ratio between the maximum specific growth for the control sample and those measured with polymer additions, by a simple relation as:
As If increases, greater can be considered the antibacterial effect. Regarding the turbidity data as measured in this work, the derivative relation (2) can be graphically represented for both types of bacteria as a function of polymer concentration as plotted in Fig. 5.
Variation of Inhibitory proportional factor (If), according to Eq. (2). The curves indicate the proportional bacterial reduction in each analyzed culture (S. aureus and E. coli) in function of polymer concentration in the medium.
The graphic indicates a higher antimicrobial activity of both polymers against the Gram-positive specie S. aureus. For this microorganism, all tested concentrations of TMC resulted in an inhibition as close as 50% over the bacterial colonies relative to growth as measured in the control culture medium. The maximum activity is attained when 1 g/l of commercial chitosan is added (for this the peak indicates a population reduction of 96% in the colonies with respect to control). A subsequent reduction in efficiency is observed as the amount of polymer increases, reaching approximately the same as measured to TMC when 2 g/l of chitosan was added.
The decrease on antibacterial activity as the concentration increases can be discussed in terms of the spatial arrangement of the polymer chains: low polymer concentrations yields a better molecular distribution in the solvent with a relatively small number interaction between the neighboring chains, so the charged sites available for external coupling are maximized (Palermo and Kuroda, 2010Palermo, E.F., Kuroda, K., 2010. Structural determinants of antimicrobial activity in polymers which mimic host defense peptides. Appl. Microbiol. Biotechnol. 87, 1605-1615). Additionally, as stated by Gabriel et al. (2009)Gabriel, G.J., Maegerlein, J.A., Nelson, C.F., Dabkowski, J.M., Eren, T., Nüsslein, K., Tew, G.N., 2009. Comparison of facially amphiphilic versus segregated monomers in the design of antibacterial copolymers. Chem-Eur. J. 15, 433-439, few numbers of chain–chain bonds makes easy the mobility of active moieties by positioning them on opposite sides of the polymer backbone, which favors interfacial interactions. As the concentration increases, the formation of hydrogen and covalent bonds amongst the functional groups of the chitosan chains is facilitated; reducing the dispersion and leads the structure to assume a densely overlapping coiled conformation (Freitas et al., 2010Freitas, R.A., Drenski, M.F., Alb, A.M., Reed, W.F., 2010. Characterization of stability, aggregation, and equilibrium properties of modified natural products: the case of carboxymethylated chitosans. Mater. Sci. Eng. C. 30, 34-41). Such random-coil shape of chitosan in solution is largely cited in the literature and attributed to the extensive intra and intermolecular bonds at lager molecular densities (Hejazi and Amiji, 2002Hejazi, R., Amiji, M., 2002. Chitosan-based delivery system: physicochemical properties and pharmaceutical applications. In: Dumitriu, S. (Ed.), Polymeric Biomaterials, Revised and Expande, 2nd ed. Marcel Dekker Inc., New York, pp. 213–238.; Morris et al., 2009Morris, G.A., Castile, J., Smith, A., Adams, G.G., Harding, S.E., 2009. Macromolecular conformation of chitosan in dilute solution: a new global hydrodynamic approach. Carbohydr. Polym. 76, 616-621; Halabalova et al., 2011Halabalova, V., Simek, L., Mokrejs, P., 2011. Intrinsic viscosity and conformational parameters of chitosan chains. Rasayan J. Chem. 4, 223-241). This in turn imposes spatial restrictions to functional groups (Solomonidou et al., 2001Solomonidou, D., Cremer, K., Krumme, M., Kreuter, J., 2001. Effect of carbomer concentration and degree of neutralization on the mucoadhesive properties of polymer films. J. Biomat. Sci-Polym. E. 12, 1191-1205). Consequently, an inferior number of charged sites will be available to interaction thereby hampering binding to bacterial cell walls. This was recently confirmed in our group by analyzing the variation of chitosan concentration in film processing and its effect on antimicrobial activity (Goy and Assis, 2014Goy, R.C., Assis, O.B.G., 2014. Antimicrobial analysis of films processed from chitosan and N,N,N-trimethylchitosan. Braz. J. Chem. Eng. 31, 643-648). On the other hand, this effect is not registered for the TMC, which can be understood as the result of its high charge density enhancing the repulsion between the chains.
Concerning the efficiency against the Gram-negative E. coli strain, both polymers showed inferior activity when compared to the effect measured on the Gram-positive S. aureus. Neither chitosan nor the TMC attained activity superior to 25% in reducing the bacterial growth. A small concentration of polymers also appears to be more effective against E. coli with peaks of maximum efficiency also attained when 1 g/l of TMC and 1.5 g/l of chitosan were added (Fig. 5).
In general, the antimicrobial effectiveness of chitosan and its derivative against Gram-positive and Gram-negative bacteria is somewhat controversial. In some published works, the literature presents that unmodified chitosan generally acts stronger on Gram-negative than on Gram-positive strains (No et al., 2002No, H.K., Park, N.Y., Lee, S.H., Meyers, S.P., 2002. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 74, 65-72; Silva et al., 2010Silva, L.P., Britto, D., Seleghim, M.H.R., Assis, O.B.G., 2010. In vitro activity of water-soluble quaternary chitosan chloride salt against E coli . World J. Microbiol. Biotechnol. 26, 2089-2092). Such better antimicrobial efficiency has been attributed to bacterium wall characteristics, considering that the Gram-negative cell wall is thinner and consequently more susceptible than the Gram-positive's.
There are however, contradictory evidences presented by several other authors, for whom greater activities of chitosan and its derivatives over Gram-positive strains were reported as predominant (Sudarshan et al., 1992Sudarshan, N.R., Hoover, D.G., Knorr, D., 1992. Antibacterial action of chitosan. Food Biotechnol. 6, 257-272; Eaton et al., 2008Eaton, P., Fernandes, J.C., Pereira, E., Pintado, M.E., Malcata, F.X., 2008. Atomic force microscopy study of the antibacterial effects of chitosans on Escherichia coli and Staphylococcus aureus . Ultramicroscopy. 108, 1128-1134). Although the electrostatic interaction between positively charged chitosan groups and negatively charged sites on microbial cell is assumed as the main antimicrobial mechanism (Rabea et al., 2003Rabea, E.I., Badawy, M.E.-T., Stevens, C.V., Smagghe, G., Steurbaut, W., 2003. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules. 4, 1457-1465), the thickness of the peptidoglycan layer can play an important role in providing a rigid structure which can act as a barrier against chitosan interactions (Zheng and Zhu, 2003Zheng, L-Y., Zhu, J-F., 2003. Study on antimicrobial activity of chitosan with different molecular weights. Carbohydr. Polym. 54, 527-630). The cell wall of E. coli, for example, has a thickness of 7–8 nm while the wall of S. aureus (Gram-positive) is around 20–80 nm (Eaton et al., 2008Eaton, P., Fernandes, J.C., Pereira, E., Pintado, M.E., Malcata, F.X., 2008. Atomic force microscopy study of the antibacterial effects of chitosans on Escherichia coli and Staphylococcus aureus . Ultramicroscopy. 108, 1128-1134), what can difficult the cellular lysis.
By using the agar well diffusion assay testing, the activity intensity can be visually checked by assessing the local inhibition (Rayn et al., 1996Rayn, M.P., Rea, M.C., Hill, C., Ross, R.P., 1996. An application in cheddar cheese manufacture for a strain of Lactococcus lactis producing a novel broad-spectrum bacteriocin, lacticin 3147. Appl. Environ. Microbiol. 62, 612-619), as illustrative examples showed in Fig. 6. Due to high solubility of TMC, it was not possible to assess the results from this material. Its dissolution and fast infiltration into the culture medium makes holding the gel in a limited well impractical and thus not reliable for any definition or inhibition zone measurements.
Examples of well inhibitory zones formed by the presence of chitosan gel, in the concentrations of 1 and 2.0 g/l, in culture medium inoculated with E. coli and S. aureus bacteria after 24 h of interaction. Following Dutta et al. (2009)Dutta, P.K., Tripathi, S., Mehrotra, G.K., Dutta, J., 2009. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 114, 1173-1182, proposed method.
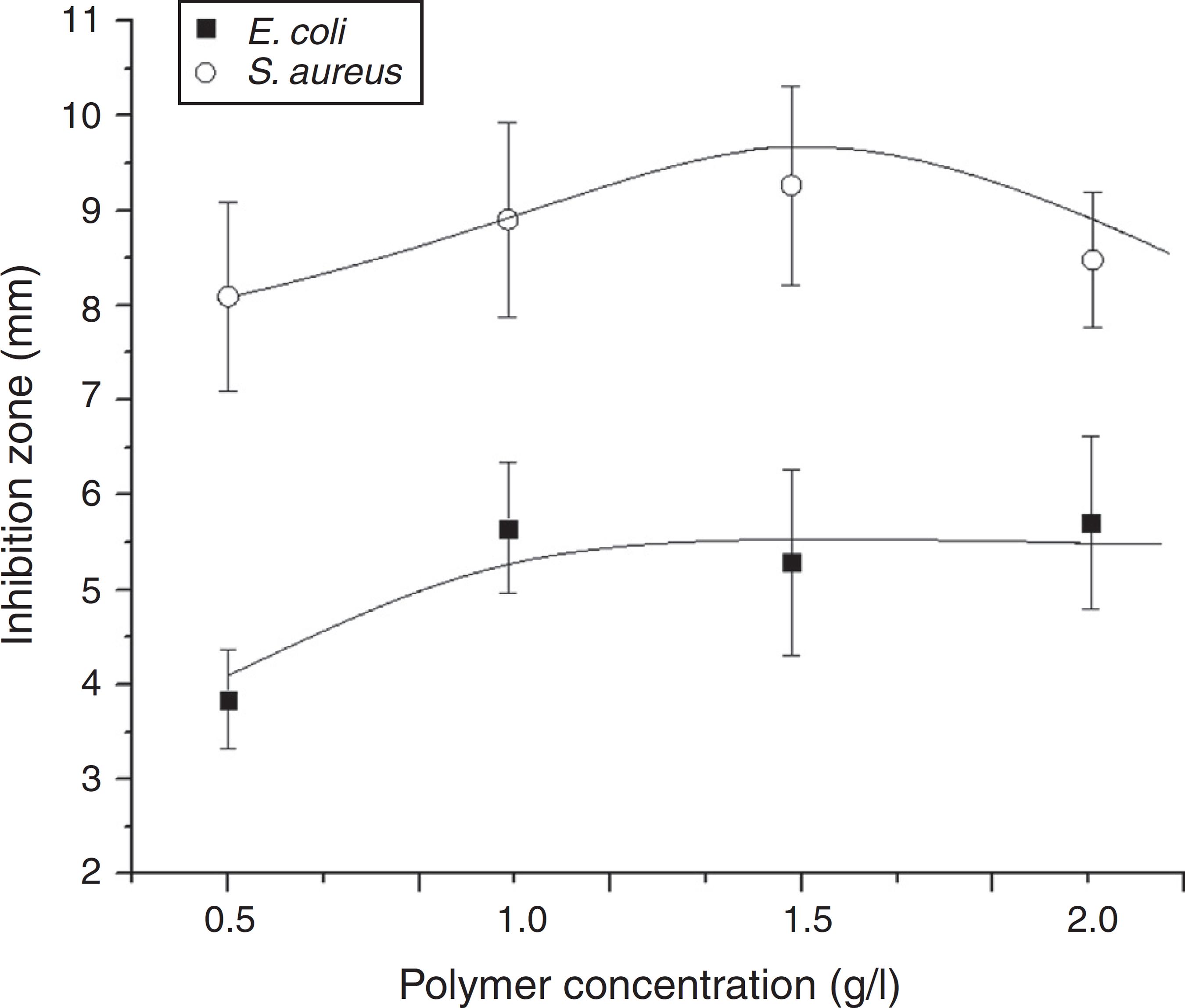
The variation of the inhibition for commercial chitosan as concentrations changes is graphically represented in Fig. 7. By comparing the action on both types of bacteria, the inhibition zone method confirms an activity more effective of this polymer against the Gram-positive bacterium S. aureus (larger areas) in agreement to results attained from the turbidity tests.
Size of inhibition zone as a function of commercial chitosan concentration in the gel, as measured against the Gram-positive S. aureus and the Gram-negative E. coli bacteria.
It should be noted that the real antimicrobial activity of chitosan is a complex process and for a complete appraisal several other factors, both intrinsic and extrinsic, should be considered such as:
-
(i) The polymer molecular weight and the size of coiled of chitosan chains are reported to have important effect on microorganism growth and multiplication (Omura et al., 2003Omura, Y., Shigemoto, M., Akiyama, T., Saimoto, H., Shigemasa, Y., Nakamura, I., Tsuchido, T., 2003. Antimicrobial activity of chitosan with different degrees of acetylation and molecular weights. Biocontrol. Sci. 8, 25-30; Zheng and Zhu, 2003Zheng, L-Y., Zhu, J-F., 2003. Study on antimicrobial activity of chitosan with different molecular weights. Carbohydr. Polym. 54, 527-630; Goy et al., 2009Goy, R.C., Britto, D., Assis, O.B.G., 2009. A review of the antimicrobial activity of chitosan. Polímeros. 19, 241-247).
-
(ii) The degree of acetylation: some studies suggested that as lower the degree of acetylation, higher will be the antimicrobial effectiveness (No et al., 2002No, H.K., Park, N.Y., Lee, S.H., Meyers, S.P., 2002. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 74, 65-72; Rabea et al., 2003Rabea, E.I., Badawy, M.E.-T., Stevens, C.V., Smagghe, G., Steurbaut, W., 2003. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules. 4, 1457-1465) and,
-
(iii) The interacting pH. As lower the pH more intense will be the ion interaction, since the amine groups are protonated and a pair of electrons on the amine nitrogen became available for ion donation generating a hydrolysis of the peptidoglycans microorganism wall constituents (Sudarshan et al., 1992Sudarshan, N.R., Hoover, D.G., Knorr, D., 1992. Antibacterial action of chitosan. Food Biotechnol. 6, 257-272; Ngamviriyavong et al., 2010Ngamviriyavong, P., Thananuson, A., Pankongadisak, P., Tanjak, P., Janvikul, W., 2010. Antibacterial hydrogels from chitosan derivatives. J. Met. Mater. Miner. 20, 113-117).
Conclusions
Chitosan and its water soluble derivative N,N,N-trimethylchitosan (TMC), showed antimicrobial activity against both Gram-positive and Gram-negative bacterium strains. In gel form, the antibacterial tests carried out by turbidity and well inhibition zone showed that the chitosan is consistently more active against the Gram-positive S. aureus than Gram-negative E. coli. The chitosan concentration in the gel also plays an important role, where a peak of efficiency is attained for addition of 1 g/l. The derivative TMC, in the conditions here tested, showed good activity despite of inferior comparative results in all evaluated concentration. Concerning the action on the Gram-negative specie E. coli, both materials resulted in inferior inhibition, independent of the used concentration.
Acknowledgments
The author thanks to FAPESP (grant 07/58715-4) and Rede AgroNano(Embrapa) for financial support.
Ethical disclosures
-
Protection of human and animal subjects . The authors declare that no experiments were performed on humans or animals for this study.
-
Confidentiality of data . The authors declare that no patient data appear in this article.
-
Right to privacy and informed consent . The authors declare that no patient data appear in this article.
References
- Assis, O.B.G., Britto, D., 2008. Formed-in-place polyelectrolyte complex membranes for atrazine recovery from aqueous media. J. Polym. Environ. 16, 192-197
- Aneja, K.R., Jain, P., Aneja, R., 2009. A Textbook of Basic and Applied Microbiology. New Age International Publishers Ltd., New Delhi, India.
- Bohinc, K., Mojca, J., Rok, F., Goran, D., Peter, R., 2015. Surface characteristics dictate microbial adhesion ability. In: Prokopovich, P. (Ed.), Biological and Pharmaceutical Applications of Nanomaterials. , 1st ed. CRC Press, Boca Raton, p. p208.
- Britto, D., Campana-Filho, S.P., Assis, O.B.G., 2005. Mechanical properties of N,N,N-trimethylchitosan chloride films. Polímeros. 5, 129-132
- Britto, D., Assis, O.B.G., 2007. Synthesis and mechanical properties of quaternary salts of chitosan-based films for food application. Int. J. Biol. Macromol. 41, 198-203
- Britto, D., Assis, O.B.G., 2007. A novel method for obtaining quaternary salt of chitosan. Carbohydr. Polym. 69, 305-310
- Britto, D., Assis, O.B.G., 2007. Obtenção de N,N,N-Trimetilquitosana por método simplificado empregando dimetilsulfato como agente metilante – Patent deposition at 16/Mar/2007. Protocol number 0120700262 INPI/DE-DF. Brasília, 2007.
- Duffy, G., Whiting, R.C., Sheridan, J.J., 1999. The effect of a competitive microflora, pH and temperature on the growth kinetics of Escherichia coli O157:H7. Food Microbiol. 16, 299-307
- Dutta, P.K., Tripathi, S., Mehrotra, G.K., Dutta, J., 2009. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 114, 1173-1182
- Eaton, P., Fernandes, J.C., Pereira, E., Pintado, M.E., Malcata, F.X., 2008. Atomic force microscopy study of the antibacterial effects of chitosans on Escherichia coli and Staphylococcus aureus . Ultramicroscopy. 108, 1128-1134
- Freitas, R.A., Drenski, M.F., Alb, A.M., Reed, W.F., 2010. Characterization of stability, aggregation, and equilibrium properties of modified natural products: the case of carboxymethylated chitosans. Mater. Sci. Eng. C. 30, 34-41
- Fujikawa, H., Morozumi, S., 2006. Modeling Staphylococcus aureus growth and enterotoxin production in milk. Food Microbiol. 23, 260-267
- Gabriel, G.J., Maegerlein, J.A., Nelson, C.F., Dabkowski, J.M., Eren, T., Nüsslein, K., Tew, G.N., 2009. Comparison of facially amphiphilic versus segregated monomers in the design of antibacterial copolymers. Chem-Eur. J. 15, 433-439
- Goy, R.C., Britto, D., Assis, O.B.G., 2009. A review of the antimicrobial activity of chitosan. Polímeros. 19, 241-247
- Goy, R.C., Assis, O.B.G., 2014. Antimicrobial analysis of films processed from chitosan and N,N,N-trimethylchitosan. Braz. J. Chem. Eng. 31, 643-648
- Halabalova, V., Simek, L., Mokrejs, P., 2011. Intrinsic viscosity and conformational parameters of chitosan chains. Rasayan J. Chem. 4, 223-241
- Hejazi, R., Amiji, M., 2002. Chitosan-based delivery system: physicochemical properties and pharmaceutical applications. In: Dumitriu, S. (Ed.), Polymeric Biomaterials, Revised and Expande, 2nd ed. Marcel Dekker Inc., New York, pp. 213–238.
- Ji, Q.X., Chen, X.G., Zhao, Q.S., Liu, C.S., Cheng, X.J., Wang, L.C., 2009. Injectable thermosensitive hydrogel based on chitosan and quaternized chitosan and the biomedical properties. J. Mater. Sci. Mater. Med. 20, 1603-1610
- Jia, Z., Shen, D., Xu, W., 2001. Synthesis and antibacterial activities of quaternary ammonium salt of chitosan. Carbohydr. Res. 333, 1-6
- Ngamviriyavong, P., Thananuson, A., Pankongadisak, P., Tanjak, P., Janvikul, W., 2010. Antibacterial hydrogels from chitosan derivatives. J. Met. Mater. Miner. 20, 113-117
- Kurita, K., 2006. Chitin and chitosan: functional biopolymers from marine crustaceans. Mar. Biotechnol. 89, 2203-2226
- Morris, G.A., Castile, J., Smith, A., Adams, G.G., Harding, S.E., 2009. Macromolecular conformation of chitosan in dilute solution: a new global hydrodynamic approach. Carbohydr. Polym. 76, 616-621
- Ngamviriyavong, P., Thananuson, A., Pankongadisak, P., Tanjak, P., Janvikul, W., 2010. Antibacterial hydrogels from chitosan derivatives. J. Met. Mater. Miner. 20, 113-117
- No, H.K., Park, N.Y., Lee, S.H., Meyers, S.P., 2002. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 74, 65-72
- Palermo, E.F., Kuroda, K., 2010. Structural determinants of antimicrobial activity in polymers which mimic host defense peptides. Appl. Microbiol. Biotechnol. 87, 1605-1615
- Omura, Y., Shigemoto, M., Akiyama, T., Saimoto, H., Shigemasa, Y., Nakamura, I., Tsuchido, T., 2003. Antimicrobial activity of chitosan with different degrees of acetylation and molecular weights. Biocontrol. Sci. 8, 25-30
- Patel, J.K., Jivani, N.P., 2009. Chitosan based nanoparticles in drug delivery. Int. J. Pharm. Sci. Nanotech. 2, 517-522
- Rabea, E.I., Badawy, M.E.-T., Stevens, C.V., Smagghe, G., Steurbaut, W., 2003. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules. 4, 1457-1465
- Rayn, M.P., Rea, M.C., Hill, C., Ross, R.P., 1996. An application in cheddar cheese manufacture for a strain of Lactococcus lactis producing a novel broad-spectrum bacteriocin, lacticin 3147. Appl. Environ. Microbiol. 62, 612-619
- Silva, L.P., Britto, D., Seleghim, M.H.R., Assis, O.B.G., 2010. In vitro activity of water-soluble quaternary chitosan chloride salt against E coli . World J. Microbiol. Biotechnol. 26, 2089-2092
- Singh, D.K., Ray, A.R., 2000. Biomedical applications of chitin, chitosan, and their derivatives. JMS Rev. Macromol. Chem. Phys. C40, 69-83
- Solomonidou, D., Cremer, K., Krumme, M., Kreuter, J., 2001. Effect of carbomer concentration and degree of neutralization on the mucoadhesive properties of polymer films. J. Biomat. Sci-Polym. E. 12, 1191-1205
- Sudarshan, N.R., Hoover, D.G., Knorr, D., 1992. Antibacterial action of chitosan. Food Biotechnol. 6, 257-272
- Tripathi, S., Mehrotra, G.K., Dutta, P.K., 2008. Chitosan based antimicrobial films for food packaging applications. e-Polymers. 93, 1-7
- Zheng, L-Y., Zhu, J-F., 2003. Study on antimicrobial activity of chitosan with different molecular weights. Carbohydr. Polym. 54, 527-630
Publication Dates
-
Publication in this collection
Jan-Feb 2016
History
-
Received
25 May 2015 -
Accepted
8 Sept 2015