Abstracts
INTRODUCTION: Phenylketonuria (PKU) is characterized by deficiency of the enzyme phenylalanine hydroxylase, leading to accumulation of phenylalanine. Early diagnosis and subordination to low-phenylalanine diet are important to prevent the harmful effects of hyperphenylalaninemia. In case the diet is not strictly followed, some possible effects are imbalance in the neutral amino acids that use the same carrier of phenylalanine to cross the blood-brain barrier, causing hence reduction in tryptophan entry, the precursor of serotonin in the brain. This neurotransmitter has been implicated in the regulation of mood states, and its high production is linked to central fatigue in individuals subjected to prolonged exercise. Physical exercise increases free tryptophan levels in the blood, which facilitates its influx in the brain, and therefore, may be useful in hyperphenylalaninemia states. OBJECTIVE: To assess whether aerobic exercise is able to normalize the concentrations of tryptophan in the brain of rats with hyperphenylalaninemia. METHODS: 32 rats were randomly assigned to sedentary (Sed) and exercise (Exe) groups, and then divided into control (HEA) and hyperphenylalaninemia (PKU). Hyperphenylalaninemia was induced by administration of alpha-metylphenylalanine and phenylalanine for three days, while the HEA groups received saline. Exe groups held a session of aerobic exercise lasting 60 minutes and speed of 12 m.min-1. RESULTS: The concentration of tryptophan in the brain of PKU groups was significantly lower than HEA groups (both in Sed and Exe groups), compatible with the condition of hyperphenylalaninemia. The exercise increased brain tryptophan levels comparing to sedentary animals. The most interesting finding was that the brain tryptophan levels of ExePKU group were not different from SedHEA group. CONCLUSION: The results indicate an important role of aerobic exercise to restore the concentration of tryptophan in the brain in hyperphenylalaninemic rats.
phenylketonuria; phenylalanine; serotonin
INTRODUÇÃO: A fenilcetonúria (PKU) é caracterizada pela deficiência da enzima fenilalanina hidroxilase, causando acúmulo de fenilalanina. O diagnóstico precoce e a subordinação à dieta pobre em fenilalanina são importantes para prevenir os efeitos prejudiciais da hiperfenilalaninemia. Não aderir estritamente à dieta provoca, entre outros efeitos, um desequilíbrio entre os aminoácidos neutros que usam o mesmo transportador da fenilalanina na barreira hematoencefálica, causando, então, a diminuição da entrada de triptofano, o precursor de serotonina no cérebro. Esse neurotransmissor tem sido implicado na regulação dos estados de humor, sendo sua alta produção ligada à fadiga central em indivíduos submetidos a exercício prolongado. O exercício físico aumenta os níveis de triptofano livre no sangue, o que facilita seu influxo no cérebro, podendo, portanto, ser útil nos estados hiperfenilalaninêmicos. OBJETIVO: Avaliar se o exercício aeróbico é capaz de normalizar as concentrações de triptofano no cérebro de ratos com hiperfenilalaninemia. MÉTODOS: Trinta e dois ratos foram separados nos grupos sedentário (Sed) e exercício (Exe), e cada um deles subdividido em controle (SAL) e hiperfenilalaninemia (PKU). A hiperfenilalaninemia foi induzida pela administração de alfa-metilfenilalanina e fenilalanina durante três dias, enquanto os grupos SAL receberam salina. Os grupos Exe realizaram uma sessão de exercício aeróbico com duração de 60min e velocidade de 12m.min-1. RESULTADOS: A concentração de triptofano no cérebro nos grupos PKU foi significativamente menor que nos grupos SAL, tanto Sed como Exe, compatível com a condição hiperfenilalaninêmica. O exercício aumentou a concentração cerebral de triptofano comparada aos animais sedentários. O achado mais interessante foi que a concentração cerebral de triptofano no grupo ExePKU não foi diferente do SedSAL. CONCLUSÃO: Os resultados indicam um importante papel do exercício aeróbico para restaurar a concentração de triptofano no cérebro em ratos hiperfenilalaninêmicos.
fenilcetonúria; fenilalanina; serotonina
ORIGINAL ARTICLE
EXERCISE AND SPORTS SCIENCES
Acute aerobic exercise restores tryptophan concentration in the brain of rats with hyperphenylalaninemia
Priscila Nicolao Mazzola; Tarsila Barros Moraes; Carolina Didonet Pederzolli; Andrea Rosa; Fernanda Rech Zanin; Juliana Coelho; Carlos Severo Dutra-Filho
Biochemistry Department, Basic Health Sciences Institute, Federal University of Rio Grande do Sul Porto Alegre, RS
Mailing address
ABSTRACT
INTRODUCTION: Phenylketonuria (PKU) is characterized by deficiency of the enzyme phenylalanine hydroxylase, leading to accumulation of phenylalanine. Early diagnosis and subordination to low-phenylalanine diet are important to prevent the harmful effects of hyperphenylalaninemia. In case the diet is not strictly followed, some possible effects are imbalance in the neutral amino acids that use the same carrier of phenylalanine to cross the blood-brain barrier, causing hence reduction in tryptophan entry, the precursor of serotonin in the brain. This neurotransmitter has been implicated in the regulation of mood states, and its high production is linked to central fatigue in individuals subjected to prolonged exercise. Physical exercise increases free tryptophan levels in the blood, which facilitates its influx in the brain, and therefore, may be useful in hyperphenylalaninemia states.
OBJECTIVE: To assess whether aerobic exercise is able to normalize the concentrations of tryptophan in the brain of rats with hyperphenylalaninemia.
METHODS: 32 rats were randomly assigned to sedentary (Sed) and exercise (Exe) groups, and then divided into control (SAL) and hyperphenylalaninemia (PKU). Hyperphenylalaninemia was induced by administration of alpha-metylphenylalanine and phenylalanine for three days, while the SAL groups received saline. Exe groups held a session of aerobic exercise lasting 60 minutes and speed of 12 m.min-1.
RESULTS: The concentration of tryptophan in the brain of PKU groups was significantly lower than SAL groups (both in Sed and Exe groups), compatible with the condition of hyperphenylalaninemia. The exercise increased brain tryptophan levels comparing to sedentary animals. The most interesting finding was that the brain tryptophan levels of ExePKU group were not different from SedSAL group.
CONCLUSION: The results indicate an important role of aerobic exercise to restore the concentration of tryptophan in the brain in hyperphenylalaninemic rats.
Keywords: phenylketonuria, phenylalanine, serotonin.
INTRODUCTION
Penylketonuria (PKU) is the most common innate error among the amino acids metabolism disorders and it occurs with the frequency of 1:14.000 births1. The classical form of this condition is caused by genetic mutations of the hepatic enzyme phenylalanine hydroxylase, located in the 12 cromosome2, making the action of this enzyme responsible for the transformation of the essential amino acid phenylalanine in tyrosine impossible. When this route is inactivated, the phenylalanine accumulates and takes alternative routes, causing disorders, mainly in the central nervous system. If not early treated, the child with phenylketonuria presents a clinical scenario characterized by important episodes to the central nervous system, presenting severe mental retardation, microcephalia, seizures, motor alterations, among other neurological symptoms3.
In order to avoid these consequences, the phenylketonuric patients adopt a hypoprotein diet especially poor in phenylalanine for maintenance of the blood levels of this amino acid within normality. However, the protein needs do not allow its total elimination from diet, causing oscillations in the plasma phenylalanine concentration even in those individuals early and continuously submitted to a special diet. Phenylalanine follows alternative metabolization routes and the increase of phenylalanine and its metabolites causes the toxic effects to the body whose mechanisms are still not completely known3,4.
These substances inhibit important enzymes necessary for the synthesis of neurotransmitter, such as 5-hydroxytryptophane decarboxylase, which originates serotonin5. The high concentration of phenylalanine causes decrease in the concentration of other neutral amino acids, harming hence their passage through the hematoencephalic barrier3,6,7. Moreover, the carriers present there evidence more intimacy with phenylalanine8, causing therefore decrease of availability of tryptophan in the brain, and consequently, lower serotonin production. In phenylketonuric individuals early treated, but who do not strictly follow the diet, lower concentrations of tryptophan and serotonin in the plasma9 and decreased serotonin excretion in the urine are found10. This situation is very common among these patients due to the great difficulty in strictly respecting the dietetical restriction, which may lead to cognitive harm10,11.The lower production of this neurotransmitter is even more pronounced in untreated patients, and reduction of 30-40% in serotonin concentration and of 40-50% in the tryptophan levels in the brain was observed post mortem12.
Serotonin (5-hydroxytryptamine or 5-HT) is an important monoaminergic neurotransmitter of the central nervous system, and is derived from the essential aromatic amino acid tryptophan which is carried by the albumin protein in the blood stream. The free tryptophan is picked by the encephal, after having crossed the hematoencephalic barrier and transformed in 5-hydroxytryptophan, being subsequently decarboxylated into active neurotransmitter, serotonin13. Due to its activity in many receptors, serotonin has been implied in the mood regulation, including depression, anxiety, food intake and compulsive violence4. While low production of serotonin causes disturbance in the mental health of an individual, its high production is related to central fatigue, as demonstrated by many studies with aerobic exercise in animal15,16 and human models 17-19.
Physical exercise is able to promote increase in the production of serotonin due to higher availability of its precursor amino acid. In aerobic conditions of low intensity and long duration, the adipose tissue is recruited by the hormone system, releasing fatty acids for energy production. These lipids will be carried away by albumin in the blood stream until they reach the active musculature 13. The need to make free fatty acids available for oxidation ends up releasing tryptophan, since both use the same carrier protein. Therefore, the free tryptophan concentration in the hematoencephalic barrier becomes increased20,21.
Thus, the aim of this study was to evaluate the effect of one session of aerobic exercise in the tryptophan concentration in the brain of rats with hyperphenylalaninemia.
METHODS
Animals
All the animal experiments were performed according to the Brazilian resolutions under the Bioethics in Animal Experimentation (Law # 6.638, from May 8, 1979 and legal opinion # 24.645, from July 10, 1934). In this study, 32 male Wistar rats, approximate age of 30 days were used. The animals were kept in polyethylene collective cages and kept in rooms with controlled temperature and exposed to light/dark cycles of 12:12 hours, with free water access and food ad libitum.
Experimental groups
At 28 days of life, the animals were randomly sorted in four groups: 1) sedentary control (SedSAL) healthy rats; 2) sedentary PKU (SedPKU) rats with hyperphenylalaninemia; 3) exercise control (ExeSAL) healthy rats which exercised ; and 4) exercise PKU (ExePKU) rats with hyperphenylalaninemia which exercised.
PKU model
The PKU groups received an injection with α-metyl-phenylalanine (phenylalanine hydroxylase inhibitor) during three days at 9h and with phenylalanine in the 1.6µmol.g-1 and 2.1µmol.g-1 concentrations, respectively and at 18h they received the same dose of phenylalanine22. The SAL groups received saline solution at the same conditions.
Exercise and tryptophan measurement
On the fourth day, the Sed groups remained in their cages, while the Exe groups performed run on treadmill specific for rodents (Insight EP 131) during 60 minutes and velocity of 12m.min-1 23. Immediately after, the animals were sacrificed by decapitation without anesthesia and the brains were quickly removed, cleaned (cerebellum, olfactory bulb, superficial bridge and blood vessels removal) and kept in ice. The brain mass was measured and they were cleaned with buffering solution of sodium phosphate (Na3PO4 20mM) with potassium chloride (KCl 140mM), in 1:5 dilution. The product was placed in eppendorfs for centrifuging during 10min at velocity of 1,000g and temperature of 4ºC (Eppendorf Centrifuge 5417R). The supernatant was used for measurement of the tryptophan by spectrophotofluorimetry (Molecular Devices Espectra Max Gemini XPS), with excitation of 280nm and emission of 360nm24. The total proteins of the samples were also measured by Lowry et al.25 technique, using bovine albumin as standard. The results were normalized (µM.mgprotein-1) and calculated in % of the control (SedSAL group) and expressed as mean and standard deviation. Data normality was verified through the Shapiro-Wilk test, and the differences between groups were examined by two-way ANOVA, followed by Bonferroni test, using the statistical package SPSS, version 12.0 for Windows. The significance level adopted was of 0.05.
RESULTS
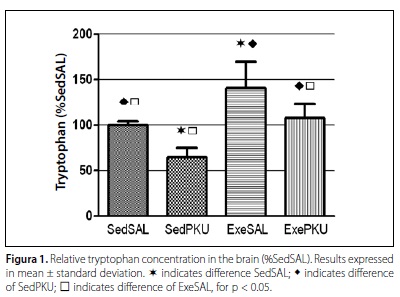
In the analysis of the tryptophan in the brain, significant differences were found between the treatments saline and hyperphenylalaninemia [F(1.28) = 83.53 p < 0.001], and also between the sedentary and exercise conditions [F(1.28) = 102.06 p < 0.001], figure 1. No interaction effect between the studies factors was observed [F(1.28) = 3.58 p = 0.069].
In the sedentary groups, the hyperphenylalaninemia model used (SedPKU group) significantly decreased the tryptophan concentration in the brain compared with the control group (SedSAL) (p < 0.001). The same has occurred with the groups which performed exercise, in which the tryptophan concentration was lower in the ExePKU group when compared with the ExeSAL group (p < 0.001).
Concerning the exercise effect, the tryptophan in the brain of exercised animals was significantly higher when compared with their controls, with higher values for the ExeSAL group compared with the SedSAL (p < 0.001), and higher for the ExePKU group compared with SedPKU (p < 0.001). Significant differences were not found between the trained hyperphenylalaninemic animals (ExePKU) and the controls which did not perform physical exercise (SedSAL) (p = 0.903).
DISCUSSION
The hyperphenylalaninemic model used in this study22 was effective in significantly decreasing the tryptophan concentration in the brain derived from increase in phenylalanine. The majority of the phenylalanine concentration in the plasma, and consequently in the hematoencephalic barrier, harms the passage to the brain of other amino acids which use the same transportation system, decreasing the tryptophan influx in an important way3,6,7,26,27. Phenylalanine is able to saturate the membrane proteins, which guarantee the permeability of the hematoencephalic barrier, making the entrance of the tryptophan and of other amino acids in the brain even more difficult9, probably due to its higher affinity with this transportation system8.
Tryptophan decrease means lower contribution of substrate for production of serotonin by the hypothalamic system, making the individual more sensitive to mood disorders14. Ardis et al.28 found negative alterations in the behavior and cognition of rats, derived from the diet poor in tryptophan to which they were submitted.to, lower tryptophan concentration in the plasma and lower serotonin concentration in the brain, evidencing the importance of the serotoninergic system in cognitive variables. Negative influence in the synthesis of amines in the brain was observed in animal model of chronic hyperphenylalaninemia, which caused cognitive deficit in adult rats29.
The proposed physical exercise23 was significant in the increase of the tryptophan concentration in the brain, both in control and hyperphenylalaninemic animals. Our results agree with many studies with healthy animals15,16,23,30,which found increase of free tryptophan concentration in the plasma and in the brain and consequently, higher serotonin production. Increase in serotonin production is caused by the increase of the activity of the sympatoadrenergic system, characteristic of physical stress. Lypolisis stimulation causes higher availability of fatty acids released in the blood stream and increases the albumin need to lead them to the musculature under exercise, causing the tryptophan ligated to albumin to be released. The Free tryptophan in increased concentration reaches the hematoencephalic barrier in advantage compared to other amino acids, resulting in its higher influx in the brain, and consequently, in higher serotonin production.
The most interesting finding of this study was that the triptophan value in the brain of the ExePKU group did not present statistical difference from the SedSAL group. Therefore, aerobic exercise was able to revert decrease of tryptophan found in the hyperphenylalaninemic rats which did not exercise, reaching values similar to the control animals. It is expected that serotonin production increases with the higher contribution of substrate, since the tryptophan supplementation presents this effect in hyperphenylalaninemic patients3,4.
The results obtained in this investigation showed that aerobic physical exercise may be an interesting strategy for restoration of the tryptophan concentration in the brain in hyperphenylalaninemic status. No studies which could relate aerobic exercise with hyperphenylalaninemic individuals were found in the scientific literature. Thus, further investigation should be carried out to support the use of aerobic physical exercise under hyperphenylalaninemia, a characteristic of individuals with PKU, as strategy of increase in the tryptophan levels in the brain. It is extremely important to know the acute responses to physical exercise as the ones presented in this investigation for the conduction of future research which assesses the effects of physical training in hyperphenylalaninemia.
REFERENCES
- 1. Walter JH, White FJ, Hall SK, MacDonald A, Rylance G, Boneh A, et al. How practical are recommendations for dietary control in phenylketonuria? Lancet 2002;360:55-7.
- 2. Kohli S, Saxena R, Thomas E, Rao P, Verma IC. Indian J Med Res 2005;122:400-3.
- 3. Surtees R, Blau N. The Neurochemistry of Phenylketonuria. Eur J Pediatr 2000;2:S109-13.
- 4. Güttler F, Lou H. Dietary problems of phenylketonuria: effect on CNS transmitters and their possible role in behaviour and neuropsychological function. J Inherit Metab Dis 1986;9:169-77.
- 5. Nyhan WL. Abnormalities in amino acid metabolism in clinical medicine. Appleton-century crofts. Norwalk, USA, 1984.
- 6. Milovanović DD, Milovanović L, Vranjesević D. Serum tryptophan to large neutral amino acid ratio and urinary tryptophan in three patients with phenylketonuria in a family. A clinical and biochemical study. Adv Exp Med Biol 1999;467:289-95.
- 7. Herrero E, Aragon MC, Gimenez C, Valdivieso F. Inhibition by L-phenylalanine of tryptophan transport by synaptosomal plasma membrane vesicles: implications in the pathogenesis of phenylketonuria. J Inherit Metab Dis 1983;6:32-5.
- 8. Malloy-Diniz LF, Martins CC, Carneiro KC, Cerqueira MMM, Ferreira APA, Aguiar MJB, et al. Funções Executivas em Crianças Fenilcetonúricas: Variações em Relação ao Nível de Fenilalanina. Arquivos de Neuro-psiquiatria 2004;62:473-9.
- 9. Schulpis KH, Papassotiriou I, Vounatsou M, Karikas GA, Tsakiris S, Chrousos GP. Morning preprandial plasma ghrelin and catecholamine concentrations in patients with phenylketonuria and normal controls: evidence for catecholamine-mediated ghrelin regulation. J Clin Endocrinol Metab 2004;89:3983-7.
- 10. Krause W, Halminski M, McDonald L, Dembure P, Salvo R, Freides D, Elsas L. Biochemical and neuropsychological effects of elevated plasma phenylalanine in patients with early-treated PKU. J Clin Invest 1985;75:40-8.
- 11. Park SB, Coull JT, McShane RH, Young AH, Sahakian BJ, Robbins TW, et al. Tryptophan depletion in normal volunteers produces selective impairments in learning and memory. Neuropharmacology 1994;33:575-88.
- 12. McKean CM. The effects of high phenylalanine concentrations on serotonin and catecholamine metabolism in the human brain. Brain Res 1972;47:469-76.
- 13. McArdle WD, Katch FI, Katch VL. Fisiologia do exercício: energia, nutrição e desempenho humano. 5a ed. Guanabara Koogan. Rio de Janeiro, Brasil, 2003.
- 14. Kandel ER, Schwartz JH, Jessell TM. Princípios da Neurociência. 4a ed. Manole. São Paulo, Brasil, 2003.
- 15. Soares DD, Lima NR, Coimbra CC, Marubayashi U. Evidence that tryptophan reduces mechanical efficiency and running performance in rats. Pharmacol Biochem Behav 2003;74:357-62.
- 16. Blomstrand E, Perrett D, Parry-Billings M, Newsholme EA. Effect of sustained exercise on plasma amino acid concentrations and on 5-hydroxytryptamine metabolism in six different brain regions in the rat. Acta Physiol Scand 1989;136:473-81.
- 17. Rossi L, Tirapegui J. Implicações do Sistema Serotoninérgico no Exercício Físico. Arquivos Brasileiros de Endocrinologia & Metabologia 2004;48:227-33.
- 18. Nybo L, Nielsen B, Blomstrand E, Moller K, Secher N. Neurohumoral Responses During Prolonged Exercise in Humans. J Appl Physiol 2003;95:1125-31.
- 19. Davis JM, Alderson NL, Welsh RS. Serotonin and central nervous system fatigue: nutritional considerations. Am J Clin Nutr 2000;72:573S-8S.
- 20. Chaouloff F. Effects of acute physical exercise on central serotonergic systems. Med Sci Sports Exerc 1997;29:58-62.
- 21. McMenamy RH. Binding of indole analogues to human serum albumin. Effects of fatty acids. J Biol Chem 1965;240:4235-43.
- 22. Kienzle Hagen ME, Pederzolli CD, Sgaravatti AM, Bridi R, Wajner M, Wannmacher CM, et al. Experimental hyperphenylalaninemia provokes oxidative stress in rat brain. Biochim Biophys Acta 2002;1586:344-52.
- 23. Meeusen R, Thorré K, Chaouloff F, Sarre S, De Meirleir K, Ebinger G, et al. Effects of tryptophan and/or acute running on extracellular 5-HT and 5-HIAA levels in the hippocampus of food-deprived rats. Brain Res 1996;740:245-52.
- 24. Hsia DY, Inouye T. Inborn Errors of Metabolism. Part 2 Laboratory Methods: Year Book Medical Publishers Inc. Chicago, EUA 1966.
- 25. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with Folin-phenol reagent. J Biol Chem 1951;193:265-75.
- 26. Dunlop DS, Yang XR, Lajtha A. The effect of elevated plasma phenylalanine levels on the protein synthesis rates in adult rat brain. Biochem J 1994;302:601-10.
- 27. Feoli AM, Siqueira IR, Almeida L, Tramontina AC, Vanzella C, Sbaraini S, et al. Effects of protein malnutrition on oxidative status in rat brain. Nutrition 2006;22:160-5.
- 28. Ardis TC, Cahir M, Elliott JJ, Bell R, Reynolds GP, Cooper SJ. Effect of acute tryptophan depletion on noradrenaline and dopamine in the rat brain. J Psychopharmacol 2009;23:51-5.
- 29. Diamond A. Phenylalanine levels of 6-10 mg/dL may not be as benign as once thought. Acta Paediatr Suppl 1994;407:89-91.
- 30. Caperuto EC, dos Santos RV, Mello MT, Costa Rosa LF. Effect of endurance training on hypothalamic serotonin concentration and performance. Clin Exp Pharmacol Physiol 2009;36:189-91.
Publication Dates
-
Publication in this collection
30 Nov 2012 -
Date of issue
Oct 2012