Abstracts
The species Alpinia purpurata is scarcely cited as to ethnopharmacology and phytochemistry. This study aimed to analyze bioactive compounds through high-performance liquid chromatography (HPLC). Hydroalcoholic crude extract was obtained from A. purpurata dried leaves. Folin-Ciocalteau method was used to quantify total phenols, using gallic acid as standard. The obtained result was 15.6 mg GAE g-1. The crude extract was partitioned with the solvents ethyl acetate and butanol, followed by thin-layer chromatography (TLC) and HPLC. The flavonoids kaempferol-3-O-glucuronide and rutin were detected at a higher concentration in ethyl acetate and butanolic extracts. The butanolic extract contains the highest flavonoid percentage (94.3%). A. purpurata presents important flavonoids of therapeutic use, already verified for A. zerumbet. This is the first study verifying the presence of flavonoids in A. purpurata extracts.
TLC; polyphenols; medicinal plants; rutin; Zingiberaceae
A espécie Alpinia purpurata apresenta poucas citações referentes a etnofarmacologia e fitoquímica. Este estudo propõe a análise de substâncias bioativas através da técnica de cromatografia líquida de alta eficiência (CLAE). O extrato bruto hidroalcóolico foi obtido a partir de folhas secas de A. purpurata. A quantificação de fenóis totais foi realizada pelo método de Folin-Ciocalteau, usando ácido gálico como padrão. Como resultado, foi verificado 15,6 mg EAG g-1. O extrato bruto foi particionado com os solventes acetato de etila e butanol e depois analisado por cromatografia em camada delgada e CLAE. Nos extratos acetato de etila e butanólico foi detectada a presença dos flavonóides kaempferol-3-O-glicuronídeo e rutina, em maior concentração. O extrato butanólico contém a maior porcentagem de flavonóides (94,3%). Esta espécie possui flavonóides importantes no uso terapêutico, já antes verificados para a espécie A. zerumbet. Este é o primeiro trabalho que verifica a presença de flavonóides em extratos de A. purpurata.
CCF; polifenóis; plantas medicinais; rutina; Zingiberaceae
Detection of flavonoids in Alpinia purpurata (Vieill.) K.Schum. leaves using high-performance liquid chromatography
Detecção de flavonóides em folhas de Alpinia purpurata (Vieill.) K. Schum. por cromatografia líquida de alta eficiência.
Victório, C.P.I*; Kuster, R.M.II; Lage, C.L.S.I
ILaboratório de Fisiologia Vegetal, Instituto de Biofísica Carlos Chagas Filho, Universidade Federal do Rio de Janeiro, Av. Carlos Chagas Filho, s/n, CCS, Bloco G, sala G2-050. Rio de Janeiro - RJ, 21941-902. Brazil
IILaboratório de Fitoquímica, Núcleo de Pesquisas de Produtos Naturais (NPPN), Universidade Federal do Rio de Janeiro, Av. Carlos Chagas Filho, s/n, CCS, Bloco H, Rio de Janeiro - RJ, 21941-902. Brazil
*E-mail: crispv@biof.ufrj.br
ABSTRACT
The species Alpinia purpurata is scarcely cited as to ethnopharmacology and phytochemistry. This study aimed to analyze bioactive compounds through high-performance liquid chromatography (HPLC). Hydroalcoholic crude extract was obtained from A. purpurata dried leaves. Folin-Ciocalteau method was used to quantify total phenols, using gallic acid as standard. The obtained result was 15.6 mg GAE g-1. The crude extract was partitioned with the solvents ethyl acetate and butanol, followed by thin-layer chromatography (TLC) and HPLC. The flavonoids kaempferol-3-O-glucuronide and rutin were detected at a higher concentration in ethyl acetate and butanolic extracts. The butanolic extract contains the highest flavonoid percentage (94.3%). A. purpurata presents important flavonoids of therapeutic use, already verified for A. zerumbet. This is the first study verifying the presence of flavonoids in A. purpurata extracts.
Key words: TLC, polyphenols, medicinal plants, rutin, Zingiberaceae
RESUMO
A espécie Alpinia purpurata apresenta poucas citações referentes a etnofarmacologia e fitoquímica. Este estudo propõe a análise de substâncias bioativas através da técnica de cromatografia líquida de alta eficiência (CLAE). O extrato bruto hidroalcóolico foi obtido a partir de folhas secas de A. purpurata. A quantificação de fenóis totais foi realizada pelo método de Folin-Ciocalteau, usando ácido gálico como padrão. Como resultado, foi verificado 15,6 mg EAG g-1. O extrato bruto foi particionado com os solventes acetato de etila e butanol e depois analisado por cromatografia em camada delgada e CLAE. Nos extratos acetato de etila e butanólico foi detectada a presença dos flavonóides kaempferol-3-O-glicuronídeo e rutina, em maior concentração. O extrato butanólico contém a maior porcentagem de flavonóides (94,3%). Esta espécie possui flavonóides importantes no uso terapêutico, já antes verificados para a espécie A. zerumbet. Este é o primeiro trabalho que verifica a presença de flavonóides em extratos de A. purpurata.
Palavras-chave: CCF, polifenóis, plantas medicinais, rutina, Zingiberaceae
INTRODUCTION
The genus Alpinia (Zingiberaceae family, Alpinioideae subfamily, Alpinieae tribe) is native to tropical and subtropical Asia (Kress et al., 2002). Nowadays, it is cultivated in several places around the world due to the attractive beauty of its inflorescences and its therapeutic potential (Soares de Moura et al., 2005; Victório, 2008). In addition, these plants are important sources of raw material for many useful products: foods, spices, medicines, perfumes, dyes and fiber paper (Tomlinson, 1969).
A. purpurata (Vieill.) K. Schum (red inflorescence) is an herbaceous perennial plant, internationally known in the ornamental plant market as potted plant, landscape accent and cut flower (Morón, 1987; Kress et al., 2002). An ethnobotanical study developed in a community from Trujillo State, Venezuela, investigated the use of its flowers as decoction for cough (Bermúdez & Velásquez, 2002). Zoghbi et al. (1999) analyzed A. purpurata essential oil composition, which showed notable antibacterial activity. However, studies about A. purpurata phytotherapeutic potential are scarce; most scientific works with this species are directed to the improvement of its production as an ornamental plant, including the evaluation of postharvest treatment as an alternative to chemical insecticides, indications of harvest and postharvest procedures, tolerance to fumigation, biological control of pests, in vitro storage of multiple shoots and micropropagation (Morón, 1987; Dekkers et al., 1991; Illg & Faria, 1995; Hara et al., 1997; Chen & Paull, 1998; Anderson & Gardner, 1999; Gonzalez & Mogollon, 2001; Sangwanangkul et al., 2008).
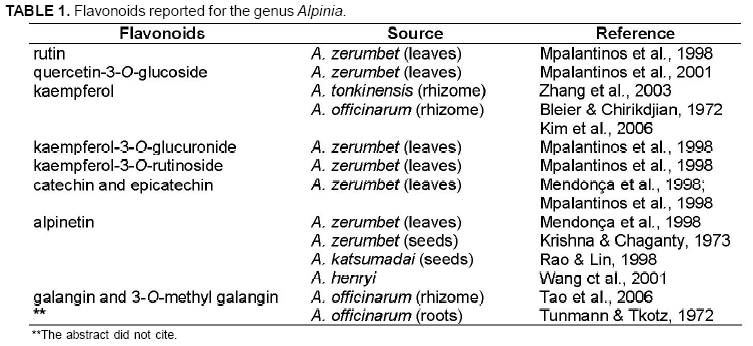
The presence of A. purpurata in several parts of Brazil allows easy access to it. Thus, this species is an available resource to phytotherapeutic treatment. Several species of the Zingiberaceae family present antioxidant property mainly due to the considerable presence of flavonoids such as rutin, quercetin, alpinetin and different types of kaempferol in the genus Alpinia (Table 1) (Williams & Harborne, 1977; Mpalantinos et al., 1998; Vankar et al., 2006). Pugialli et al. (1993) studied Zingiberaceae chemotaxonomy and considered that flavonoids and their structural variety are taxonomic markers. Zingiberaceae family is at a higher level within the superorder Zingiberiflorae due to the use of protection mechanisms of phenolic hydroxyl groups: glycosylation and methylation. Investigations of phytochemical compounds have been important tools to study plant classification and evolution (Kaplan & Gottlieb, 1982). This study proposes a phytochemical approach to A. purpurata based on the chemotaxonomy of the genus Alpinia, which presents high therapeutic potential (Bleier & Chirikdjian, 1972; Mendonça et al., 1998; Mpalantinos, 2001; Kim et al., 2006).
The aim of the present study was to evaluate the phytochemistry of hydroalcoholic extracts from A. purpurata leaves for the presence of the flavonoids rutin, kaempferol-3-O-rutinoside and kaempferol-3-O-glucuronide, reported in the scientific literature for their therapeutic action.
MATERIAL AND METHOD
Plant material
A. purpurata leaf samples were collected from plants growing in the city of Rio de Janeiro in the Federal University of Rio de Janeiro (Rio de Janeiro State, Brazil). The voucher specimen was identified and deposited at the Herbarium of Rio de Janeiro Botanical Garden under the accession number RB 433484.
Preparation of extracts and Fractions
A. purpurata leaves were collected from adult plants in the morning; then, the plant material was dried and ground in 70% ethanol for a week. After the first extraction, leaves were kept in ethanol (100%) until exhaustive extraction. Crude extracts were filtered and dried through evaporation at 60ºC in a rotary evaporator and through freeze-drying. From 1009 g dried leaves, 112.5 g dried crude extract was obtained. The yield was calculated as percentage, according to the formula: (crude extract weight/plant material weight) x 100. A 59.4g crude extract fraction was resuspended in methanol:water (9:1, v/v) and partitioned in different solvents of increasing polarity range: hexane, dichloromethane, ethyl acetate and n-butanol. Hexane partition was separated and evaporated to dryness (4.1 g). The residue was dried using a rotary evaporator until methanol elimination; then, the aqueous layer was further partitioned using solvents and evaporated to dryness in order to obtain dichloromethane(0.29 g), ethyl acetate (0.31 g) and n-butanol (4.8 g) partitions. Each solvent extractor (50 to 60 mL) was used five times.
Flavonoid standards
Flavonoids of the kaempferol class were isolated from Alpinia zerumbet Roxb. and identified using Nuclear Magnetic Resonance (NMR) (Mpalantinos et al., 1998). Rutin was purchased from Merck. Kaempferol-3-O-glucuronide had 82% purity, kaempferol-3-O-rutinoside, 91%, and rutin, 98%. Purity was verified using three replicate injections of standards into HPLC.
Evaluation of phenolic compound content
Total phenolic compounds were determined using the Folin-Ciocalteau method. Hydroalcoholic extracts were dissolved in ethanol (70%) at 1mg mL-1. A 0.5 mL aliquot of diluted extract and 2 mL Folin-Ciocalteau reagent (10%) were added after 3 min, together with 2 mL of 7.5% sodium carbonate, and mixed. The mixture was homogenized and incubated at 50oC for 30 min. Absorbance was measured at 740 nm in a spectrophotometer using gallic acid as standard. Two controls were used: (1) Folin-Ciocalteau + sodium carbonate and (2) crude extract solution. Phenolic compounds in crude extracts were quantified through the regression equation of calibration curves: y = 0.0229x + 0.0968 (R2 = 0.9993), and expressed as mg gallic acid equivalents (GAE) per 1 g dried leaves. All measurements were done in triplicate. The analyses included A. purpurata and A. zerumbet samples collected in June (2006) and February (2007).
Thin-layer chromatography (TLC)
Aliquots of standards, and crude, ethyl acetate and butanolic extracts were spotted on TLC plate (silica gel 60 F254 nm, Merck) and developed in ethyl acetate, formic acid and distilled water (65/20/15, v/v/v) mobile phase. TLC was observed under UV spectrum at 254 and 360 nm before and after spraying with NP/PEG reagent. The flavonoid standards of rutin (Rf = 0.69), kaempferol-3-O-rutinoside (Rf = 0.76) and kaempferol-3-O-glucuronide (Rf = 0.83) were verified in the extracts after concomitant running with standards (Victório et al., 2007).
High-performance liquid chromatography (HPLC)
Crude, ethyl acetate and butanolic extracts were dissolved in methanol (70%) at 20 mg mL-1 and filtered under vacuum; HPLC-UV analyses were performed in a Shimadzu apparatus equipped with SPD-M10A diode array detector, LC-10AD pump and CBM-10 interface. Data were obtained and processed in a reversed phase column (Lichrosorb RP-18, 25 cm x 5 mm), at room temperature. Separation was done in the following mobile phase: MilliQ water + 0.1% phosphoric acid (A) and methanol (B): 1-10 min (30% B); 20 min (40% B); 60 min (100% B). The prepared mobile phase was degassed using ultrasonic agitation. After 61 min, the gradient was recycled to the initial conditions and held for 10 min before a new injection. The flow rate was kept constant at 1 mL min-1 and peaks were detected at 254 nm and 360 nm. All chemicals used in the analysis, such as methanol and phosphoric acid, were of HPLC grades and were purchased from Merck. MilliQ water was used in HPLC mobile phase and sample preparation. Standards were dissolved in 70% methanol at 1 mg.mL-1 and analyzed in the same elution. Injections were done in triplicate. Linearity was observed in the concentration range 0.0078 - 0.0625 mg mL-1 rutin and 0.01325 - 0.25 mg mL-1 kaempferol-3-O-glucuronide. Flavonoids in the extracts were quantified against calibration curves of standards, where y is the peak area and x the concentration in mg mL-1 (Figure 1).
Flavonoid detection
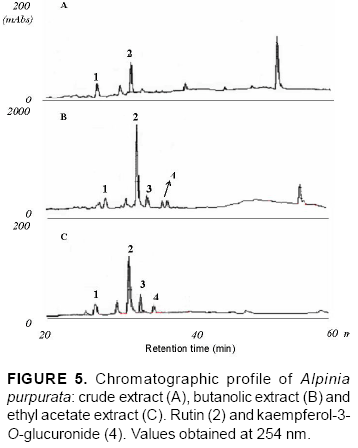
Flavonoids were detected through retention times (RT), ultraviolet spectrum compared with flavonoid standard spectrum (Figure 2) and coinjection with authentic samples analyzed under the same conditions. For coinjection, a mixture (1:1, v/v) of extracts at 20 mg mL-1 and standard at 1 mg mL-1 was prepared. The purity of each flavonoid peak in Figures 4 and 5 was assessed by comparing the UV spectra at upslope and downslope inflexion points for both wavelengths (254 and 360 nm).
RESULT AND DISCUSSION
Phenolic compounds present antioxidant activity; thus, they are considered important therapeutic agents. As already known, antioxidants reduce the effects of excessive free radical production in critically ill patients affected by diseases like cancer, cardiovascular disturbances, and brain dysfunction (Atoui et al., 2005). Folin-Ciocalteau method can quantify the presence of flavonoids and other phenolic compounds in plant material. There were significant differences in total phenolic content (p<0.03) between A. purpurata and A. zerumbet in both evaluated months (Figure 3). A. zerumbet was used for comparison since it is greatly employed in folk medicine and has been extensively reported concerning phytochemistry and phytotherapy.
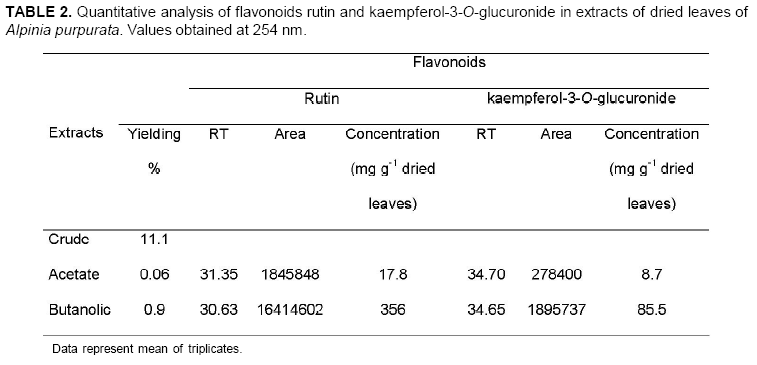
The chromatographic profile of A. purpurata shows rutin and kaempferol-3-O-glucuronide peaks, similarly to those for A. zerumbet (Figure 4) in opposite proportions. These flavonoids were verified in ethyl acetate and butanolic extracts between 20 and 40 min (Figure 5). The main difference was detected between 40 and 60 min, when A. zerumbet crude extract showed more compounds in the chromatographic profile than A. purpurata (Figure 4). The flavonoid kaempferol-3-O-rutinoside was not detected through TLC or HPLC in the extracts. HPLC and TLC methods were reproducible. UV spectrum of peaks 1 and 3 of the chromatogram (Figure 5) are characteristic of flavonoids; however, for an accurate identification, they should be isolated in SEPHADEX column using polar solvents from ethyl acetate and butanolic fractions, followed by structural elucidation using the spectroscopy technique. Rutin and kaempferol-3-O-glucuronide were isolated from A. zerumbet and also detected in A. purpurata at the following concentrations, respectively: 17.8 and 8.7 mg g-1 dried leaves (ethyl acetate) and 356 and 85.5 mg g-1 dried leaves (butanol) (Table 2). Rutin had the highest concentration in both extracts. This flavonoid is widely distributed in the Plant Kingdom and, despite its hydrophilic character, it presents several therapeutic applications such as antioxidant activity, besides reducing arteriosclerosis risks and increasing vein tone, which improves the blood flow (Wojcicki, 1995; Kreft et al., 2006). Recent studies have reported the application of flavonoids of the kaempferol class to treat Alzheimer's disease (De Melo & Costa, 2005). Mpalantinos et al. (1998) suggested that rutin, kaempferol-3-O-glucuronide and kaempferol-3-O-rutinoside are responsible for the effects of lower blood pressure. In an analysis of A. purpurata crude extract other compounds were obtained between 40 and 60 min RT but were not identified (Figure 5). No previous research has been conducted into A. purpurata phytochemistry. This study is the first to report such flavonoids in this species, also confirming the significant presence of flavonoids in different Alpinia species. In addition, these results indicate the close phytochemical relationship between A. zerumbet and A. purpurata, which represent important phytoterapeutic resources.
ACKNOWLEDGMENT
The authors are grateful to Coordination for the Improvement of Higher Education Personnel (CAPES) for the fellowship to the first author and PROAP/PROEX for financial support.
REFERENCE
Recebido para publicação em 29/02/2008
Aceito para publicação em 06/10/2008
- Anderson, R.C.; Gardner, D.E. An Evaluation of the wilt-causing bacterium Ralstonia solanacearum as a potential biological control agent for the alien kahili ginger (Hedychium gardnerianum) in Hawaiian Forests. Biological Control, v.15, n.2, p.89-6, 1999.
- Atoui, A.K. et al. Tea and herbal infusions: their antioxidant activity and phenolic profile. Food Chemistry, v.89, n.1, p.27-6, 2005.
- Bermúdez, A.; Velázquez, D. Etnobotánica médica de una comunidad campesina del estado Trujillo, Venezuela: un estudio preliminar usando técnicas cuantitativas. Revista Facultad Farmacia, v.44, p.2-6, 2002.
- Bleier, W.; Chirikdjian, J.J. Flavonoids from galanga rhizome (Alpinia officinarum Hance). Planta Medica, v.22, n.2, p.145-51, 1972.
- Chen, C.C.; Paull, R.E. Tolerance of tropical fruits and a flower to carbonyl sulfide fumigation. Postharvest Biology and Technology, v.14, n.2, p.245-50, 1998.
- De Melo, G.O.; Costa, S.S. Herbal Natural Products for the treatment of Alzheimer`s disease: promise and challenge. Revista Fitos, v.1, n.2, p.41-7, 2005.
- Dekkers, A.J.; Rao, A.N.; Goh, C.J. In vitro storage of multiple shoot cultures of gingers at ambient temperatures of 24-29ºC. Science Horticulturae, v.47, n.1/2, p.157-67, 1991.
- González, M.T.; Mogollón, N.J. Fertilización nitrogenada sobre el crecimiento y desarrollo de la inflorescencia en plantas de Alpinia purpurata (Vieill.) K. Schum. "Jungle King" provenientes de cultivo in vitro y de sección de rizoma. Revista de la Facultad de Agronomía, v.18, p.124-34, 2001.
- Hara, A.H. et al. Hot-air induced thermotolerance of red ginger flowers and mealybugs to postharvest hot-water immersion. Postharvest Biology and Technology, v.12, n.1, p.101-8, 1997.
- Illg, R.D.; Faria, R.T. Micropropagation of Alpinia purpurata from inflorescence buds. Plant Cell, Tissue and Organ Culture, v.40, p.183-5, 1995.
- KAPLAN, M.A.C.; GOTTLIED, O.R. Plant systematics and phylogeny. XVI. Iridoids as systematics markers in dicotyledons. Biochemical Systematics and Ecology, v.10, p.329-47, 1982.
- Kim, H. J. et al. Vasorelaxation effect of the flavonoids from the rhizome extracts of Alpinia officinarum on isolated rat thoracic aorta. Saengyak Hakhoechi, v.37, n.1, p.56-9, 2006.
- Kreft, I.; Fabjan, N.; Yasumoto, K. Rutin content in buckwheat (Fagopyrum esculentum Moench.) food materials and products. Food Chemistry, v.98, n.3, p.508-12, 2006.
- Kress, W.J.; Prince, L.M.; Williams, K.J. The phylogeny and a new classification of the gingers (Zingiberaceae): evidence from molecular data. American Journal of Botany, v.89, n.10, p.1682-96, 2002.
- KRISHNA, B.M.; CHAGANTY, R.B. Cardamonin and alpinetin from the seeds of Alpinia speciosa. Phytochemistry, v.12, n.1, p.238-42, 1973.
- Mendonça, L.A. et al. Flavonoids with antihypertensive activity from Alpinia zerumbet (Pers.) Burt et Smith (colônia). Revista Brasileira de Farmácia, v.79, n.3/4, p.96-8, 1998.
- Morón, A. In vitro clonal propagation of Alpinia purpurata K. Schum (red ginger) 1987. 97p. Dissertação (Mestrado) - Centro Agronomico Tropical de Investigación y Ensenanza, Turrialba, Costa Rica.
- Mpalantinos, M.A. et al. Biologically active flavonoids and kava pyrones from the aqueous extract of Alpinia zerumbet Phytotherapy, v.12, p. 442-4, 1998.
- Mpalantinos, M.A. Identificação de substâncias anti-hipertensivas de Alpinia zerumbet Pers. 2001. 197p. Tese (Doutorado) - Núcleo de Pesquisas de Produtos Naturais, Universidade Federal do Rio de Janeiro, Rio de Janeiro.
- PUGIALLI, H.R.L.; KAPLAN, M.A.C.; GOTTLIEB, O.R. Chemotaxonomy of superorder Zingiberiflorae (sensu Dahlgren) I. Flavonoids. Acta Botanica Brasilica, v.7, n.2, p.135-48, 1993.
- Rao, W.; Lin, Y. Quantitative analysis of alpinetin and cardamomin in Alpinia katsumadai by HPLC. Chinese Pharmaceutical Journal, v.33, n.12, p.743-5, 1998.
- Sangwanangkul, P.; SARADHULDHAT, P.; PAULL, R.E. Survey of tropical cut flower and foliage responses to irradiation. Postharvest Biology and Technology, v.48, n.2, p.264-71, 2008.
- SOARES DE Moura, R. et al. Antihypertensive and endothelium-dependent vasoditlator effects of Alpinia zerumbet, a medicinal plant. Journal of Cardiovascular Pharmacology, v.46, n.3, p.288-94, 2005.
- Tao, L. et al. HPLC analysis of bioactive flavonoids from the rhizome of Alpinia officinarum South African Journal of Botany, v.72, n.1, p.163-6, 2006.
- Tomlinson, P.B. Commelinales - Zingiberales. In: Metcalfe, C.R (Org.). Anatomy of the monocotyledons Oxford: Clarendon Press, 1969. v.3, p.341-59.
- TUNMANN, P.; TKOTZ, H. Flavonols and sterol glycosides in root of Alpinia officinarum Hance. Zeitschrift fur Naturforschung part B-chemie Biochemie Biophysik Biologie und Verwandten Gebiete, v.27, n.3, p.323-4, 1972.
- VANKAR, P.S. et al. Antioxidant properties of some exclusive species of Zingiberaceae family of Manipur. Agronomy and Food Chemistry, v.5, n.2, p.1318-22, 2006.
- Victório, C.P.; Kuster, R.M.; Lage, C.L.S. Detecção de flavonóides de Alpinia purpurata por CCD e CLAE. Jornal Brasileiro de Fitomedicina, v.5, p.137, 2007.
- VICTÓRIO, C.P. Cultura de tecidos e metabólitos especiais em colônia (Alpinia zerumbet Pers. Burtt et Smith) e A. purpurata (Vieill) K. Schum e estudos preliminares de atividade biológica. 2008. 186p. Tese (Doutorado) - Instituto de Biofísica Carlos Chagas Filho, Universidade Federal do Rio de Janeiro, Rio de Janeiro.
- WANG, Z.T. et al. Vasorelaxant Effects of cardamonin and clpinetin from Alpinia henryi K. Schum. Journal of Cardiovascular Pharmacology, v.37, n.5, p.596-606, 2001.
- WILLIAMS, C.A.; HARBORNE, J.B. The leaf flavonoids of the Zingiberales. Biochemical Systematics and Ecology, v.5, n.3, p.221-9, 1977.
- Wojcicki, J. et al. Extractum Fagopyri reduces artheriosclerosis in high-fat diet fed rabbits. Die Pharmazie, v.50, p.560-2, 1995.
- Zoghbi, M.G.B.; Andrade, E.H.A.; Maia, J.G.S. Volatile constituents from leaves and flowers of Alpinia speciosa K. Schum. and A. purpurata (Viell.) Schum. Flavour and Fragrance Journal, v.14, p.411-4, 1999.
- Zhang, J.; Guo, Q.H.; Kong, L.Y. Flavonoids from rhizome of Alpinia tonkinensis Zhongguo Zhong Yao Za Zhi, v.28, n.1, p.41-3, 2003.
Publication Dates
-
Publication in this collection
29 Feb 2012 -
Date of issue
2009
History
-
Received
29 Feb 2008 -
Accepted
06 Oct 2008