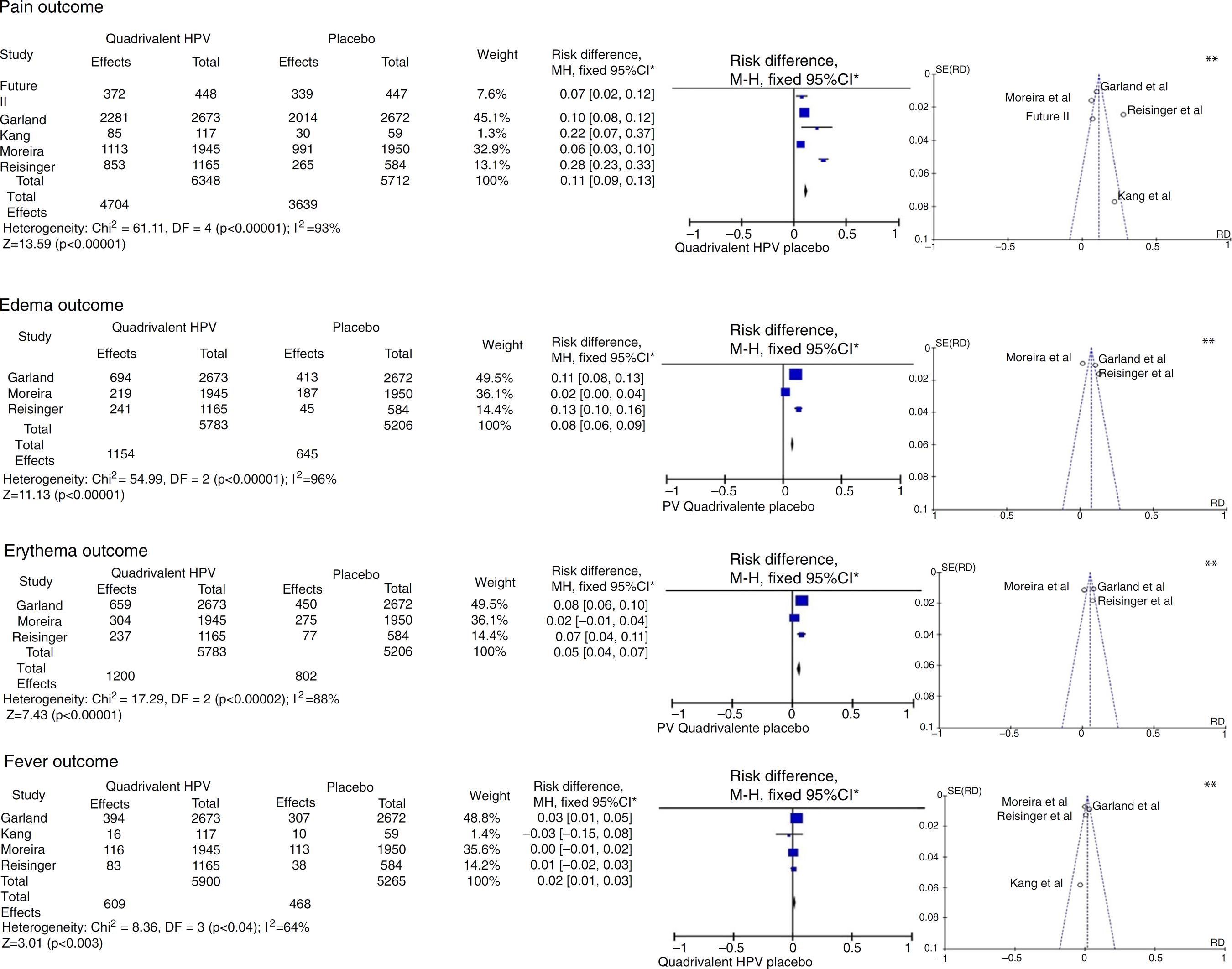
| Moreira et al. [3434 Moreira ED Jr, Palefsky JM, Giuliano AR, et al. Safety and reactogenicity of a quadrivalent human papillomavirus (types 6, 11, 16, 18) L1 viral-like-particle vaccine in older adolescents and young adults. Hum Vaccines. 2011;7:768-75.] (2011/5) |
RCT, DB, PC |
HPV QV (n=1945) vs. Placebo (n=1950) |
M (16–26 yrs) |
Local pain |
| Kim et al. [2323 Kim YJ, Kim KT, Kim JH, et al. Vaccination with a human papillomavirus (HPV)-16/18 AS04-adjuvanted cervical cancer vaccine in Korean girls aged 10–14 years. J Korean Med Sci. 2010;25:1197-204.] (2010/5) |
RCT |
HPV DV (n=474) vs. hepatitis A vaccine (n=483) |
F (10–14 yrs) |
Local pain, erythema, edema and myalgia |
| Sow et al. [3535 Sow PS, Watson-Jones D, Kiviat N, et al. Safety and immunogenicity of human papillomavirus-16/18 AS04-adjuvanted vaccine: a randomized trial in 10–25-year-old HIV seronegative African girls and young women. J Infect Dis. 2013;207:1753-63.] (2013/5) |
RCT IIIb, DB, PC, MULTI (aluminum hydroxide) |
HPV DV (n=1298) vs. Placebo (n=643) |
F (10–25 yrs) |
Local pain |
| Reisinger et al. [3636 Reisinger KS, Block SL, Lazcano-Ponce E, et al. Safety and persistent immunogenicity of a quadrivalent human papillomavirus types 6, 11, 16, 18 L1 virus-like particle vaccine in preadolescents and adolescents: a randomized controlled trial. Pediatr Infect Dis J. 2007;26:201-9.] (2007/5) |
RCT, DB, PC |
HPV QV (n=1165) vs. Placebo (n=584) |
M and F (1:1) (9–15 yrs) |
Local pain, erythema and edema |
| Szarewski et al. [3737 Szarewski A, Poppe WA, Skinner SR, et al. Efficacy of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine in women aged 15–25 years with and without serological evidence of previous exposure to HPV-16/18. Int J Cancer. 2012;131:106-16.] (2012/5) |
RCT, DB |
HPV DV (n=9319) vs. hepatitis A vaccine (n=9325) |
F exposed to HPV (15–25 yrs) |
Local pain, erythema and edema |
| Petäjä et al. [3838 Petäjä T, Keränen H, Karppa T, et al. Immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine in healthy boys aged 10–18 years. J Adolesc Health. 2009;44:33-40.] (2009/3) |
RCT, phase I/II, blind observer |
HPV DV (n=181) vs. hepatitis B vaccine (n=89) |
M (10–18 yrs) |
Local pain, edema and myalgia |
| FUTURE II [3333 The FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915-27.] (2007/5) |
RCT, DB, PC |
HPV QV (n=448) vs. Placebo (n=447) |
F (15–26 yrs) |
Local pain, seasonal allergies |
| Medina et al. [3939 Medina DM, Valencia A, de Velasquez A, et al. Safety and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine: a randomized, controlled trial in adolescent girls. J Adolesc Health. 2010;46:414-21.] (2010/3) |
RCT, MULTI |
HPV DV (n=1017) vs. hepatitis A vaccine (n=1010) |
F (10–14 yrs) |
Local pain, erythema, edema, headache, myalgia, fatigue, hives and syncope |
| Romanowski et al. [4040 Romanowski B, Schwarz TF, Ferguson LM, et al. Immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose schedule compared with the licensed 3-dose schedule: results from a randomized study. Hum Vaccines. 2011;7:1374-86.] (2011/3) |
RCT phase I/II, PB; 2 vs. 3 doses of divalent HPV |
2 Doses FOR20/20 M0/6 (n=240); 2 doses FOR40/40 M0/6 (n=241); 2 doses FOR40/40 M0/2 (n=240); 3 doses FOR20/20 0/1/6 (n=239) |
F (9–25 yrs) |
– |
| Garland et al. [3232 Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928-43.] (2007/5) |
RCT, DB, PC, MULTI |
HPV QV (n=2673) vs. Placebo (n=2672) |
F (16–24 yrs) |
Local pain, erythema, edema and local pruritus, fever >38.9 °C, bronchospasm |
| Petäjä et al. [4141 Petäjä T, Pedersen C, Poder A, et al. Long-term persistence of systemic and mucosal immune response to HPV-16/18 AS04-adjuvanted vaccine in preteen/adolescent girls and young women. Int J Cancer. 2011;129:2147-57.] (2011/3) |
RCT phase III, MULTI |
HPV DV (n=616) |
F (10–25 yrs) |
– |
| Esposito et al. [4242 Esposito S, Birlutiu V, Jarcuska P, et al. Immunogenicity and safety of human papillomavirus-16/18 AS04-adjuvanted vaccine administered according to an alternative dosing schedule compared with the standard dosing schedule in healthy women aged 15 to 25 years: results from a randomized study. Pediatr Infect Dis J. 2011;30:e49-55.] (2011/3) |
RCT, standard schedule (0/1/6 m) vs. alternative (0/1/12 m) |
HPV DV standard (n=1195) vs. HPV DV alternative (n=1188) |
F (15–25 yrs) |
– |
| Kang et al. [4343 Kang S, Kim KH, Kim YT, et al. Safety and immunogenicity of a vaccine targeting human papillomavirus types 6, 11, 16 and 18: a randomized, placebo-controlled trial in 176 Korean subjects. Int J Gynecol Cancer. 2007;18:1013-9.] (2008/5) |
RCT, DB, PC |
HPV QV (n=117) vs. Placebo (n=59) |
F (9–23 yrs) |
Local pain, erythema, edema and fever |
| Li et al. [4444 Li R, Li Y, Radley D, et al. Safety and immunogenicity of a vaccine targeting human papillomavirus types 6, 11, 16 and 18: a randomized, double-blind, placebo-controlled trial in Chinese males and females. Vaccine. 2012;30:4284-91.] (2011/3) |
RCT, DB, PC |
HPV QV (n=302) vs. Placebo (n=298) |
M (9–15 yrs) and F (9–45 yrs) |
Local pain, edema and pruritus |