Abstract
The biosorption process, characterized by the use of biomass for removing metals from aqueous solutions, is an attractive technology using inactive and dead biomasses to remove heavy metals from aqueous solutions in the absence of metabolic activity necessary for intracellular accumulation. The desorption process, which concentrates the metal previously absorbed for possible reuse, is also important. The desorption of copper (Cu) (II) associated with the biomass of Cladosporium cladosporioides (Fres) de Vries was evaluated following the biosorption process (adsorption). Specifically, four eluents were used (all at a concentration 0.1 mol L-1), sulfuric acid, hydrochloric acid, ethylenediaminetetraacetate disodium and calcium chloride, as well as the recovery of Cu (II) desorbed as copper sulfate. After 120 h, 97 % of Cu in solution had been adsorbed. C. cladosporioides can efficiently adsorb Cu (II). Further, 0.1 mol L-1 sulfuric acid was viable and the most efficient for the desorption of the absorbed metal, while ensuring viability of C. cladosporioides after desorption, which is important for the reuse of the biomass in cycles of sorption-desorption.
biosorption; metal; desorption
AGRICULTURAL MICROBIOLOGY
Recovery of copper (II) absorbed in biomass of Cladosporium cladosporioides
Juliana Ribeiro do Carmo* * Corresponding author < julianaribeirocarmo@hotmail.com> ; Carlos José Pimenta; Júlia Ferreira da Silva§ § Present address: UFBA/Instituto de Ciências Ambientais e Desenvolvimento Sustentável, R. Professor José Seabra, s/n 47805-100 Barreiras, BA Brasil ; Sara Maria Chaulfoun de Souza
UFLA/DCA Depto. de Ciência dos Alimentos, C.P. 3037 37200-000 Lavras, MG Brasil
ABSTRACT
The biosorption process, characterized by the use of biomass for removing metals from aqueous solutions, is an attractive technology using inactive and dead biomasses to remove heavy metals from aqueous solutions in the absence of metabolic activity necessary for intracellular accumulation. The desorption process, which concentrates the metal previously absorbed for possible reuse, is also important. The desorption of copper (Cu) (II) associated with the biomass of Cladosporium cladosporioides (Fres) de Vries was evaluated following the biosorption process (adsorption). Specifically, four eluents were used (all at a concentration 0.1 mol L1), sulfuric acid, hydrochloric acid, ethylenediaminetetraacetate disodium and calcium chloride, as well as the recovery of Cu (II) desorbed as copper sulfate. After 120 h, 97 % of Cu in solution had been adsorbed. C. cladosporioides can efficiently adsorb Cu (II). Further, 0.1 mol L1 sulfuric acid was viable and the most efficient for the desorption of the absorbed metal, while ensuring viability of C. cladosporioides after desorption, which is important for the reuse of the biomass in cycles of sorption-desorption.
Keywords: biosorption, metal, desorption
Introduction
Biosorption is a technology that is based on the removal of heavy metals by biomass present in aqueous solutions through passive means; that is, the mechanism of removal is not metabolically controlled (Davis et al., 2003). Biosorption is a potentially important mechanism for heavy metal cleanup relative to conventional methods due to its low cost, high efficiency and the possibility of the regeneration and recovery of the metals (Cruz et al., 2004; Luo et al., 2010). The biosorption does not replace methodologies used for removal of metals (such as precipitation, reduction, ion exchange, adsorption and coagulation); however, it can act as a system polishing in processes that are not completely efficient. The desorption of heavy metals in biosorbents can be accomplished with the use of eluents (agent competitors), which have different modes of interaction with biomass that has accumulated metals, allowing some percentage of recovery (Seolatto et al., 2009; Suhasinia et al., 1999).
The biomass of Cladosporium is an efficient biosorbent of copper Cu (II), cyanide, nickel, organochlorine pesticides, cadmium, silver, gold, and several organic compounds, including aromatic hydrocarbons, ketones and organic acids (Buszman et al., 2006; Juhasz et al., 2002; Pethkar et al., 2001a,b; Qi et al., 2002). While Cu (II) is an important trace element for the growth of these fungi, it can be toxic in high concentrations. An effective biosorbent for Cu (II) must be able to withstand high concentrations of this metal found in contaminated wastes (Melgar et al, 2007), for example, with pesticides used in agricultural production (Andreazza et al., 2010; Dell'Amico et al., 2008). The process of desorption of the metals that are biosorbed from the water, and its concentration for subsequent use, is as important as the biosorption process. Thus, the aim of the present study was to evaluate the desorption of Cu (II) by Cladosporium cladosporioides (Fres) de Vries biomass through biosorption (adsorption) using sulfuric acid, hydrochloric acid, ethylenediaminetetraacetate disodium and calcium chloride, as well as Cu (II) desorbed as copper sulfate (CuSO4).
Materials and Methods
This study focused on the filamentous fungi, C. cladosporioides, isolate G088, obtained from coffee beans. Cladosporium cladosporioides was first cultivated in the middle of solidified potato-dextrose-agar (PDA), inoculated with chloramphenicol antibiotic, and incubated at 25 °C for 10 days. After this period, three discs (6 mm in diameter each) of the fungus were transferred to Erlenmeyer flasks of 250 mL with 100 mL of medium potato-dextrose (PD). Twenty flasks were used, each containing three discs of C. cladosporioides. After 5 h-agitation at 120 rpm at 25 °C in an orbital shaker, the flasks were incubated at 25 °C for 20 days. After this period, the biomass was filtered and washed with distilled water four times to remove the middle of the culture.
For adsorption, 20 g of wet biomass of C. cladosporioides were transferred to a 250 mL-Erlenmeyer flask containing 150 mL of copper sulfate pentahydrate (CuSO4. 5H2O), with a concentration of 20 mg L1, which contained the initial concentration of 4.8 mg L1 of Cu (II). This procedure was replicated 12 times. The adsorption test was carried out in a finite bath for 120 h and was agitated at 120 rpm at 25 °C. The concentration factor (CF) was calculated by CF= C/Co, where C is the adsorbed Cu (II) concentration and Co is the initial Cu (II) concentration. To achieve kinetic adsorption of Cu (II), aliquots of 10 mL of the solution that contained the fungus were flooded during the following periods: 0; 0.5; 1; 2; 4 ; 6; 8; 10; 22; 24; 48; 72; 96 and 120 h. Samples were filtered on filter paper and analyzed for Cu (II) absorption by atomic spectrometry.
Viability of C. cladosporioides after adsorption: three discs (6.0 mm diameter) were removed from the Cu (II) solutions after 120 h of adsorption, transferred to Petri dishes with solidified PDA and chloramphenicol antibiotic and incubated at 25 °C for 10 days. Fungal growth was visually compared to control treatments where there was no chemical treatment.
Desorption of Cu (II) by C. cladosporioides: after 120 h of contact with the Cu (II) solution, the contaminated biomass was filtered and rinsed five times with distilled water to remove superficially deposited Cu (II). After it was filtered and rinsed, the biomass was placed in contact with one of the following 0.1 mol L1 eluent solutions: sulfuric acid, hydrochloric acid, ethylenediaminetetraacetate disodium and calcium chloride, to determine the amount by which they desorbed the Cu (II) that was previously adsorbed.
For the desorption stage, 20 samples of 5 g of biomass (five samples for each eluent) were placed in contact with 100 mL of the eluent solution in 250 mL Erlenmeyer flasks. The entire system (biomass contaminated and eluent solution) were agitated at 120 rpm at 25 °C for 10 h. In order to estimate the process of Cu (II) desorption, 5 mL aliquots were removed from each system at the following times: 0, 2, 4, 6, 48 and 100 h. The amount of Cu (II) was analyzed by atomic absorption spectrometry.
Viability of C. cladosporioides after desorption: three discs (6.0 mm diameter) were removed from each eluent solutions after 10 h of desorption, transferred to Petri dishes with middle solidified PDA (potato-dextrose agar) and chloramphenicol antibiotic, and incubated at 25 °C for 10 days. The changes in growth were compared (visually) to controls that had not been exposed to chemical treatment.
Recovery of Cu (II) desorbed as hydrated copper sulfate: sulfuric acid at 0.1 mol L1 had the highest elution capacity of Cu (II) adsorbed in biomass of C. cladosporioides. To discern how this Cu (II) could be recovered, the adsorption and desorption processes were repeated for this eluent. Therefore, 12 Erlenmeyer flasks, each containing 20 g of C. cladosporioides and 100 mL CuSO4.H2O solution (at a concentration of 20 mg L1) were incubated at 25 °C and agitated for 120 h at 120 rpm. Next, the biomass was rinsed five times with distilled water. To repeat the desorption process, 12 Erlenmeyer flasks, each containing 5 g of washed and filtered biomass (after adsorption) and 100 mL of sulfuric acid 0.1 mol L1, were incubated at 25 °C and agitated at 120 rpm for 10 h. Samples were collected at the beginning and end of the adsorption and desorption processes for Cu (II) analysis using atomic absorption spectrophotometry. After desorption, 900 mL of sulfuric acid 0.1 mol L1 (containing Cu II) were obtained. For Cu (II) precipitation, this volume was reduced to 100 mL by heating on an electric plate. Five mL of thioacetamide 3 % w/v were added during this process to operate as a source of sulfur for the precipitation of copper sulfide (CuS). The CuS was dried on an electric plate. To obtain Cu (II) in the form that was used in the solution for the adsorption test (copper sulfate), hydrogen peroxide (2 mL) was added to oxidize CuS to copper sulfate.
Statistical Analyses: The experiment was carried out using a completely randomized factorial design with four types of eluent solution and five contact times. Each treatment was replicated five times. The data were analyzed using analysis of variance (ANOVA), the quantitative data (time) were analyzed using linear regression and the qualitative (type of eluent) data were compared with an average means test [Tukey's test (p < 0.05)]. Analyses were performed with SAS software (SAS, 1996).
Results and Discussion
Process of Cu (II) adsorption by C. cladosporoides: the reaction kinetics of Cu (II) adsorption relative to the Cu (II) remaining in the solution was modeled with a common decay model (Equations 1 and 2).
dC/dTc= k.C (1)
C=Co.eK.Tc(2)
where: C = Remaining Cu (II) concentration (mg L1); Co = Initial Cu (II) concentration (Tc = 0); Tc = Contact time (time); k = Reaction coefficient (time1).
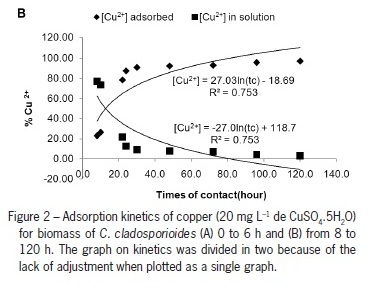
The values of the reaction coefficient (k) were correlated with the time of contact. Higher values of k have higher reactions speeds. The adsorption process occurs in two phases, the first having greater speed than the second (higher values of k) (Figures 1A and 1B). At the beginning of the adsorption process, most of the binding sites are free, and adsorption is fast. According to Silveira and Alleoni (2003), this is due the initial period of the adsorption curve corresponds to a high energy link. The second phase (Figure 1B) occurs due to a release of sites previously saturated by ligands in the first stage that result, for example, from a change in pH (Kaewsarn, 2002), and this phase occurs more slowly.
The quantity of adsorbed Cu (II) was calculated by the difference between the initial concentration and the concentration during the different contact times and was reported as the concentration of adsorbed Cu (II) and the remnants in the solution. After 30 min, 71 % of Cu (II) in the solution had been adsorbed, and after 2 h, 94 %. At the end of the process of adsorption (i.e., after 120 h), a concentration factor of 0.97 was observed. These adsorption values are close to the 90 % of Cu (II) removed after 15 min using the alga, Padina sp., as observed by Kaewsarn (2002).
The kinetics of the adsorption reaction indicates that the time of balance (point in which the concentration absorbed is the same to the concentration desorbed) was at 12.7 h of contact (Figure 2A and 2B). Finding the equilibrium time is important because this is when the adsorbed solute can, with a simple change in temperature, concentration or operating pressure, be removed from the adsorbent, hindering the subsequent adsorptive process (Peruch et al., 1998).
Viability of C. cladosporioides after adsorption: the visual comparison of the growth of C. cladosporioides after immersion in a solution of CuSO4 for 120 h showed that this does not inhibit the growth of the fungi but delays the start of growth by three days relative to when it was not immersed in a solution of Cu (II) for 10 days.
Desorption Process of Cu (II) by C. cladosporoides: The mean values of Cu (II) concentrations desorbed for each type of eluent solution, in each time period, are summarized in Table 1. A significant interaction was observed (p < 0.01) between the time of contact and the type of eluent solution.
The average percentage of desorption in relation to the final quantity of Cu (II) adsorbed by C. cladosporioides was 43 %, 36 %, 38 % and 3 % for the eluents sulfuric acid, hydrochloric acid, ethylenediaminetetraacetate disodium and calcium chloride, respectively. Similar results regarding the use of inorganic acids for the desorption of metals absorbed in biomasses were reported by Chojnacka et al. (2005), who compared the 0.1 mol L1 of the eluents EDTA-Na2 and HNO3, in the desorption of cadmium (II), chrome (III) and Cu (II) absorbed in alga Spirulina sp. They found the solution of HNO3 that removed almost all of the linked metallic ions with the biomass (98 %), and it did not cause loss of the biosorption capacity.
At all evaluated contact times, calcium chloride caused lower desorption of Cu (II) adsorbed in biomass of C. cladosporioides. This same eluent (in the same concentration and at pH = 3.0, adjusted with HCl) was used in the study by Vijayaraghavan et al. (2005), which examined the regeneration of the alga, Ulvaretigulata, after adsorption of Cu (II), and found that it is efficient in three cycles of sorption-desorption. It is likely that the HCl acidification favored the potential desorption of calcium chloride.
Until 6 h of contact, the highest average concentration of Cu (II) desorbed was observed with sulfuric acid. Between 8 and 10 h, ethylenediaminetetraacetate disodium had similar results to both sulfuric acid and hydrochloric acid. Treatment with hydrochloric acid should be avoided when reusing biomass in cycles of sorption/desorption because this eluent may affect polysaccharide ion exchange and structure.
The behavior of the Cu (II) concentration desorbed as a function of the contact time for the four eluents is shown in Figure 3 (A, B, C and D). Hydrochloric acid and calcium chloride responded linearly, while ethylenediaminetetraacetate disodium had a quadratic relationship (p < 0.01). There was no difference observed with sulfuric acid (p > 0.05, Figure 2A), suggesting that there was no variation in the concentration of Cu (II) desorbed relative to the times of contact assessed. The values desorbed concentrations of some metals in different biomasses are above those found in the present study (Table 2). However, there is a lack of published information in the literature of any test using desorption C. cladosporioides nor the viability of this microorganism after desorption.
Viability of C. cladosporioidesafter desorption: the visual comparison of the growth of C. cladosporioides after desorption for 10 h in the eluent solutions showed similar growth with 0.1 mol L1 sulfuric acid and no chemical treatment. All the other eluent solutions inhibited the growth of the C. cladosporioides for up to 12 days of incubation. The preservation of the capacity for biosorption of biomass is as important as the capacity desorbed in the choice of the eluent (Chu et al., 1997); 0.1 mol L1 sulfuric acid is the most effective for both of these.
Recovery of Cu (II) desorbed in the form of copper sulfate hydrous: after the process of sorption-desorption using C. cladosporioides, 900 mL solution of 0.1 mol L1 sulfuric acid, containing 2.02 mg L1 of Cu (II), were obtained. In order to recover this metal for the preparation of the solution designed to study the adsorption and desorption process of CuSO4, the 900 mL volume was reduced to 100 mL with an electric heating plate, resulting in a concentration of 14.05 mg L1 of Cu (II) (corresponding to a mass of 1.405 mg of Cu II). Thioacetamide (5 mL of solution 3 % w/v) was used as a sulfur source for the precipitation of Cu (II) in the form of CuS. After precipitation, the precipitate was dried on an electric plate at 60 °C, resulting in a mass of 1.8 mg of CuS, which after oxidation with 2 mL of concentrated hydrogen peroxide and drying on an electric plate (60 °C), resulted in 4.9 mg of a solid blue precipitate (CuSO4). The 4.9 mg mass of CuSO4 was diluted in up to 1000 mL of distilled water, resulting in a concentration of 1.15 mg L1 of Cu (II). As such, there was a recovery of approximately 63.3 % of the desorbed Cu (II) in the form of copper sulfate.
Conclusions
C. cladosporioides can efficiently adsorb Cu (II). 0.1 mol L1 sulfuric acid was viable and the most efficient in desorbing the absorbed metal, while ensuring viability of the in C. cladosporioides after desorption, which is important for the reuse of the biomass in cycles of sorption-desorption.
Acknowledgements
To EPAMIG/UFLA collection, for providing the C. cladosporioides used in the present study.
Received January 28, 2012
Accepted September 21, 2012
Edited by: Cláudio Marcelo Gonçalves de Oliveira
- Andreazza, R.; Pieniz, S.; Wolf, L.; Lee, M.; Camargo, F.; Okeke, B. 2010. Characterization of copper bioreduction and biosorption by a highly copper resistant bacterium isolated from copper-contaminated vineyard soil. Science of the Total Environment 408: 15011507.
- Blanco, A.; Sanz, B.; Llama, M.J.; Serra, J.L. 1999. Biosorption of heavy metals to immobilised Phormidium laminosum biomass. Journal of Biotechnology 69: 227240.
- Buszman, E.; Pilawa, B.; Zdybel, M.; Wilczynski, S.; Gondzik, A.; Witoszynska, T.; Wilczok, T. 2006. EPR examination of Zn2+ and Cu2+ binding by pigmented soil fungi Cladosporium cladosporioides. Science of the Total Environment 363: 195205.
- Chojnacka, K.; Chojnacki, A.; Górecka, H. 2005. Biosorption of Cr3+, Cd 2+ and Cu 2+ ions by blue-green algae Spirulina sp.: kinetics, equilibrium and the mechanism of the process. Chemosphere 59: 7584.
- Chu, K.H., Hashim, M.A., Phang, S.M., Samuel, V.B. 1997. Biosorption of cadmium by algal biomass: adsorption and desorption characteristics. Water Science and Technology 35: 115122.
- Cruz, C.C.V.; Costa, A.C.A.; Henriques, C.A.; Luna, A.S. 2004. Kinetic modeling and equilibrium studies during cadmium biosorption by dead Sargassum sp. biomass. Bioresource Technology 91: 249257.
- Davis, T.A.; Volesky, B.; Mucci, A. 2003. A review of the biochemistry of heavy metal biosorption by brown algae. Water Research 37: 43114330.
- Dell'amico, E; Mazzocchi, M; Cavalca, L; Allievi, L; Andreoni, V. 2008. Assessment of bacterial community structure in a long-term copper-polluted ex vineyard soil. Microbiological Research 163: 671683.
- Juhasz, A.L.; Smith, E.; Smith, J.; Naidu, R. 2002. Biosorption of organochlorine pesticides using fungal biomass. Journal of Industrial Microbiology and Biotechnology 29: 163169.
- Kaewsarn, P. 2002. Biosorption of copper (II) form aqueous solutions by pre-treated biomass of marine algae Padina sp. Chemosphere 47: 10811085.
- Luo, J.M.; Xiao, X.; Luo, S.L. 2010. Biosorption of cadmium (II) from aqueous solutions by industrial fungus Rhizopuscohnii Transactions of Nonferrous Metals Society of China 20: 11041111.
- Melgar, M.J.; Alonso, J.; García, M.A. 2007. Removal of toxic metals from aqueous solutions by fungal biomass of Agaricus macrospores Science of the Total Environment 385: 1219.
- Pethkar, A.V.; Gaikaiwari, R.P.; Paknikar, K.M. 2001a. Biosorptive removal of contaminating heavy metals from plant extracts of medicinal value. Current Science 80: 12161219.
- Pethkar, A.V.; Kulkarni, S.K.; Paknikar, K.M. 2001b. Comparative studies on metal biosorption by two strains of Cladosporium cladosporioides Bioreseource Technology 80: 211215.
- Qi, B.; Moe, W.M.; Kinney, K.A. 2002. Biodegradation of volatile organic compounds by five fungal species. Applied Microbiology and Biotechnology 58: 684689.
- Seolatto, A.A.; Câmara, M.M.; Tavares, C.R.; Cossich, E.S.; Silva, E.A. 2009. Removal of nickel (II) from aqueous solutions by Sargassum biomass filipendulain multiplecycles of sorption-desorption. Acta Scientiarum Technology 31: 5764 (in Portuguese,
- Silveira, M.L.A.; Alleoni, L.R.F. 2003. Copper adsorption in tropical oxisols. Brazilian Archives of Biology and Technology 46: 529536.
- Statistical Analysis System [SAS]. 1996. SAS/Stat User´s Guide: Statistics. SAS Institute, Cary, NC, USA.
- Suhasinia, I.P.; Sriram, G.; Abolekar, S.R.; Sureshkumar, G.K. 1999. Biosorptive removal and recovery of cobalt from aqueous systems. Process Biochemistry 34: 239247.
- Vijayaraghavan, K.; Jegan, J.; Palanivelu, K.; Velan, M. 2005. Biosorption of copper, cobalt and nickel by marine green alga Ulva reticulate in a packed column. Chemosphere 60: 419426.
- Xiao-Ming, L.; De-Xiang, L.; Xuen-Qin, X.; Qi, Y.; Guang-Ming, Z.; Wei, Z.; Liang, G. 2008. Kinetic studies for the biosorption of lead and cooper ions by Penicillium simplecissimun immobilized within loofa sponge. Journal of Hazardous Materials 159: 610615.
- Zulfadhly, Z.; Mashitah, M.D.; Bhatia, S. 2001. Heavy metals removal in fixed-bed column by the macro fungus Pycnoporus sanguineus Environmental Pollutions 112: 463470.
Publication Dates
-
Publication in this collection
14 May 2013 -
Date of issue
June 2013
History
-
Received
28 Jan 2012 -
Accepted
21 Sept 2012