Abstract
Coffee ringspot is a minor coffee disease caused by the nuclear type of Brevipalpus mite-transmitted virus, Coffee ringspot virus (CoRSV). Recently outbreaks of the disease in some growing regions of the state of Minas Gerais, Brazil, were registered with qualitative and quantitative yield losses. Coffea arabica was the only species registered as natural host. A survey was made on a germplasm collection of Coffea and related species kept at the Centro de Café "Alcides Carvalho", Instituto Agronômico, Campinas, state of São Paulo (SP), Brazil, to assess natural susceptibility of Coffee species, other than C. arabica and some interspecific hybrids of Coffea as well as other non-Coffea plant species to the Coffee ringspot virus (CoRSV). The following plants were found with ringspot symptoms on their leaves and/or fruits besides C. arabica L.: C. kapakata (IAC 4511), C. dewevrei cv. Excelsa, C. canephora cv. Robusta, hybrid derivative of the C. arabica × C. racemosa (IAC1195-5-6-2), C. arabica × C. dewerei (Piatã IAC 387), Híbrido de Timor CIFC 832/1 (derivative from a natural crossing between C. arabica × C. canephora) and C. racemosa. Also Psilanthus ebracteolatus, a species close to the genus Coffee was also found with ringspot lesions on their leaves. All these plants were also found infested by Brevipalpus mites identified as B. phoenicis. Infection of these plants by CoRSV was confirmed by the observation of characteristic cytopathic effects in the tissues of the lesion and by RT-PCR using a pair of primer specific for CoRSV. Only with C. racemosa RT-PCR failed to amplify the CoRSV genome. The susceptibility of P. ebracteolatus to CoRSV adds new dimension regarding its controversial taxonomic position.
Coffea arabica; C. kapakata; C. racemosa; C. dewevrei; Híbrido de Timor
NOTE
Natural infection of several Coffea species and hybrids and Psilanthus ebracteolatus by the coffee ringspot virus (CoRSV)
Elliot Watanabe KitajimaI,* * Corresponding author < ewkitaji@esalq.usp.br> ; César Martins ChagasI; Masako Toma BraghiniII; Luiz Carlos FazuoliII; Eliane Cristina Locali-FabrisIII; Renato Barbosa SalaroliI
IUSP/ESALQ - Depto. de Fitopatologia e Nematologia, C.P. 9 - 13418-900 - Piracicaba , SP - Brasil
IIInstituto Agronômico/Centro de Café "Alcides Carvalho", C.P. 28 - 13102-280 - Campinas, SP - Brasil
IIICentro Citros "Sylvio Moreira", C.P. 4 - 13490-970 - Cordeirópolis, SP - Brasil
ABSTRACT
Coffee ringspot is a minor coffee disease caused by the nuclear type of Brevipalpus mite-transmitted virus, Coffee ringspot virus (CoRSV). Recently outbreaks of the disease in some growing regions of the state of Minas Gerais, Brazil, were registered with qualitative and quantitative yield losses. Coffea arabica was the only species registered as natural host. A survey was made on a germplasm collection of Coffea and related species kept at the Centro de Café "Alcides Carvalho", Instituto Agronômico, Campinas, state of São Paulo (SP), Brazil, to assess natural susceptibility of Coffee species, other than C. arabica and some interspecific hybrids of Coffea as well as other non-Coffea plant species to the Coffee ringspot virus (CoRSV). The following plants were found with ringspot symptoms on their leaves and/or fruits besides C. arabica L.: C. kapakata (IAC 4511), C. dewevrei cv. Excelsa, C. canephora cv. Robusta, hybrid derivative of the C. arabica × C. racemosa (IAC1195-5-6-2), C. arabica × C. dewerei (Piatã IAC 387), Híbrido de Timor CIFC 832/1 (derivative from a natural crossing between C. arabica × C. canephora) and C. racemosa. Also Psilanthus ebracteolatus, a species close to the genus Coffee was also found with ringspot lesions on their leaves. All these plants were also found infested by Brevipalpus mites identified as B. phoenicis. Infection of these plants by CoRSV was confirmed by the observation of characteristic cytopathic effects in the tissues of the lesion and by RT-PCR using a pair of primer specific for CoRSV. Only with C. racemosa RT-PCR failed to amplify the CoRSV genome. The susceptibility of P. ebracteolatus to CoRSV adds new dimension regarding its controversial taxonomic position.
Keywords:Coffea arabica, C. kapakata, C. racemosa, C. dewevrei, Híbrido de Timor
Introduction
Ringspot symptoms on leaves (Figure 1 A) and berries of coffee (Coffea arabica L.) were first observed in the State of São Paulo, Brazil, and named "mancha anular" (Coffee ringspot) (Bitancourt, 1938). The disease has been observed in several regions of Brazil (Chagas et al., 2003; Kitajima and Chagas, 2009) and outside Brazil, confirmed only in Costa Rica (Rodrigues et al., 2002). The viral nature of the Coffee ringspot was inferred by electron microscopy which revealed the presence of short rod-like particles in the nucleus and cytoplasm and a characteristic electron lucent inclusion (viroplasma) in the nucleus (Kitajima and Costa, 1972; Chagas, 1980), its transmission by the tenuipalpid mite Brevipalpus phoenicis (Geijskes) (Acari: Tenuipalpidae) (Chagas, 1973), and mechanical transmission to Chenopodium quinoa Willd., C. amaranticolor Coste & Reyn. and Gomphrena globosa L. (Chagas et al., 1981). The causal virus was named Coffee ringspot virus (CoRSV). Coffee ringspot has been a minor disease in coffee plantations, but in some localities of the state of Minas Gerais, it caused significant yield loss (Chagas et al., 2003; Kitajima and Chagas, 2009) and affected the quality of the beverage (Boari et al., 2006). The virus was purified (Boari et al., 2004) and part of its genome was sequenced (GenBank accession GQ 979998). So far, CoRSV has been reported only in C. arabica. On the other hand, coffee is undergoing intense breeding program to produce plants with better agronomic properties not only selecting new lines of C. arabica but also through crossing with other Coffee species. Thus a survey was made on different Coffee species and hybrids of the germplasm bank maintained at the Centro de Café "Alcides Carvalho", of the Instituto Agronômico, Campinas, SP, Brazil, to detect cases of natural infection of some of these plants by the CoRSV. This article reports the finding of different species of Coffea and hybrids as well as a non-Coffea plant susceptible to CoRSV.
Material and Methods
Coffee germplasm are kept either under slate wood roof nursery or in the field at the above mentioned germplasm bank (22º52'20" S, 47º04'44" W), where no chemical control of diseases or pests is made. Visual inspections were made throughout this collection and samples were taken whenever leaves and/or fruits showed ringspot-like symptoms. Samples were kept in plastic bags for later analysis.
Presence of mites was assessed by visual inspection under binocular. When present, they were fixed in ethanol 90% and mounted for scanning electron microscopy after air-dried for species identification. Some mites were transferred to a leaf of C. arabica and left few hours. The leaf was instantly frozen by contact with a block of stainless steel at liquid nitrogen temperature and subsequently fixed in the vapor of osmium tetroxide, air dried, sputter coated before examination in a Zeiss 940A DSM scanning electron microscope.
Tissues from lesions on the leaves and fruits were fixed in a modified Karnovsky solution (2.5% glutaraldehyde, 2% paraformaldehyde in 0.05 M sodium cacodylate buffer, pH 7.2) for at least 1-2 h at room temperature and post-fixed in a 1% solution of osmium tetroxide in the same buffer for 1 h. After dehydration in an increasing series of concentration of acetone, fixed tissues were infiltrated and embedded in the Spurr's low viscosicty epoxy resin (Kitajima and Nome, 1999). Blocks were sectioned in a Leica UTC ultramicrotome equipped with a diamond knife. After staining with 3% uranyl acetate and Reynold's lead citrate, the ultrathin sections were examined under a Zeiss EM 900 transmission electron microscope and the images registered digitally.
For the confirmation of virus presence, total RNA was extracted from symptomatic leaf tissues and health leaf tissues (negative control), according to Gibbs and Mackenzie (1997) and used for RT-PCR to detect CoRSV. RNA concentration and purity were estimated by spectrophotometry and denaturing agarose gel electrophoresis (1% agarose, 6.7% formaldehyde, MOPS 10X [200 mM MOPS, 5 mM sodium acetate, 10 mM EDTA and DEPC-treated H2O]). Two hundred U of M-MLV reverse transcriptase (Invitrogen), 1.5 µL of 50 mM MgCl2, 100 ng of total RNA and 100 ng of random primers (3 µg µL-1) were used for RT reaction. Samples were denatured at 95ºC for 10 min and placed into ice. Then, 4 µL of 5X buffer were added along with 1 µL dNTP mix (10 mM), 0.5 µL (2 mM) DTT, 15 U RNAse inhibitor (Invitrogen), and sterile Milli-Q water to a 20 µL final volume. The reaction was incubated at 37ºC for 2 h. All PCR amplifications were conducted in a PTC 100 (MJ Research, Waltham, MA) thermocycler. The amplification reactions consisted of 2.5 mM MgCl2, 10 mM dNTP mix (Invitrogen), 100 ng of each especific primer: CoRSVF (5' - GGACCATGAGACAGGAGGTG - 3') and CoRSVR (5' CTCTGCCAGTCCTCAATGTG - 3'), 2 µL of cDNA used as template, 1 U of Taq DNA polymerase (Invitrogen), and sterile Milli-Q water for a final volume of 25 µL. These primers were designed based on the sequence of the virus polymerase gene, the sequence of which is deposited in the GenBank (accession GQ 979998). An initial denaturing cycle at 94ºC for 2 min was followed by 32 cycles of denaturation at 94ºC for 30 sec, annealing at 56ºC for 30 sec, and extension at 72ºC for 40 sec. A final 5 min extension was added. An aliquot of eight microliters of the PCR product were run in a 1% agarose gel.
Results and Discussion
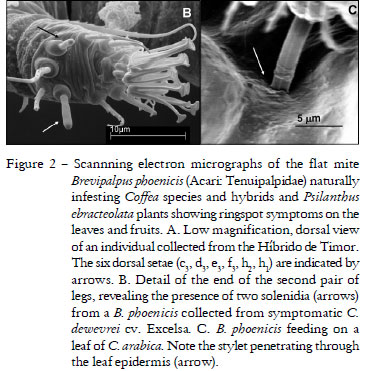
All sampled plants (several Coffea species and hybrids and the related species P. ebracteolatus) were infested by the flat mite identified as Brevipalpus phoenicis. Scanning electron microscopy (Figure 2) revealed the presence of two solenidia at the end of the second pair of legs (Figure 2 B) and six dorsal setae in the opistosome (Figure 2 A) which identify these mites as B. phoenicis (Welbourn et al., 2003). In frozen leaves, some B. phoenicis individuals were preserved in feeding position with the stylet perforating the epidermal layer (Figure 2 C). There are preliminary data indicating that CoRSV multiplies in the vector (E.W. Kitajima, unpublished data), showing that the virus vector relationship is of circulative/replicative type.
The following plants were found with ringspot symptoms on their leaves and/or fruits: C. kapakata (IAC 4511) (A. Chev.) Bridson (Figure 1 C), C. dewevrei (De Wild. and T. Duran) Lebrun cv. Excelsa (Figure 1 D), C. canephora Pierre ex. A. Froehner cv. Robusta (Linden) A. Chev. (Figure 1 B), hybrid derivative of the C. arabica x C. racemosa Lour. (IAC1195-5-6-2) (Figure 1 E), C. arabica x C. dewevrei (Piatã IAC 387) (Figure 1 G), Híbrido de Timor CIFC 832/1 (derivative from the natural crossing between C. arabica x C. canephora) (Figure 1 F), C. racemosa (Figure 1 H) e P. ebracteolatus Hiern. (IAC 3461-7) (Figure 1 I).
Transmission electron microscopy detected the previously described cytopathic effects induced by CoRSV (Kitajima and Costa, 1972; Chagas, 1980) in the cells of the leaf and fruit lesions. Several nuclei of the parenchymal and epidermal cells contained an electron lucent inclusion in the nucleus, referred to as viroplasma. Short, rodlike particles ca. 40 nm wide and 100-110 nm long were present in the nucleus, either in the nucleoplasm and/or within the viroplasma. These particles were also present in the cytoplasm, commonly associated with the membranes of the endoplasmic reticulum (Figure 3 A-J). This intracellular behavior of CoRSV is typical of the so-called nuclear type of Brevipalpus-transmitted virus (Kitajima et al., 2003). The best studied virus of this group is Orchid fleck virus (OFV). Its genome was completely sequenced revealing to be bipartite (ca. 6 kb each) and negative sense ss-RNA with organization similar to that of rhabdoviruses and a new genus Dichorhabdovirus was proposed (Kondo et al., 2006). Though distinct from OFV, CoRSV has biological and molecular similarities with OFV (Chagas et al., 2003; Kondo et al., 2003) and may belong to the same genus.
RT-PCR assays using primers specific for CoRSV consistently amplified genomic fragments of the expected size in all, except one (C. racemosa) symptomatic samples (Figure 4). These results clearly reveal that these Coffea species and hybrids were naturally infected by CoRSV. The virus must have been transmitted by the mite B. phoenicis present in all symptomatic plants, and known as the vector for CoRSV (Chagas, 1973). A plant of C. racemosa was found also with leaves exhibiting ringspot symptoms (Figure 2 H). Electron microscopy demonstrated the presence of cytopathic effect typical for CoRSV (Figure 3 H) but RT-PCR failed to amplify the viral genome in at lest three attempts. The cause of this discrepancy is being investigated.
P. ebracteolatus is distributed in the African continent (Ghana, Ivory Coast, Nigeria, Cameroon). It was first described by Hiern in 1877 but was suggested to be a member of the genus Coffea (C. ebracteolata) by Brennan in 1953. Experimental hybrids were obtained between C. arabica and P. ebracteolatus (Couturon et al., 1998). Recent studies applying cytological and molecular techniques did not clearly resolve the taxonomic relationship between the genera Coffea and Psilanthus (Lombello and Pinto-Maglio, 2003; Davis et al., 2006; Maurin et al., 2007) though Kumar et al. (2008) showed diversity between Indian species of Psilanthus and West and Central African species of Coffea using RAPD and ISSR assays. Though several non-Rubiaceae plant species have been described as experimental host for CoRSV (Chagas et al., 2003; Kitajima and Chagas, 2009), P. ebracteolatus is the only non-Coffea natural host for this virus reported so far. This susceptibility to a virus affecting naturally many Coffea species may add a new factor for the discussion of the taxonomical position of the genus Psilanthus.
Acknowledgements
This work received financial support from FAPESP (2007/50809-0) and CNPq (47.1268/2006-2).
Received July 19, 2010
Accepted November 05, 2010
Edited by: Jorge Alberto Marques Rezende
- Bitancourt, A.A. 1938. Coffee ringspot, a new disease of coffee. O Biológico 4: 404-405. (in Portuguese).
- Boari, A.J.; Freitas-Astúa, J.; Ferreira, P.T.O.; Neder, D.G.; Nogueira, Rossi, M.L.; Kitajima, E.W. 2004. Purification and serology of the Coffee ringspot virus. Summa Phytopathologica 30: 453-458.
- Boari, A.J.; Figueira, A.R.; Neder, D.G.; Santos, R.C.; Goussain, M.M.; Nogueira, N.L.; Rossi, M.L. 2006. Coffee ringspot virus: influence on the quality of the beverage and yield of coffee beans. Summa Phytopathologica 32: 192-194. (in Portuguese).
- Chagas, C.M. 1973. Association of the mite Brevipalpus phoenicis (Geijskes) to the coffee ringpsot. O Biológico 39: 229-232. (in Portuguese).
- Chagas, C.M. 1980. Morphology and intracellular behavior of coffee ringspot virus (CRV) in tissues of coffee (Coffea arabica L.). Phytopathologische Zeitschrift 99: 301-309.
- Chagas, C.M.; July, J.R.; Alba, A.P.C. 1981. Mechanical transmission and structural features of Coffee ringspot virus (CRV). Phytopathologisches Zeitschrift 102: 100-106.
- Chagas, C.M.; Kitajima, E.W.; Rodrigues, J.C.V. 2003. Coffee ringspot virus vectored by Brevipalpus phoenicis (Acari: Tenuipalpidae) in coffee. Experimental and Applied Acarology 30: 203-213.
- Couturon, E.; Lashermes, P.; Charrier, A. 1998. First intergeneric hybrids (Psilanthus ebracteolatus Hiern Coffea arabica L.) in coffee trees. Canadian Journal of Botany 76: 542-546.
- Davis, A.P.; Govaerts, R.; Bridson, D.M.; Stoffelen, P. 2006. An annotated taxonomic conspectus of the genus Coffea (Rubiaceae). Botanical Journal of the Linnean Society 152: 465-512.
- Gibbs, A.; Mackenzie, A. 1997. A primer pair for amplifying part of the genome of all potyvirids by RT-PCR. Journal of Virological Methods 63: 9-16.
- Kitajima, E.W.; Chagas, C.M. 2009. Virus disease in coffee. p. 550-556. In: Wintgens, J.N., ed. Coffee: growing, processing, sustainable production. 2ed. Wiley-VCH, Weinheim, Germany.
- Kitajima, E.W.; Costa, A.S. 1972. Bacilliform particles associated to the coffee ringspot. Ciência e Cultura 24: 542-545. (in Portuguese).
- Kitajima, E.W.; Nome, C.F. 1999. Electron microscopy in plant virology. p. 59-87. In: Docampo, D.M.; Lenardon, S.L., eds. Methods to detect systemic pathogens. IFFIVE/INTA/JIC, Córdoba, Argentina. (in Spanish).
- Kitajima, E.W. Chagas, C.M., Rodrigues, J.C.V. 2003. Brevipalpus-transmitted plant vírus and virus-like diseases: cytopathology and some recent cases. Experimental and Applied Acarology 30: 135-160.
- Kondo, H.; Maeda, T.; Tamada, T. 2003. Orchid fleck virus: Brevipalpus californicus mite transmission, biological properties and genome structure. Experimental and Applied Acarology 30: 215-223.
- Kondo, H.; Maeda, T.; Shirako, Y.; Tamada, T. 2006. Orchid fleck virus is a rhabdovirus with an unusual bipartite genome. Journal of General Virology 87: 2413-2421.
- Kumar, S.A.; Sudisha, J.; Sreenath, H.L. 2008. Genetic relation of Coffea and Indian Psilanthus species as revealed through RAPD and ISSR markers. International Journal of Integrative Biology 3: 150-158.
- Lombello, R.A.; Pinto-Maglio, C.A.F. 2003. Cytogenetic studies in Psilanthus ebracteolatus Hiern, a wild diploid coffee species. Cytologia 68: 425-429.
- Maurin, O.; Davis, P.L.; Chester, M.; Mzungi, E.F.; Jaufeerally-Fakim, Z.; Faëz, M.F. 2007. Towards a phylogeny for Coffea (Rubiaceae): Identifying well-supported lineages based on nuclear and plastid DNA sequences. Annals of Botany 100: 1565-1583.
- Rodrigues, J.C.V.; Rodriguez, C.M.; Moreira, L.; Villalobos, W.; Rivera, C.; Childers, C.C. 2002. Occurrence of Coffee ringspot virus, a Brevipalpus mite-borne virus in coffee in Costa Rica, Central America. Plant Disease 86: 564.
- Welbourn, W.C.; Ochoa, R.; Kane, E.C.; Erbe, E.F. 2003. Morphological observations on Brevipalpus phoenicis (Acari: Tenuipalpidae) including comparisons with B. californicus and B. obovatus Experimental and Applied Acarology 30: 107-133.
Publication Dates
-
Publication in this collection
22 Sept 2011 -
Date of issue
Aug 2011
History
-
Received
19 July 2010 -
Accepted
05 Nov 2010