Abstracts
Coffee (Coffea arabica) plants show a positive relationship between stomatal closure and formation and accumulation of H2O2. However, for coffee plants under water restriction such relationship has never been studied. The objective of the present study was evaluate the stomatal movement and the antioxidant capacity of coffee seedlings under different water regimes. Eight months old coffee seedlings of cv. Catuaí IAC 99 were submitted to field capacity, gradual and total suspension of irrigation during a period of 21 days. Evaluations of leaf water potential (Ψw) were performed in the beginning of the morning, and stomatal resistance, transpiration rate and vapor pressure deficit were determined at 10 am and 5 pm. All biochemical and enzymatic determinations were performed in leaves collected at 5 pm. Evaluations and samplings were performed at three days intervals. There was no variation in Ψw during the evaluated period for plants in field capacity. However, an expressive decrease of Ψw following day 12, reaching values near -2.5 MPa at the end of the experiment was observed for plants submitted to gradual suspension of irrigation. For plants submitted to total suspension of irrigation, Ψw decreases after the sixth day, reaching -2.5 MPa at day 15. The decay of Ψw in plants submitted to gradual and total suspension of irrigation reflected in increased stomatal resistance and in a decreased transpiration rate leading to an increase in hydrogen peroxide formation and, on final stages, increase in lipid peroxidation. As a conclusion, an increase in the activity of antioxidant enzymes as well as in the levels of ascorbate and dehydroascorbate was observed, which act in the detoxification of free radicals formed as result of the water stress.
Coffea arabica; oxidative stress; antioxidants; water stress
Para o cafeeiro (Coffea arabica) existe uma comprovada relação positiva entre fechamento estomático e formação e acúmulo de H2O2. Entretanto, tal relação para a cultura sob restrição hídrica ainda não foi estudada. Avaliou-se o movimento estomático e a capacidade antioxidante em mudas de cafeeiro sob diferentes regimes hídricos. Mudas de cafeeiro cv. Catuaí IAC 99, com oito meses de idade, foram submetidas à capacidade de campo, suspensão gradativa e suspensão total da irrigação por um período de 21 dias. Foram realizadas avaliações do potencial hídrico (Ψw) foliar na antemanhã e resistência estomática, taxa transpiratória e déficit de pressão de vapor foram avaliados as 10h00 e 17h00. As determinações bioquímicas e enzimáticas foram realizadas em folhas coletadas às 17h00. Todas as avaliações e coletas foram realizadas em intervalos de três dias. Nas plantas em capacidade de campo não houve variação no Ψw durante o período de avaliação. Para a suspensão gradativa da irrigação, houve queda expressiva a partir dos 12 dias, chegando próximo a -2,5 Mpa, ao final do experimento. Já nas plantas em suspensão total da irrigação observou-se queda no Ψw a partir do sexto dia, chegando a -2,5 MPa aos 15 dias. A queda no Ψw para as plantas em suspensão gradual e total da irrigação refletiu em aumentos na resistência estomática e diminuição da taxa transpiratória, ocasionando aumento na formação de peróxido de hidrogênio e nos períodos finais, aumentos na peroxidação de lipídios. Em conseqüência obervaram-se aumentos na atividade das enzimas antioxidantes, bem como nos teores de ascorbato e dehidroascorbato, atuando na detoxificação dos radicais livres formados em função do estresse.
Coffea arabica; estresse oxidativo; antioxidantes; estresse hídrico
PLANT PHYSIOLOGY AND BIOCHEMISTRY
Stomatal behavior and components of the antioxidative system in coffee plants under water stress
Comportamento estomático e componentes do sistema antioxidante em cafeeiros sob estresse hídrico
Sidnei DeunerI; José Donizeti AlvesII,* * Corresponding author < jdalves@dbi.ufla.br> ; Ilisandra ZanandreaIII; Patrícia de Fátima Pereira GoulartIV; Neidiquele Maria SilveiraII; Paôla de Castro HenriqueII; Alessandro Carlos MesquitaV
IUFPel - Depto. de Botânica, Instituto de Biologia, C.P. 354 - 96010-900 - Pelotas, RS - Brasil
IIUFLA - Depto. de Biologia, Setor de Fisiologia Vegetal, C.P. 37 - 37200-000 - Lavras, MG - Brasil
IIIEmbrapa Clima Temperado, C.P. 403 - 96001-970 - Pelotas, RS - Brasil
IVUNILAVRAS - Centro Universitário de Lavras, 37200-000 - Lavras, MG - Brasil
VEmbrapa Café, C.P. 3037 - 37200-000 - Lavras, MG - Brasil
ABSTRACT
Coffee (Coffea arabica) plants show a positive relationship between stomatal closure and formation and accumulation of H2O2. However, for coffee plants under water restriction such relationship has never been studied. The objective of the present study was evaluate the stomatal movement and the antioxidant capacity of coffee seedlings under different water regimes. Eight months old coffee seedlings of cv. Catuaí IAC 99 were submitted to field capacity, gradual and total suspension of irrigation during a period of 21 days. Evaluations of leaf water potential (Ψw) were performed in the beginning of the morning, and stomatal resistance, transpiration rate and vapor pressure deficit were determined at 10 am and 5 pm. All biochemical and enzymatic determinations were performed in leaves collected at 5 pm. Evaluations and samplings were performed at three days intervals. There was no variation in Ψw during the evaluated period for plants in field capacity. However, an expressive decrease of Ψw following day 12, reaching values near -2.5 MPa at the end of the experiment was observed for plants submitted to gradual suspension of irrigation. For plants submitted to total suspension of irrigation, Ψw decreases after the sixth day, reaching -2.5 MPa at day 15. The decay of Ψw in plants submitted to gradual and total suspension of irrigation reflected in increased stomatal resistance and in a decreased transpiration rate leading to an increase in hydrogen peroxide formation and, on final stages, increase in lipid peroxidation. As a conclusion, an increase in the activity of antioxidant enzymes as well as in the levels of ascorbate and dehydroascorbate was observed, which act in the detoxification of free radicals formed as result of the water stress.
Key words:Coffea arabica, oxidative stress, antioxidants, water stress
RESUMO
Para o cafeeiro (Coffea arabica) existe uma comprovada relação positiva entre fechamento estomático e formação e acúmulo de H2O2. Entretanto, tal relação para a cultura sob restrição hídrica ainda não foi estudada. Avaliou-se o movimento estomático e a capacidade antioxidante em mudas de cafeeiro sob diferentes regimes hídricos. Mudas de cafeeiro cv. Catuaí IAC 99, com oito meses de idade, foram submetidas à capacidade de campo, suspensão gradativa e suspensão total da irrigação por um período de 21 dias. Foram realizadas avaliações do potencial hídrico (Ψw) foliar na antemanhã e resistência estomática, taxa transpiratória e déficit de pressão de vapor foram avaliados as 10h00 e 17h00. As determinações bioquímicas e enzimáticas foram realizadas em folhas coletadas às 17h00. Todas as avaliações e coletas foram realizadas em intervalos de três dias. Nas plantas em capacidade de campo não houve variação no Ψw durante o período de avaliação. Para a suspensão gradativa da irrigação, houve queda expressiva a partir dos 12 dias, chegando próximo a -2,5 Mpa, ao final do experimento. Já nas plantas em suspensão total da irrigação observou-se queda no Ψw a partir do sexto dia, chegando a -2,5 MPa aos 15 dias. A queda no Ψw para as plantas em suspensão gradual e total da irrigação refletiu em aumentos na resistência estomática e diminuição da taxa transpiratória, ocasionando aumento na formação de peróxido de hidrogênio e nos períodos finais, aumentos na peroxidação de lipídios. Em conseqüência obervaram-se aumentos na atividade das enzimas antioxidantes, bem como nos teores de ascorbato e dehidroascorbato, atuando na detoxificação dos radicais livres formados em função do estresse.
Palavras-chave:Coffea arabica, estresse oxidativo, antioxidantes, estresse hídrico
Introduction
Brazil is the main country exporter of coffee (Coffea arabica) and it is responsible for one third of the world production (Pereira et al., 2007). Brazilian production could be even more significant if unfavorable environmental conditions, such as drought, did not occur frequently (Gomes et al., 2008). Particularly for coffee trees, decreases in the water supply imposes a substantial reduction in plant growth (Oliveira et al., 2002; Freitas et al., 2007), although no features of water deficiency can be seen (Grisi et al., 2008). In these cases, rapid stomatal closure in leaves occurs in response to water deficiency (Nascimento et al., 2008) and optimizes water use efficiency, thereby playing crucial roles in drought stress tolerance. When plants are drought-stressed, abscisic acid (ABA) induces stomatal closure by reducing the turgor of guard cells under water deficit (An et al., 2008). By opening and closing stomata, the guard cells control transpiration to regulate water loss or retention.
ABA and H2O2 are produced and accumulated in plants under adverse environmental conditions such as drought, and are crucial in signaling adaptive responses, including stomatal closure (Zhang et al., 2001) and antioxidant defense (Zhang et al., 2006). Zhang et al. (2001) reported the accumulation of H2O2 in the guard-cells under water deficit, activated by an increase in the ABA production. These authors clearly show that H2O2 is an essential signal in ABA-induced stomatal closure.
Environmental stresses promote enhanced production reactive oxygen species (ROS), such as superoxide anion (O2-), hydrogen peroxide (H2O2), and many free radicals and its consequent accumulation in plants and thereby damage DNA, proteins and lipids (Gratão et al., 2005; Vasconcelos et al., 2009). To protect its cells from ROS, drought tolerant plants activate an oxidative defense system, formed by enzymatic and non-enzymatic components capable of maintaining a favorable balance between ROS and the detoxifying capacity (Pompeu et al., 2008; Vasconcelos et al., 2009). Moreover, plant responses to different types of stresses are associated with generation of ROS, suggesting that ROS may function as a common signal in signaling pathways of plant stress responses (Gratão et al., 2005).
Coffee studies are consolidated in the sense that there is a positive relationship between stomatal closure and formation and accumulation of H2O2 (Deuner et al., 2008). In this previous report we evaluated the exogenous application of ascorbic acid and hydrogen peroxide on enzyme activity of the antioxidant system and its relation to stomatal opening. Application of H2O2 reduced the stomatal conductance and consequently the transpiration rate compared to the control plants, and at the same time, promoted higher antioxidant activity of the enzymes. When plants were sprayed with ascorbic acid, higher stomatal conductance and transpiration rate were found in the first hour of evaluation. Coffee under water restriction has the level of H2O2 increased and triggers mechanisms related to stomatal closure and detoxification of H2O2 in order to retain its degree of hydration. These findings provide an interesting insight into the mechanism of stomata-regulated drought stress tolerance in coffee trees and important contributions to better understanding of the management of this important crop coffee under water stress. Therefore, this paper aimed to evaluate the stomatal movement and the antioxidant capacity of coffee seedlings under different water regimes.
Material and Methods
Eight months old seedlings of Coffea arabica, cultivar Catuaí IAC 99, were grown in 3L plastic bags containing as substrate a mixture of soil and cattle manure. The plants remained for two months in a greenhouse, covered with a black-film that intercepts 30% solar radiation. During this period, the plants were watered regularly, maintaining the water level within the field capacity until the treatments were started. The seedlings were subjected then to three water regimes: field capacity (FC), gradual suspension of irrigation (GS) and total suspension of irrigation (TS). In the FC treatment, the plastic bags were weighed and the soil maintained near the field capacity. Every three days, these were weighed once again for reposition of water lost by evapotranspiration. For the regime of gradual suspension of irrigation, the water lost after the first day was restored in 80%, 60%, 40%, 20% and 0%, on every three day interval. For the regime of total suspension of irrigation, the water was totally suspended from the first day. The experiment was conducted in a completely randomized design with three replicates per treatment, and the evaluations were performed every three days, during a period of 21 days.
The water status was evaluated by measurements potential of pre-dawn (before sunrise) leaf water potential with a Scholander-type pressure chamber on six fully expanded leaves of the fourth pair. Leaf transpiration (E) and stomatal resistance (rs) measurements were taken on six fully expanded leaves of the fourth pair with a steady state porometer at 10 am and 5 pm. The mean vapor pressure deficit (VPD) was the vapor pressure at leaf temperature minus the mean vapor pressure of the chamber gas (computed as the average vapor pressure of the gas entering and that exiting the chamber). The timing of determinations followed the recommendations of Deuner et al. (2008) since at 10 am the photosynthesis and transpiration were higher and stomatal resistance was minimal and 5 pm there was the maximum expression of antioxidant enzymes. Thus measurements of stomatal resistance, transpiration and VPD were also carried out at 5 pm in order to compare the activity of enzymes (in its time of maximum expression) with these features.
At 5 pm, following the recommendations of (Deuner et al., 2008), leaves of the third pair were collected for the biochemical (hydrogen peroxide, lipid peroxidation, ascorbate - Asc and dehydroascorbate - DHA) and enzymatic (superoxide dismutase - SOD, catalase - CAT, ascorbate peroxidase - APX and glutathione reductase - GR) analyses. The collected material was frozen in liquid nitrogen and stored in ultra-freezer, at -80ºC, for subsequent analysis.
Hydrogen peroxide (H2O2) content was determined according to methodology described by Sinha et al. (2005). Leaf tissues were macerated in a solution of 0.1% (w/v) trichloroacetic acid (TCA) and the homogenate centrifuged at 12,000 x g for 15 min., at 4ºC. Next, 0.5 mL of the supernatant was added to 0.5 mL of 10 mM potassium phosphate buffer (pH 7.0) and 1 mL of 1 M KI. Absorbance measurements were carried out in spectrophotometer at 390 nm. The H2O2 content was calculated by comparing the reads with a standard curve obtained from concentrations of H2O2.
Lipid peroxidation was determined by quantification of the thiobarbituric acid reactive species (TBARS) as described by Buege and Aust (1978). Two hundred milligrams of leaf tissue were macerated in liquid N2 plus 20% of PVPP (polivinilpolipirolidone) and homogenized in trichloroacetic acid (TCA) 0.1% (w/v). The homogenate was then centrifuged at 10,000 x g for 10 min. Two hundred and fifty microliters of the supernatant were added to 1 mL of the reaction medium [0.5% (w/v) of thiobarbituric acid (TBA) and 10% (w/v) of TCA], incubating it then at 95ºC for 30 minutes. The reaction was stopped by rapid cooling in ice, and the absorbance measurements were determined in a spectrophotometer at 535 nm and 600 nm. The concentration of the complex MDA/TBARS was calculated using the extinction coefficient of 1.55 mmol L-1 cm-1.
Ascorbate (Asc) and dehydroascorbate (DHA) were quantified as described by Arakawa et al. (1981) with some modifications. One hundred milligrams of leaf tissue were macerated in 2 mL of trichloroacetic acid (TCA) 5% (w/v) homogenate and centrifuged at 10,000 × g for 15 min at 4ºC. Total ascorbate (Asc + DHA) was determined after reduction of DHA by dithiothreitol (DTT). Then, 30 µL of the supernatant were added to a reaction medium containing: 70 µL of 5% TCA (w/v), 125 µL of 0.06% DTT (w/v) and 125 µL sodium phosphate 0.2 M (pH between 7 and 8 adjusted with 1.2 M NaOH). After incubation at room temperature for 10 min, 125 µL of N-ethylmaleimide 0.24% (w/ v) was added and the pH of each tube adjusted to between 1 and 2 with the addition of 20% TCA (w/v). After that, 125 µL of phosphoric acid (H3PO4) 4% (v/v), 250 µL of bathophenanthroline 0.5% (w/v) and 125 µL of FeCl3 0.03% (w/v) were added, mixing the mixture vigorously and incubating it at 30ºC for 90 minutes. The reads were performed in a spectrophotometer at 534 nm. The ascorbate was determined as described above, but replacing the DTT by absolute ethanol in equal volume. The values for DHA were obtained by the difference between the values of total ascorbate and ascorbate.
Two hundred milligrams of leaf tissue were macerated in liquid N2 with 50% of polyvinylpyrrolidone (PVPP) and homogenized in 1.5 mL of extraction buffer as following: 100 mM potassium phosphate (pH 7.8), 0.1 mM EDTA and 10 mM ascorbic acid. After centrifugation at 13,000 g for 10 minutes at 4ºC, the supernatant was collected and desalinated in a Sephadex G-25 Column (PD-10). The proteins were eluted with the same buffer and monitored at 595 nm. The eluate was used for the enzymes activity assays and quantification of protein by the method of Bradford (1976).
SOD activity was assessed by the ability the enzyme to inhibit the blue of nitrotetrazole (NBT) photoreduction (Giannopolitis and Ries, 1977) in a reaction medium composed of 100 mM potassium phosphate (pH 7.8), 14 mM methionine, EDTA 0.1 µM, 75 mM NBT and riboflavin 2 mM. The tubes with the reactions and the samples were illuminated for seven minutes in a box by a fluorescent lamp of 20 W. For the control, the same reaction medium without the sample was illuminated and as blank was used the tube kept in the dark. The readings were performed at 560 nm. One unit of SOD is the amount of enzyme capable to inhibit by 50% the photoreduction of NBT under the tested conditions.
The activity of CAT was determined as described by Azevedo et al. (1998) with minor modifications. Activity was monitored by the decrease in absorbance at 240 nm for two minutes in a reaction medium incubated at 28ºC, containing 100 mM potassium phosphate buffer (pH 7.0) and 12.5 mM H2O2.
The activity of APX was determined as Nakano and Asada (1981), tracking the oxidation rate of ascorbate at 290 nm. The reaction medium that was incubated at 28ºC was composed of 100 mM potassium phosphate buffer (pH 7.0), ascorbic acid 0.5 mM and 0.1 mM H2O2. The decrease in absorbance was monitored for a period of two minutes from the beginning of the reaction.
The activity of GR was based on the method of Cakmak et al. (1993), tracking the rate of oxidation of NADPH by the decrease in absorbance, at 340 nm for two minutes. The reaction medium incubated at 28ºC consisted of 50 mM potassium phosphate buffer (pH 7.8), oxidized glutathione 1 mM and 0.075 mM NADPH.
Data sets were subjected to analyses of variance (ANOVA) with three water regimes and days of stress as main factors. Tukey HSD tests were carried out to determine differences among treatment means, using the STATISTICA software (ver. 5.0, Statsoft, Inc. Tulsa, OK, USA)
Results and Discussion
In plants at field capacity (FC), the water potential (ΨW) was constant, close to -0.2 MPa throughout the whole assessment period (Figure 1). However, in plants which the irrigation was suspended gradually (GS), a decrease was observed in ΨW only at 18 d, when the plants were no longer receiving water for three days. During this period, the ΨW dropped to -2.0 MPa, a value considered critical for coffee plants (Rena and Maestri, 2000; Oliveira et al., 2002; Pinheiro et al., 2005; Grisi et al., 2008) since it may cause reductions in the photosynthetic rates (Grisi et al., 2008). Following this period, the reduction was accentuated reaching values close to -2.5 MPa at 21 days. On the other hand, the total suspension of irrigation (TS) promoted a rapid decrease in water potential after the sixth day of evaluation, reaching -2.4 MPa at 15 days and -3.0 MPa at the end of the experiment. Coffee plants supports long periods of drought, adjusting mechanisms that lead to water saving, proportionally to the intensity of water deficit (Deuner et al., 2008; Nascimento et al., 2008).
The stomatal resistance measured at 10 am (Figure 2A) and 5 pm (Figure 2B), in plants that were kept at FC, showed no difference during the assessment period (Figure 2A). However, coffee plants under the regime of GS showed an increase in stomatal resistance measured at 10 am starting 12 d after the onset the experiment, being this effect more pronounced after 15 d, when the irrigation was completely stopped and when ΨW reached -0.7 MPa (Figure 1). For plants under TS, differences from the control FC were observed after sixth day, when ΨW reached -0.5 MPa, and continued to increase to values near -2.5 MPa, at 15th day, a value considered critical. Until the end of the experiment, with total restriction of irrigation, the stomatal resistance continued to increase, even with the potential falling below the critical values. The sensitivity of coffee plants stomata to water deficit is evidenced, since even small decreases in Ψw were sufficient to promote its initial closure (Oliveira et al., 2002; Pinheiro et al., 2005), which continued until 21 days, when it reached -2.3 and -3.0 MPa for the GS and TS treatments, respectively.
The increase in stomatal resistance, when the leaves were still turgid, shows that the coffee stomata participate actively in the process of water loss reduction, initiating its closure at the first signs of water restriction (Pinheiro et al., 2005). This process certainly enables coffee plants to support long periods of drought, without compromising its degree of cellular hydratation.
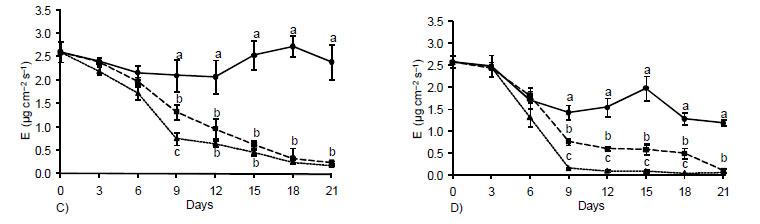
In general, values of stomatal resistance at 5 pm were higher than those verified at 10 am (Figure 2B). After nine days of evaluation, there were differences among the three water regimes and this difference accentuated with the progress of the experiment. The recovery in stomata opening in the morning (Figure 2A), after its closured had occurred in the previous afternoon (Figure 2B), reinforces that the coffee stomata remained functional even with the leaf under strong dehydration (Oliveira et al., 2002), such as that shown after 21 d of water restriction.
The transpiratory rates virtually did not vary between the two periods of the day (Figure 2C and D) and for plants subjected to water deficit (GS and ST) a rate inversely proportional to the stomatal resistance was observed (Figure 2A and B), i.e., while it fell the stomatal resistance increased. The evaporative pattern of leaves explains variations in ΨW (Figure 1). In the case of control FC plants, a reduction in transpiration by the 9th day was observed at 5 pm, as a result of the higher evaporative demand during this period (Figure 2E and F), without causing, however, decreases in water status of leaves (Figure 1). In general, the observed transpiration behavior, at both 10 am and 5 pm, reflected the stomatal movement which, in turn, reflected in the pattern set by the water potential (Deuner et al., 2008).
Pinheiro et al. (2005), Deuner et al. (2008) and Nascimento et al. (2008) also concluded that the closure of stomata appears to be one of the first strategies to minimize losses of water occurring with the transpiration under conditions of low leaf water potentials The effects of water stress on plants may be mediated by the production and accumulation of reactive oxygen species, leading to extensive membranes damage, and triggering of lipid peroxidation processes (Smirnoff, 1993). In this experiment, the levels of H2O2 in leaves of plants subjected to water stress were higher than those observed in control plants after three days (Figure 3A). In the course of the period of stress, the values increased almost linearly, reaching at the end of the experiment, values 33 and 100% higher than those of the controls, for plant submitted to GS and TS, respectively.
The H2O2 production occurs in the plants tissues under a variety of factors that include extreme temperatures, excessive excitement energy, water stress, heavy metal and pathogen action (Deuner et al., 2008; Gomes-Júnior et al., 2006; Pompeu et al., 2008). The H2O2 production may occur in plant cells via a number of patways, such as NAD(P)H oxidase, peroxidation of lipids or by the transport of electrons in photosynthesis (Montillet et al., 2004). By means of the analysis of the behavior of the H2O2 production in coffee plants under water restriction, we found that its metabolic route is activated before the plants show any signs of the water deficit, due to the high values of ΨW or due to the low values of stomatal resistance or transpiration (Figure 2).
The accumulation of reactive oxygen species, here represented by the levels of H2O2, can cause peroxidation of cell membranes, impairing its function and integrity, with damage, often irreversible, to the functioning of the cell. In the current case, the lipid peroxidation (Figure 3B) in control plants (FC) increased, until the first six days, from 8.7 to 11.4 nmol of MDA g-1 MF, remaining, however, in that level until the 12th day, falling from thereafter to its initial levels. Lipid peroxidation is a metabolic process that occurs normally under natural conditions (Blokina et al. 2003), which explains the values of peroxidation found in these plants.
Plants that were in gradually (GS) and immediately (TS) submitted to irrigation suspension, showed an increase in lipid peroxidation in the first six days, returning to baseline values during the 9th and the 15th day from which increased again until the end of the experiment. During this period the H2O2 concentration in plants that were under gradual (GS) and total suspension (TS) of irrigation, was 33 and 100% greater than that found in control plants (FC).
Differences in lipid peroxidation between irrigated and non-irrigated plants were observed only after 15 days, for plants with total suspension of irrigation (TS) and 21 days for plants in gradual suspension of irrigation (GS). These increases occurred when the values of water potential of plants with water deficit fell to -2.5 MPa (Figure 1), and when the levels of H2O2 in GS and TS plants were, respectively, 66 and 33% higher than their controls (Figure 3A). Therefore, the thresholds range of water potential that coffee plants may tolerate from -2.0 to -2.5 MPa. Until this critical point is reached, the coffee plants, through a stomatal control, maintained its water status at the expense of the preservation of its membranes integrity. From thereafter, the increase in H2O2 concentrations with its deleterious effects on the peroxidation of lipids prevails, causing cellular damage that, depending on its duration and intensity, may compromise the physiological processes. As an example, there is a greater stomata movement towards stomatal closure, virtually stopping the transpiration (Figure 2), exactly when the plants reached -2.5 MPa, i.e. at 15 and 21 days for GS and TS plants, respectively. In this case, it was observed that plants under total suspension irrigation were, until the end of the experiment, under stress for at least six days, while plants under gradual suspension, reached the water stress status at after 21 days, i.e. at the last assessment period.
Under normal conditions, plants usually are well adapted to minimize damage due to the inevitable formation of reactive oxygen species in photosynthesis (Gratão et al., 2005). However, drought intensifies the formation of free radicals by limiting the pool of NADP+ available to accept electrons from photosystem I. Thus, increases the likelihood of excitation energy transfer to the O2, leading to the production of O2- and 1O2, which react with the membranes fatty acids, causing lipid peroxidation (Smirnoff, 1993).
In some drought tolerant plants, increase in the production of antioxidants may limit lipid peroxidation (Yoshimura et al., 2000; Blokina et al., 2003). In this experiment, the levels of ascorbic acid and dehydroascorbate, in plants kept at field capacity exhibited little variation (Figure 3C and D) during the whole evaluation period. However, levels of ascorbic acid increased in plants subjected to gradual suspension of irrigation after the ninth day, the highest levels occurring at days 18 and 21. For plants under total suspension of irrigation, the values were similar to the previous treatment; however, there was a drop at day 21. The dehydroascorbate content of plants in gradual suspension of irrigation (Figure 3D) also was higher than those found in control plants after the ninth day, remaining constant until the end of the experiment. The plants under total suspension of irrigation showed increases in the levels of dehydroascorbate in relation to the control plants after the third day of stress. In these plants, the highest levels were observed at 15 and 18 days.
The protective effect of antioxidants was sufficient to maintain the functionality of the membranes and its effects on stomatal movement and ΨW for a period of 15 and 18 d for plants under GS and TS, respectively. These results confirm those obtained by Deuner et al. (2008) who observed that application of exogenous H2O2 and ascorbic acid in coffee leaves, respectively, reduced and increased stomatal conductance. Induction of stomatal closure in vivo has been attributed to the accumulation of H2O2 in guard cells in response to ABA (Suhita et al., 2004). Once accumulated in the cells, H2O2 activates the passage of calcium channels in vacuole membrane, increasing its concentration in the cytosol (Kohler and Blatt, 2002), leading thereby to a depolarization of guard cells, efflux of potassium, loss of turgor and, consequently, closing the stomata (Schroeder et al., 2001).
The activation of enzymes of the antioxidant system interferes in the levels of some compounds involved these reactions. The ascorbic acid, besides being a non-enzymatic antioxidant, acts as coenzyme of APX and therefore its intercellular content can be changed in function of a stress. The same may occur with dehydroascorbate, since it is the product of the reaction of this same enzyme. Ascorbic acid is an essential component of plant tissues and has been the focus of numerous studies regarding the enzymatic and non-enzymatic oxidation in biological systems. It serves as an excellent antioxidant and plays a key role in the H2O2 removal by the ascorbate/glutathione cycle and produces DHA (Arakawa et al., 1981). ROS are involved in the oxidation of ascorbic acid to form dehydroascorbate, which is subsequently regenerated to ascorbic acid again. Antioxidants such as ascorbic acid and glutathione, which are found in high concentrations (5-20 mM and 1-5 mM, respectively) in chloroplasts and other cellular compartments, are important for the plants defense against oxidative stress (Blokhina et al., 2003).
The activity of superoxide dismutase (SOD) in plants under GS differed from control FC after 12 days of stress, remaining constant up to 15 days, when it started to increase until the end of the experiment (Figure 4A). This increase occurred just when the water potential fell from -0.7 to -2.5 MPa, approximately (Figure 1) and when stomatal resistance significantly increased for the evaluation of 5 pm (Figure 2B). For plants kept under TS, the activity of SOD increased constantly from the beginning of the experiment until the 15th day comparing to the control plants. After 15 d of stress, this increase was higher, coinciding with the values of water potential considered below the critical threshold (-2.5 MPa), and sudden increases in stomatal resistance and lipid peroxidation. However, in the period between days 18 and 21, there was a decrease in SOD activity which coincided with the highest values of lipid peroxidation.
Among the various enzymes involved in the elimination of ROS, SOD can be considered a key enzyme and is usually the first line of defense mechanism against oxidative stress (Gratão et al., 2005; Pompeu et al., 2008). Water deficit induced a higher activity of SOD, which determines the concentration of O2- and H2O2, being central in the defense mechanisms required to prevent the formation of OH radical (Yoshimura et al., 2000). Analysis by non-denaturing PAGE followed by activity staining, revealed the existence of nine SOD isoenzymes in coffee cells suspension cultures in response to cadmium (Gomes-Júnior et al., 2006). Therefore, we can not dismiss the possibility that the increase in SOD activity in response to water stress may have occurred due to the appearance of other forms of isoenzymes. In this case, not only the variations in activity but also the isoenzymes may be responsible for the production of excess H2O2.
Although SOD is part of the first line of adjustments of the tolerance to oxidative stress, its product, H2O2, is also a ROS as harmful as superoxide. In any case, a higher protection against oxidative damage may require a rapid metabolism of H2O2 generated by the action of SOD. Therefore, for the proper functioning of the detoxification of free radicals, subsequent antioxidant enzymes in the system, such as catalase (CAT) and the ascorbate peroxidase (APX), may work in synchrony to remove H2O2 (Gratão et al., 2005; Pompeu et al., 2008). In this case, CAT (Figure 4B) and APX (Figure 4C) had similar behavior to SOD for plants under both stressful conditions. For plants under total suspension of irrigation, a higher activity after the 15th day aws observed, just when cell damage occurred, as evidenced by lipid peroxidation. In plants under gradual suspension, the greatest damage was observed when there was abrupt decline in water potential between 12 and 21 days.
APX uses ascorbate as the specific electron donor to reduce H2O2 to water, generating monodehydroascorbate, which in turn needs to be regenerated again to ascorbate to maintain the antioxidant system active. For that, other enzymatic reactions are involved, intermediated by glutathione, which is oxidized (Montillet et al., 2004). The maintenance of the pool of reduced glutathione for the process depends on the activity of glutathione reductase (GR). Here, again, GR activity showed similar pattern to that observed for other enzymes under study (Figure 4D).
The increase in activity of the enzymes verified in plants that were under gradual or total suspension of irrigation is due, probably, to the induction of oxidative stress caused by the water deficiency condition. It is known that for coffee plants that the water potential of 2.5 MPa is considered critical (Rena and Maestri, 2000; Grisi et al., 2008).
The capability to maintain, in high levels, the activity of SOD, CAT and APX, under conditions of environmental stress, is essential for maintaining the balance between the formation and the removal of H2O2 from the intracellular environment (Zhang and Kirkam, 1996). However, reduced CAT activity and increased activity of other peroxidases indicate that in plants kept under stress conditions, the H2O2 generated is preferably consumed in oxidative processes, such as peroxidation of lipids, than eliminated by the metabolism (Cakmak et al., 1993).
The increased production of antioxidants, combined with the activity of antioxidant enzymes, seems to be the main strategy to limit lipid peroxidation in plants (Buege et al., 1978). Thus the action of these enzymes, in addition to the action of low molecular weight antioxidants such as ascorbate, may, indeed, eliminate, sweep and immobilize ROS (Gratão et al., 2005). Deuner et al. (2008) showed that in coffee sprayed with H2O2, SOD, CAT, APX and GR activities increased to values above those observed in control plants. Furthermore, after application of ascorbic acid, SOD remained throughout the evaluation period with activity close to that observed in control plants.
In the final assessment the activity of all enzymes in plants under water deficit declined, proportionally to the level of stress. In the case of plants under TS in the penultimate evaluation (18 days), the water potential had exceeded the tolerable value of -2.5 MPa and a higher level of lipid peroxidation, that was accentuated in the last day, had already been observed (Figure 3B). For the plants which the speed of stress was delayed (GS), the water potential only reached the critical value at 21th day, and when compared to control differences were observed regarding lipid peroxidation. Therefore, this decrease in activity of antioxidative enzymes may be due to destruction of the cellular membrane system, proportionally to the stress level, represented by the low water potential and high concentration of hydrogen peroxide, in the penultimate and final assessments of TS and GS, respectively.
Conclusions
Eight months old plants of Catuaí IAC 99 coffee, subjected to the restriction of water in the soil, triggers mechanisms related to stomatal closure and detoxification of free radicals in order to keep the cellular hydration. Until the 15th and 18th days of moderate and severe stress, respectively, there is an increase in stomatal resistance in response to H2O2 elevation, which remained at acceptable levels, counterbalanced by the production of compounds and synchronized action of antioxidant enzymes. Until then, there was then an efficient stomatal control, maintaining a good water status at the expense of the preservation of the membranes integrity. With the persistence of stress, there was an imbalance in favor of production and removal of H2O2 until a critical point when the water potential fell to -2.5 MPa was reached. Thereafter, the priority is the increase in concentrations of H2O2, with its deleterious effects on the peroxidation of lipids, leading to stomata closure, paralyzing the transpiration.
Acknowledgements
To FAPEMIG (Fundação de Amparo a Pesquisa do Estado de Minas Gerais) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico).
Received July 14, 2009
Accepted May 19, 2010
- An, Z.; Jing, W.; Liu, Y.; Zhang, W. 2008. Hydrogen peroxide generated by copper amine oxidase is involved in abscisic acid-induced stomatal closure in Vicia faba Journal of Experimental Botany 59: 815-825.
- Arakawa, N.; Tsutsumi, K.; Sanceda, N.G.; Kurata, T.; Inagaki, C. 1981. A rapid and sensitive method for the determination of ascorbic acid using 4,7-diphenyl-1,10-phenanthroline. Agricultural and Biological Chemistry 45: 1289-1290.
- Azevedo, R.A.; Alas, R.M.; Smith, R.J.; Lea, P.J. 1998. Response from elevated carbon dioxide to air and ozone fumigation in leaves and roots of wild type and a catalase-deficient mutant of barley. Physiologia Plantarum 104: 280-292.
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. 2003. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Annals of Botany 91: 179-194.
- Bradford, M.M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72: 48-254.
- Buege, J.A.; Aust, S.D. 1978. Microsomal lipid peroxidation. Methods in Enzymology 52: 302-310.
- Cakmak, I.; Strbac, D.; Marschner, H. 1993. Activities of hydrogen peroxide-scavenging enzymes in germination wheat seeds. Journal of Experimental Botany 44: 127-132.
- Deuner, S.; Alves, J.D.; Fries, D.D.; Zanandrea, I.; Lima, A.A.; Henrique, P.C.; Goulart, P.F.P. 2008. Hydrogen peroxide and ascorbic acid effects on antioxidant enzyme activity in coffee seedlings. Revista Ceres 55: 134-140. (in Portuguese, with abstract in English).
- Freitas, R.B.; Alves, J.D.; Magalhães, M.M.; Goulart, P.F.P.; Nascimento, M.N.; Fries, D.D. 2007. Coffee tree fertilization with potassium nitrate via leaf and soil, in autumn-winter and spring-summer: effects on nitrate reductase activity, on plant growth and production. Ciência e Agrotecnologia 31: 945-952. (in Portuguese, with abstract in English).
- Giannopolitis, C.N.; Ries, S.K. 1977. Superoxide dismutases. I. Occurrence in higher plants. Plant Physiology 59: 309-314.
- Gomes, I.A.C.; Castro, E.M.; Soares, A.M.; Alves, J.D.; Alvarenga, M.I.N.; Alves, E.; Barbosa, J.P.R.A.D.; Fries, D.D. 2008. Morphophysiological alterations in leaves of Coffea arabica L. cv. 'Oeiras' shaded by Acacia mangium Willd. Ciência Rural 38: 109-115. (in Portuguese, with abstract in English).
- Gomes-Júnior, R.A.; Moldes, C.A.; Delite, F.S.; Pompeu, G.B.; Gratão, P.L.; Mazzafera, P.; Lea, P.J.; Azevedo, R.A. 2006. Antioxidant metabolism of coffee cell suspension cultures in response to cadmium. Chemosphere 65: 1330-1337.
- Gratão, P.L.; Polle A.; Lea P.J.; Azevedo R.A. 2005. Making the life of heavy metal-stressed plants a little easier. Functional Plant Biology 32: 481-494.
- Grisi, F.A.; Alves, J.D.; Castro, E.M.; Oliveira, C.; Biagiotti, G.; Melo, L. 2008. Leaf anatomical evaluations in 'Catuaí' and 'Siriema' coffee seedlings submitted to water stress. Ciência e Agrotecnologia 32: 1730-1736. (in Portuguese, with abstract in English).
- Kohler, B.; Blatt, M.R. 2002. Protein phosphorylation activates the guard cell Ca channel and is a requisite for gating by abscisic acid. Plant Journal 32:185-194.
- Montillet, J.L.; Cacas, J.L.; Garnier, L.; Montane, M.H.; Douki, T. 2004. The upstream oxylipin proWle of Arabidopsis thaliana: a tool to scan for oxidative stresses. Plant Journal 40: 439-451.
- Nascimento, M.N.; Alves, J.D.; Soares, A.M.; Castro, E.M.; Magalhães, M.M.; Alvarenga, A.A.; Silva, G.H. 2008. Biochemical alterations of plants and bud morphology of coffee tree associated to events on flowering in response to meteorological elements. Ciência Rural 38: 1300-1307. (in Portuguese, with abstract in English).
- Oliveira, M.A.J.de; Bovi, M.L.A.; Machado, E.C.; Gomes, M.M. de A.; Habermann, G.; Rodrigues, J.D. 2002. Photosynthesis, stomatal conductance and transpiration in peach palm under water stress. Scientia Agricola 59: 59-63. (in Portuguese, with abstract in English).
- Pereira, S.P.; Guimarães, R.J.; Bartholo, G.F.; Guimarães, P.T.G.; Alves, J.D. 2007. Vegetative growth and yield of coffee plants (Coffea arabica L.) in two different pruning times, conducted at different spacings. Ciência e Agrotecnologia 31: 643-649. (in Portuguese, with abstract in English).
- Pinheiro, H.A.; DaMatta, F.M.; Chaves, A.R.M.; Loureiro, M.E.; Ducatti, C. 2005. Drought tolerance is associated with rooting depth and stomatal control of water use in clones of Coffea canephora Annals of Botany 96: 101-108.
- Pompeu, G.B.; Gratão, P.L.; Vitorello, V.A.; Azevedo, R.A. 2008. Antioxidant isoenzyme responses to nickel-induced stress in tobacco cell suspension culture. Scientia Agricola 65: 548-552.
- Rena, A.B.; Maestri, M. 2000. Water relations in coffee. ITEM 48: 34-41. (in Portuguese).
- Sinha, S.; Saxena, R.; Singh, S. 2005. Chromium induced lipid peroxidation the plants of Pistia stratiotes L.: Role of antioxidants and antioxidant enzymes. Chemosphere 58: 595-604.
- Smirnoff, N. 1993. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytologist 125: 27-58.
- Schroeder, J.I.; Kwak, J.M.; Allen, G.J. 2001. Guard cell abscisic acid signaling and engineering drought hardiness in plants. Nature 410:327-330.
- Suhita, D.; Raghavendra, A.S.; Kwak, J.M.; Vavasseur, A. 2004. Cytoplasmic alkalization precedes ROS production during methyl jasmonate- and abscisic acid-induced stomatal closure. Plant Physiology 134:1536-1545.
- Vasconcelos, A.C.F.; Zhang, X.; Ervin, E.H.; Kiehl, J.C. 2009. Enzymatic antioxidant responses to biostimulants in maize and soybean subjected to drought. Scientia Agricola 66: 395-402.
- Zhang, J.; Kirkam, M.B. 1996. Lipid peroxidation in sorghum and sunflower seedlings as affected by ascorbic acid, benzoic acid and propyl gallate. Journal of Plant Physiology 149: 498-493.
- Zhang, X.; Ervin, E.; Evanylo, G.; Sherony, C.; Peot, C. 2005. Biosolids impact on tall fescue drought resistance. Journal of Residuals Science & Technology 2: 173-180.
- Zhang, X.; Zhang, L.; Dong, F.; Gao, J.; Galbraith, D.W.; Song, C.P. 2001. Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiology 126: 1438-1448.
Publication Dates
-
Publication in this collection
13 Jan 2011 -
Date of issue
Feb 2011
History
-
Received
14 July 2009 -
Accepted
19 May 2010