Abstracts
A retrospective analysis of 24 patients with established osteoporosis and with ten or more years of menopause treated with conjugated estrogen, progesterone and calcium followed for one year has been performed. Treated women received 0.625 mg/day of conjugated estrogen from day 1 to 25, 5 mg/day of medroxiprogesterone from day 13 to 25, of each cycle, plus calcium (500 - 1000 mg/day), during one year (12 cycles). As control group was used 18 age-matched that received only calcium (500 a 1000 mg/day). All patients had at least two dual-photon spine and proximal femur (neck, Ward's triangle and trocanter) densities measurements performed 12 months apart. Estrogen treatment was associated with increased bone mineral density at spine and trocanter. Control group did not present any statistically change after one year in any site studied. We concluded that women with ten or more years of menopause and established osteoporosis treated with replacement hormonal therapy and calcium results in improvement of bone mineral density. These data support that women with ten or more years of menopause respond to estrogen replacement therapy with absolute increments in bone density similar to those seen in younger women, in the early menopause.
established osteoporosis; hormonal replacement therapy; women with ten or more years of menopause
No presente estudo foi realizada uma análise retrospectiva de 24 pacientes com osteoporose estabelecida, com 10 ou mais anos de menopausa, tratadas com estrogênio conjugado, progesterona e cálcio, seguidas por um ano. As mulheres tratadas receberam 0,625mg/dia de estrogênio conjugado do 1º ao 259 dia, 5mg/dia de medroxiprogesterona do 13º ao 25º dia, de cada ciclo, e cálcio (500 a 1000mg/dia), durante um ano (12 ciclos). Como grupo controle foram estudadas 18 mulheres pareadas para idade, peso, altura e anos de menopausa, que receberam apenas cálcio (500 a 1000mg/dia). Todas as pacientes tinham pelo menos duas medidas da densidade óssea na coluna e região proximal do fêmur (colo, triângulo de Ward e trocanter), feitas antes e após um ano. O tratamento com estrogênio foi associado com aumento da densidade óssea na coluna e trocanter. O grupo controle não apresentou qualquer mudança estatisticamente significante após um ano, em nenhum dos locais avaliados. Nós concluímos que, mulheres com osteoporose estabelecida, tratadas com terapia de reposição hormonal e cálcio, mesmo quando iniciada com 10 ou mais anos após a menopausa apresentam aumento da densidade óssea. Estes dados comprovam que mulheres com 10 ou mais anos de menopausa respondem á hormonioterapia com aumento absoluto da densidade óssea de forma semelhante à observada em mulheres mais jovens, nos primeiros anos de menopausa.
ORIGINAL ARTICLE
Do Estrogens improve bone mass in osteoporotic women over ten years of menopause
Vera Lucia Szejnfeld; Jorge Saad Souen; Edmund Chada Baracat; Edgard Atra; Geraldo Rodrigues de Lima
Studies carried out at Escola Paulista de Medicina e Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brasil
Address for correspondence Address for correspondence: Dr. Vera L. Szejnfeld Rua Botucatu, 740 - CEP 04023-000 Disciplina de Reumatologia - Escola Paulista de Medicina São Paulo - SP - Brasil
ABSTRACT
A retrospective analysis of 24 patients with established osteoporosis and with ten or more years of menopause treated with conjugated estrogen, progesterone and calcium followed for one year has been performed. Treated women received 0.625 mg/day of conjugated estrogen from day 1 to 25, 5 mg/day of medroxiprogesterone from day 13 to 25, of each cycle, plus calcium (500 - 1000 mg/day), during one year (12 cycles). As control group was used 18 age-matched that received only calcium (500 a 1000 mg/day). All patients had at least two dual-photon spine and proximal femur (neck, Ward's triangle and trocanter) densities measurements performed 12 months apart. Estrogen treatment was associated with increased bone mineral density at spine and trocanter. Control group did not present any statistically change after one year in any site studied. We concluded that women with ten or more years of menopause and established osteoporosis treated with replacement hormonal therapy and calcium results in improvement of bone mineral density. These data support that women with ten or more years of menopause respond to estrogen replacement therapy with absolute increments in bone density similar to those seen in younger women, in the early menopause.
Key words: established osteoporosis, hormonal replacement therapy, women with ten or more years of menopause.
RESUMO
No presente estudo foi realizada uma análise retrospectiva de 24 pacientes com osteoporose estabelecida, com 10 ou mais anos de menopausa, tratadas com estrogênio conjugado, progesterona e cálcio, seguidas por um ano. As mulheres tratadas receberam 0,625mg/dia de estrogênio conjugado do 1º ao 259 dia, 5mg/dia de medroxiprogesterona do 13º ao 25º dia, de cada ciclo, e cálcio (500 a 1000mg/dia), durante um ano (12 ciclos). Como grupo controle foram estudadas 18 mulheres pareadas para idade, peso, altura e anos de menopausa, que receberam apenas cálcio (500 a 1000mg/dia). Todas as pacientes tinham pelo menos duas medidas da densidade óssea na coluna e região proximal do fêmur (colo, triângulo de Ward e trocanter), feitas antes e após um ano. O tratamento com estrogênio foi associado com aumento da densidade óssea na coluna e trocanter. O grupo controle não apresentou qualquer mudança estatisticamente significante após um ano, em nenhum dos locais avaliados. Nós concluímos que, mulheres com osteoporose estabelecida, tratadas com terapia de reposição hormonal e cálcio, mesmo quando iniciada com 10 ou mais anos após a menopausa apresentam aumento da densidade óssea. Estes dados comprovam que mulheres com 10 ou mais anos de menopausa respondem á hormonioterapia com aumento absoluto da densidade óssea de forma semelhante à observada em mulheres mais jovens, nos primeiros anos de menopausa.
INTRODUCTION
Prevention of postmenopausal bone loss remains a well-recognized indication for estrogen replacement therapy. It has been repeatedly demonstrated that estrogen replacement in either oophorectomized or early postmenopausal women stabilizes spinal bone density by inhibiting bone remodeling and preventing the accelerated bone loss associated with estrogen deficiency (3,7,8,9,11, 14,18,21). Since bone mineral density (BMD) is an important determinant of the risk of fracture, it is not surprising that several case-control studies have associated estrogen replacement in postmenopausal women with reduced rates of hip, wrist, or vertebral fractures (6,10,11.12.18,24). However, the role of estrogens in the treatment of established postmenopausal osteoporosis is not as clear. New studies show that estrogens increases spine density (5,15,17) and, this benefit is likely long lasting (22). The efficacy of estrogen in women with more than 10 or more years of menopause has been uncertain (14) although improved bone mineral density (BMD) in this setting has recently been demonstrated (15,16).
We retrospectively reviewed our clinic-based experience with a treatment for moderate to severe osteopenia using calcium, estrogen and progesterone in women with ten or more than 10 years of menopause. The response to treatment was assessed with measurements of spinal and proximal femur BMD.
PATIENTS AND METHODS
The medical records of all patients with two measurements of spinal and proximal femur BMD by dual-photon absorptiometry (Lunar DPX, Madison, WI) per-formed between 1991 to 1993 at the Climacteric outpatients from Escola Paulista de Medicina were reviewed. Subjects were women with spontaneous menopause and documented osteopenia at spine (BMD < 1.0 g/cm2). Fracture was not necessary for inclusion in the study. Hyperparathyroidism, hyperthyroidism, hysterectomy, oophorectomy, recent use of any drugs known to affect bone metabolism (corticosteroids, calcitonin, diphosphonates, tamoxifen, diuretics, anticonvulsivants, anticoagulants or previous estrogen or progesterone therapy); ab-normal liver or kidney function tests; presence of ostheophytes or calcification of aorta, which would inter-fere with bone density measurements and women without follow-up density measurement at one year constituted exclusion criteria.
All patients had received adequate supplementation with calcium carbonate (500 - 1000 mg/day). Pharmacologic supplementation with vitamin D was not prescribed. It was included in the treated group only Caucasian women receiving cycled therapy with 0.625 mg of conjugated estrogen from 1th to 25th day, 5 mg/ day of medroxyprogesterone from 13th to 25th, each month, during 12 months and calcium (500 a 1000 mg/day). An age-matched group, receiving only calcium, in whom bone density was measured at baseline and 12 months and who met the criteria for the study were randomly selected from our computadorized data bank to serve as controls.
The change in bone density at spine, femoral neck, Ward's triangle and trocanter were calculated by subtracting the baseline measurement from the one year measurement.
Statistical analysis was calculated by using un-paired Student' t-test to compare clinical and mean difference BMD at each site between groups and paired Student's t-test to compare bone mass within each group. The level of statistical significance was defined as p < 0.05.
RESULTS
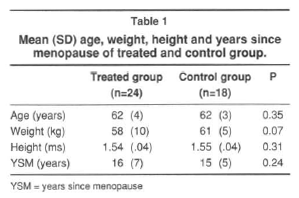
A total of 24 treated women and 18 age-matched control were included in this study. The characteristics of the patients treated with estrogen and the control group are shown in table 1.
The groups did not differ significantly in age, weight, height and years of menopause (table 1). But the control patients had baseline BMD at every site measured greater than the treated group (Table 2).
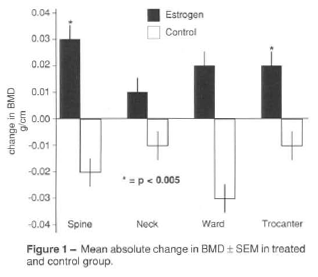
Spine density decreased in 11 of 18 subjects in the control group but the mean change was not statistically significant ( 0.93 ± 0.10 vs. 0.91 ± 0.12 g/cm2; p = 0.14). In treated group BMD increased in 19 of 24 patients (0.85 ± 0.15 vs. 0.89 ± 0.13 g/cm2; p = 0.00006).
The difference between the change in BMD in the treated group compared to control group was significant ( p = 0.003, Figure 1). At the end of the year BMD at spine of the treated group was similar to the control group (0.89 ± 0.13 vs. 0.91 ± 0.12 g/cm2; p = 0.14)
Femoral neck density decreased in 9 of 18 subjects in the control group but the mean change was not statistically significant (p = 0.16). In the treated group BMD increased in 14 of 24 patients but the mean change was not statistically significant (p = 0.13). The mean difference between final and initial BMD of both groups at femoral neck was not statistically significant (p = 0.07, Figure 1). But at the end of the year BMD at femoral neck from treated group was similar to the control group (0.74 ± 0.09 vs. 0.78 ± 0.09 g/cm2; p = 0.06).
Bone mass at Ward's triangle decreased in 10 of 18 control group but the mean change was not statistically different (p = 0.40). The mean difference between change in BMD in the treated and control groups was not significant (p = 0.08, Figure 1). Although after one year there was a significant increase ( p = 0.03) at Ward's triangle density in treated group, the final mean BMD at Ward's triangle from control group remained statistically greater than the treated group (0.64 ± 0.11 vs. 0.58 ± 0.12 g/cm2; p = 0.05).
Trocanteric density decreased in 11 of 18 subjects of control group, but the mean change was not different (p = 0.13). After one year there was a significant increase ( p = 0.001) at trocanter density in treated group. The mean difference between change in BMD in the treated and control groups was significant (p = 0.003, Figure 1) and, after one year BMD from treated group was similar to the controls (0.64 ± 0.10 vs. 0.67 ± 0.08 g/ cm2, respectively; p = 0.16).
DISCUSSION
Treatment with conjugated equine estrogen and progesterone results in increased bone mass in patients with classical postmenopausal osteoporosis after ten years of menopause. The results are particularly evident in the spine and trocanter and less in the femoral neck and Ward's triangle, although the trend is similar at those sites. These results are similar to those published by Civitelli et al (5) who showed that after 12 months of treatment the femoral shaft did not respond as well to estrogen therapy as did lumbar spine, although in that study the authors used double the dose of estrogen used in our study. These data are similar to those published by Lindsay et al (13,14) and Al-Azzawi et al (1), that in a cross-sectional analysis of bone mass in the lumbar spine and femoral neck of patients who participated in a long- term study of the effects of estrogens on cortical bone in oophorectomized showed lesser effect of estrogen on the femoral neck. One possible reason for these results could be due to a slower rate of remodeling at femoral neck (13, 14). However, in the present study the rate of bone loss observed at femoral neck, Ward's triangle and trocanter in control group did not appear to be slower than in the spine so do not support that hypothesis. Nevertheless it is important to note that there was no gain only at femoral neck in the treated group and on the contrary there was a statistically increase in bone mass at
Ward's triangle and trocanter. These differences between the three sites may be occurred because Ward and trocanter have more trabecular bone than the femoral neck and for this reason the answer was similar to that seen at spine. Indeed, the increments seen are presumably related to sudden inhibition of resorption, while formation continued unabated, filling the remodeling space created by the generation of osteoclasts that existed before treatment. Several studies have demonstrated similar effects in young individuals who did not have osteoporosis but who were presumably in a stage of fairly rapid bone loss (1,2,14).
If bone loss is relatively slow the increment in mass is less marked. That we were able to demonstrate a significant increase in mass suggests that our patients were in a state of relatively rapid turnover at the start of the study. As we did not study seric or urinary bone markers of hone remodelation we cannot conclude anything about it. Another reason for differences seen between the two groups could be that there were more patients at high turnover in the treated group than at the control. This idea is supported by the fact that there was not a significant loss at any site in the control group. Another point to be pointed is the fact that the mean bone mass of the control group was greater than the treated group suggesting a high turnover in the treated group.
For patients with established osteoporosis the most important therapeutic outcome is the prevention of fracture. This study does not address that issue but only the effects of therapy on bone mass. We have insufficient power in a study of this size and duration to be able to demonstrate any potential effects on fracture. However, given the relationship between bone mass and fracture prevalence or incidence (23), it is perhaps not unreason-able to expect that estrogen-treated patients would fare better than the control group given only calcium supplementation; this requires examination in larger prospective controlled studies.
The finding that response of the skeleton to estrogen was greater in older population with lower bone mass was seen also by other authors (2,4,15,20), but never been described in Ward and trocanter. Our data suggest that bone loss is ongoing in estrogen-deficient osteoporotic women and that correction of estrogen deficiency at any stage results in at least a slowing on bone loss.
Our data also support that the same dose of estrogen used to prevent bone loss in immediate postmenopausal women is also sufficient to slow the most aggressive bone loss that might be expected in patients with established osteoporosis. We do not know whether the association with progesterone in these patients would have exerted a greater suppressive effect on bone loss in this situation, since progesterone could act directly on bone by engaging an osteoblast receptor or indirectly through com-petition for a glucocorticoid osteoblast receptor (19). More studies using only estrogen without progesterone, with more subjects, during more time need to be done in order to shed light in this new approach of treatment of older women with established osteoporosis.
- 1. AL-AllAWI, F.; HART, D. M. & LINDSAY, R. - Long term effect of oestrogen replacement therapy on bone mass as measured by dual-photon absorptiometry. Br Med J, 294: 1261-1262, 1987.
- 2. BARZEL, U. S. - Estrogens in the prevention and treatment of postmenopausal osteoporosis: a review. Am J Med, 85: 847-850, 1988.
- 3. CHRISTIANSEN, C.; CHRISTIENSEN, M. S.; McNAIR, P.; HAGEN, C.; KNUDERICK, S. & TRANSBOL,I. - Prevention of early postmenopausal bone loss: control led 2-year study in 315 normal females. Eur J Clin Invest, 10: 273-279, 1980.
- 4. CHRISTIANSEN, C. & RIIS, B. J. - 17 beta-estradiol and continuous norethisterone: a unique treatment for established osteoporosis in elderly women. J Clin Endocrinol Metab, 71: 836-841, 1990.
- 5. CIVITELLI, R.; AGNUSDEI, D.; NARDI, P.; ZACCHEI, F.; AVIOLI, L. V. & GENNARI, C. - Effects of one-year treatment with estrogens on bone mass, intestinal calcium absorption, and 25-hydroxyvitamin D-1-alpha-hydroxylase reverse in postmenopausal osteoporosis. Calcif Tissue, Int, 42: 77-86, 1988.
- 6. ETTINGER, B.; GENANT, H. K.& CANN, C. E. - Long term estrogen therapy prevents bone loss and fracture. Ann Intern Med, 102: 319-24, 1985.
- 7. GENANT, H. K.; CANN, C. E.; ETTINGER, B. & GORDAN, G. S. - Quantitative computed tomography of vertebral spongiosa: a sensitive method for detecting early bone loss after oophorectomy. Ann Intern Med, 97: 699-705, 1982.
- 8. HORSMAN, A.; GALLAGHER, J. C.; SIMPSON, M. & NORDIN, B. E. C. -Prospective trial of oestrogen and calcium in postmenopausal women. Br Med J, 2: 789-92, 1977.
- 9. HORSMAN, A.; JONES, M.; FRANCIS, R. & NORDIN, B. E. C. - The effect of estrogen dose on postmenopausal bone loss. N Engl J Med, 309: 1405-1407, 1983.
- 10. HUTCHINSON, T. A.; POLANSKY, S. M. & FEINSTEIN, A. R. - Post-menopausal oestrogens protect against fractures of hip and distal radius: a case-control study. Lancet, 2: 705-709, 1979.
- 11. JENSEN, G. F.; CHRISTIANSEN, C. & TRANSBOL, I. - Fracture frequency and bone preservation in postmenopausal women treated with estrogen. Obstet Gynecol, 60: 493-496, 1982.
- 12. KREIGER, N.; KELSEY, J. L.; HOLFORD, T. R. & O'CONNOR, T. - An epidemiologic study of hip fracture in postmenopausal women. Am J Epidemiol, 116: 141-148, 1982.
- 13. LINDSAY, R.; COUTTS, J. R. T. & HART, D. M.-The effect of endogenous oestrogen on plasma and mineral calcium and phosphate in oophorectomized women. Clin Endocrinol, 6: 87-93, 1977.
- 14. LINDSAY, R.; HART, D. M.; AITKEN, J. M.; MacDONALD, E. B.; ANDERSON, J. & CLARKE, A. C. - Long-term prevention of postmenopausal osteoporosis by oestrogen: evidence for an increased bone mass after delayed onset of oestrogen treatment. Lancet, 1: 1038-1041, 1976.
- 15. LINDSAY, R. & TOHME, J. F. - Estrogen treatment of patients with established postmenopausal osteoporosis. Obstet Gynecol, 76(2): 290-294, 1990.
- 16. MOORE, M.; BRACKER, M.; SARTORIS, D.; SALTMAN, P. & STRAUSE, L. - Long-term estrogen replacement therapy in postmenopausal women sustains vertebral bone mineral density. J Bone Miner Res, 5(6): 659-664, 1990.
- 17. MUNK-JENSEN, N.; NIELSEN, S. P.; OBIEL, E. B.& ERIKSEN, P. B. - Reversal of postmenopausal vertebral bone loss by oestrogen and progesterone: A double-bind placebo controlled study. Br Med J, 296: 1150-1152, 1988.
- 18. NACHTIGALL, L. E.; NACHTIGALL, R. H.; NACHTIGALL, R. D. & BECKMAN, E. M. - Estrogen replacement therapy I: a 10-year prospective study in the relationship to osteoporosis. Obstet Gynecol, 53: 277-281, 1979.
- 19. PRIOR, J. C. - Progesterone as a bone-trophic hormone. Endrocrine Reviews, 11(2): 386-398, 1990.
- 20. QUIGLEY, M. E. T.; MARTIN, P. L.; BURNIER, A. M.& BROOKS, P. - Estrogen arrests bone loss in elderly women. Am J Obstet Gynecol, 156: 1516-1523, 1987.
- 21. RECKER, R. R.; SAVILLE, P. D. & HEANEY, R. P. - Effect of estrogens and calcium carbonate on bone loss in postmenopausal women. Ann Intern Med, 87: 649-655, 1977.
- 22. STEINICHE, T.; HASLING, C.; CHARLES, P.; ERIKSEN, E. F.; MOSEKILDE, L. & MELSEN, F. - A randomized study on the effects of estrogen/gestagen or high dose oral calcium on trabecular bone remodeling in postmenopausal osteoporosis. Bone, 10: 313-320, 1989.
- 23. WAHNER, W. H. - Single and duual-photon absorptiometry in osteoporosis and osteomalacia. Seminars in Nuclear Medicine, 17(4): 305-315, 1987.
- 24. WEISS. N. S.; URE, C. L. & BALLARD, J. H.-Decreased risk of fractures of the hip and lower forearm with postmenopausal use of estrogen. N Engl J Med, 303: 1195-8, 1981.
Publication Dates
-
Publication in this collection
03 July 2009 -
Date of issue
Mar 1994