Abstract
From an ecological point of view, parasites are considered important selective forces that influence all aspects of a host's life. The purpose of this study was to assess the health state of Turdus leucomelas Vieillot, 1818 (Turdidae: Passeriformes) inhabiting a Brazilian Cerrado in the breeding and molting seasons through a hematological parameter analysis and an evaluation of infection by blood parasites. The birds were collected with mist-nets, ringed and blood sampled to assess hematological parameters (hematocrit, hemoglobin concentration and white blood cells) and blood parasites. We detected blood parasites through optical microscopy and subsequently used PCR (amplification of the 18sRNA gene) to verify the presence of Plasmodium spp. (avian malaria). During the breeding season, the hemoglobin level and CHGM percentage were greater. Global leukocyte count was positively related to hemoglobin level, CHGM percentage and body weight. Of the total 31 T. leucomelas individuals examined, 18 presented Plasmodium parasites (58% of prevalence). There was a significant relationship between the presence of Plasmodium spp. and decreased CHGM. These results suggest a connection between the health parameters of wild birds and the physiological stress associated with the breeding season.
Avian health parameters; avian malaria; blood parasites; Brazilian Cerrado
MORPHOLOGY AND PHYSIOLOGY
Hematological and parasitological health conditions of the Pale-breasted Thrush (Turdus leucomelas) (Passeriformes: Turdidae) in southeastern Brazil
Débora N. C. LobatoI; Érika M. BragaII; Nayara de O. BeloII; Yasmine AntoniniIII, 1 1 Corresponding Author. E-mail: antonini.y@gmail.com
IPrograma de Pós Graduação em ECMVS, Departamento de Biologia Geral, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais. debora_bio@yahoo.com.br
IILaboratório de Malária, Departamento de Parasitologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais
IIILaboratório de Biodiversidade, Departamento de Biodiversidade, Evolução e Meio Ambiente, Instituto de Ciências Exatas e Biológicas, Universidade Federal de Ouro Preto. Campus Morro do Cruzeiro, Bauxita, 35400-000 Ouro Preto, MG, Brazil
ABSTRACT
From an ecological point of view, parasites are considered important selective forces that influence all aspects of a host's life. The purpose of this study was to assess the health state of Turdus leucomelas Vieillot, 1818 (Turdidae: Passeriformes) inhabiting a Brazilian Cerrado in the breeding and molting seasons through a hematological parameter analysis and an evaluation of infection by blood parasites. The birds were collected with mist-nets, ringed and blood sampled to assess hematological parameters (hematocrit, hemoglobin concentration and white blood cells) and blood parasites. We detected blood parasites through optical microscopy and subsequently used PCR (amplification of the 18sRNA gene) to verify the presence of Plasmodium spp. (avian malaria). During the breeding season, the hemoglobin level and CHGM percentage were greater. Global leukocyte count was positively related to hemoglobin level, CHGM percentage and body weight. Of the total 31 T. leucomelas individuals examined, 18 presented Plasmodium parasites (58% of prevalence). There was a significant relationship between the presence of Plasmodium spp. and decreased CHGM. These results suggest a connection between the health parameters of wild birds and the physiological stress associated with the breeding season.
Key words: Avian health parameters; avian malaria; blood parasites; Brazilian Cerrado.
From an ecological point of view, parasites are considered important selective forces that influence all aspects of a host's life. The presence of blood parasites, for example, affects the temporal and spatial dynamics of bird communities (HOLMES & PRICE 1986, HOLMES 1996). In birds, a decline in health negatively affects survival, growth (ATKINSON et al. 2000) and consequently reproductive success (HÕRAK et al. 1998, MARZAL et al. 2005, NORTE et al. 2009).
The physiological and biological parameters commonly used to evaluate the health of a bird include hematological exams that check the hemoglobin and hematocrit (for the evaluation of anemia), the rate of leucocytes or white blood cells (infection indicator), and the fraction of heterophile, lymphocytes (stress indicators), as well as weight and morphometric measures. The hematological parameters listed above are widely used to monitor the health of various species of poultry, and are predictive of physiological changes caused by various stress factors (FAIRBROTHER et al. 1990, MAXWELL 1993, MORENO et al. 2002, KILGAS et al. 2006).
Hemoglobin level indicates the oxygen-carrying capacity of the blood (SMITH et al. 2000). In case of nutritional deficiency or exposure to parasites, hemoglobin levels might be reduced (COLES 1984). On the other hand, white blood cell (WBC) counts are an indicator of the state of the immune system: high values may indicate inflammation/infection, whereas low values may indicate immunosuppression (CAMPBELL 1994, 1995).WBC also reflect nestling growth conditions related with brood size (OTS & HÕRAK 1996) and food supply (DUBIEC & CICHOÑ 2001). The heterophil/lymphocyte (H,L) ratio is considered a stress indicator (GROSS & SIEGEL 1983), given that stress accelerates hormone secretion from the adrenal cortex, which is associated with a decrease in lymphocytes and an increase in heterophils (POST et al. 2003).
In addition to blood parameters, the health of a bird can be evaluated through the rate of infection by hemoparasites. Avian malaria is among the most prevalent infections and can be caused by several Plasmodium protozoans, which are blood parasites. Other common hemoparasite genera found in wild birds include Haemoproteus Kruse, 1890, and Leucocytozoon Sambon, 1908 (BENNETT & BORRERO 1976, HOPINKS et al. 1990, GARVIN et al. 1993). Besides birds, Plasmodium spp. parasites also infect reptiles and mammals, including humans, and can be transmitted quite easily between different hosts through blood and consequent inoculation of the infectious forms (VALKIUNAS 2005). It is also known that the health parameters of birds oscillate among the different species according to natural factors such as sex, seasonal cycles and climate (MORENO et al. 1998). Among environmental factors, the breeding season and subsequent feather molt periods can significantly change the physiological conditions of birds (FAIRBROTHER et al. 1990).
Bird health evaluation is commonly performed in species of economic interest, especially in captive birds and poultry birds. Therefore, literature information dealing with the health of wild birds remains limited. Most of the research performed involving the health of birds in the wild has been conducted in temperate environments (EEVA et al. 1997, FAIR & RICKEFS 2002, QUILLFELDT et al. 2004, SHUTLER et al. 2004). Fewer reports are available on the health of wild birds in the tropics, especially in Brazil.
In this contribution we describe the results of a study on the prevalence of species of one genus of hematozoa, Plasmodium. We also describe the hematological parameters in a population of the Pale-breasted Thrush, Turdus leucomelas, Vieillot 1818, inhabiting a Brazilian Cerrado. Additionally, we studied the seasonal fluctuations in the prevalence of hematozoa and variations associated with the breeding and molting periods. This particular species was chosen because it presents a wide geographical distribution in South America (SICK 1997), an aspect that makes it a good model for future studies.
MATERIAL AND METHODS
This study was carried out at the Parque Estadual Rio Preto (PERPRETO), Minas Gerais, Brazil, from October to December 2006 (breeding season) and in March 2007 (molting season). The park has a total area of 10,755 hectares and is located in the city of São Gonçalo do Rio Preto, MG, 18°10'47"S, 43°21'0"W, 355 km northeast of Belo Horizonte and 57 km from Diamantina. The vegetation of the park is dominatedby Brazilian Cerrado.
All bird species were captured using mist-nets, and each received a metallic ring provided by CEMAVE/IBAMA (Ringer License, 905905; Capture and Biological Material Collection License, 158/06). Bird weight was measured using spring scales (Pesola AG from Switzerland) of a 0.1 g of precision. Biological measurements such as presence of feather molt and level of the incubatory plate were also taken. A blood sample was taken within 10 minutes of capture. All other procedures were performed within 30 minutes after capture; each bird was released near the place it was caught.
Individuals were considered to be in their molting period when the following characteristics were present: body molting, rectrices molting and flight molting. To confirm that the feather change was not due to an occasional replacement of feathers lost in an isolated event, the characters above must occur symmetrically on both wings (the same remiges).
The incubatory plate reflects the period in which the bird is incubating the brood. It was classified according to the Ringing Manual of CEMAVE (IBAMA 1994) following a progressive scale from 1 to 5 that characterizes the sequence of development and regression events of the incubatory plate. Females were considered as the individuals who had incubatory plate and eggs.
Approximately 70 ¼l of blood were taken from the brachial vein of each bird into heparinized capillary tubes for later analysis of hemoglobin concentration and hematocrit (%). Blood smears were prepared using approximately 10 ¼L of blood that was air dried, fixed in absolute methanol, and stained for 20 min in 10% Giemsa (Sigma Chemical Co., 21 St. Louis, Missouri, USA) at pH 7.4. This material was later examined in the laboratory using an optic microscope to quantify and classify the leukocyte types (lymphocyte, heterophil, eosinophil, basophil and monocyte) and to check for presence of hemoparasites. The slides were exhaustively examined and parasite density was quantified after examination of 200 microscopic fields (approximately 150 erythrocytes/field) at 1000x magnification under oil immersion (RIBEIRO et al. 2005). With respect to the parasitic infection rate, we used prevalence, which is an estimate of the percentage of birds infected by Plasmodium spp. (BUSH et al. 1997). The remaining blood sample (approximately 20 ¼l) was stored at room temperature (22-25°C) in cell lysis solution (PROMEGA, Madison, Wisconsin, USA) for approximately one day prior the DNA extraction.
Hemoglobin concentration (g/L) refers to the hemoglobin content in red blood cells, and was measured with the cyanmethemoglobin method at a wavelength of 540 nm (CAMPBELL 1994, 1995) using a commercial kit (Sigma Chemical Co., 21 St. Louis, Missouri, USA) in a spectrophotometer. The preparation of blood for storage included the centrifugation of the capillary tubes at 1200 rpm for 10 min (at 4ºC) in order to separate the plasma from the red blood cells. Hematocrit (%) was measured as the percentage of the capillary tube occupied by red blood cells in relation to the total length of the capillary tube occupied by blood after its centrifugation.
With the hematocrit measurement results, it was possible to determine the globular hemoglobin concentration rate (CHGM), which represents the volume occupied by the hemoglobin in each red blood cell. This measure constitutes the most exact index for anemia classification, as it does not require erythrocyte counting in hemocytometers, a method that can have an error of up to 20% (COLES 1984), CHGM = hemoglobin (g/100ml) x 100/ globular volume (hematocrit).
White blood cell count (WBC) was estimated by counting the number of white blood cells per approximately 10.000 red blood cells. Heterophil/lymphocyte ratio (H/L) was measured on the basis of the examination of 50 white blood cells because the repeatability of measurements on 50 and 100 white blood cells was 0.94 ± 0.01 (CAMPBELL 1995, SUTHERLAND et al. 2004). The Atlas of Hematology (LUCAS & JAMROZ 1961, MATOS & MATOS 1988) was used for the identification of each cell type.
DNA samples were processed by nested PCR to amplify genus-specific sequences of the small subunit ribosomal ribonucleic acid (18S SSU rRNA) genes of Plasmodium spp., as previously described (RIBEIRO et al. 2005). The presence of an approximately 240 bp final product was scored as a positive control of infection. It is important to note that the nested PCR assay used here cannot amplify DNA of the genus Haemoproteus and was used to identify only Plasmodium species (RIBEIRO et al. 2005).
Descriptive statistics were used to compute proportions, means, and confidence intervals. For data not conforming to a normal distribution, nonparametric statistics were performed. P values of < 0.05 were considered statistically significant.
The t-test was performed to evaluate significant hematological parameter differences between the two seasons (breeding and molting) and the Mann-Whitney U-test was used for the hemoparasitic parameters. Hematological parameters were tested against weight and presence of plate using linear regression (ZAR 1998, DYTHAM 2003).
The Mann-Whitney U-test was used to compare haematological parameters between infected and non-infected individuals. All tests were performed using the Statistica 6.0 software.
RESULTS
A total of 31 Turdus leucomelas birds were captured, 18 in the breeding season, and 13 in the molting season.
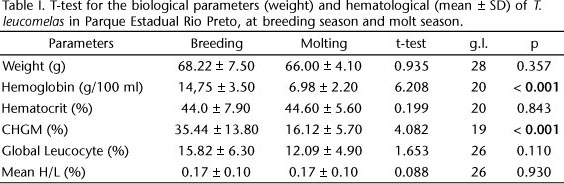
The hemoglobin measure (T20 = 6.208, p < 0.05) and the CHGM percentage (T19 = 4.082, p < 0.05) were greater in the breeding season than in the molting season. The hematocrit percentage, as well as global and differential leukocyte counts, did not significantly differ between seasons (Tab. I).
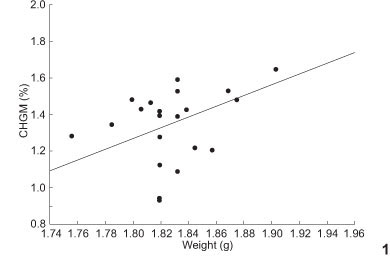
We found a positive relationship between weight and both hemoglobin concentration (R2 = 0.15, P = 0.01) and CHGM (R2 = 0. 23, p < 0.05, Fig. 1), as well as a negative correlation between weight and hematocrit (R2 = 0.12, p = 0.03, Fig. 2). A positive correlation was found between hemoglobin concentration and incubatory plate (R2 = 0.22, p <0.01, Fig. 3).
Body weights were also positively correlated with the plate values (R2 = 0.53, p < 0.05). This relationship was evident in three individuals of T. leucomelas that were at the same time the heaviest and had the highest plate values. These same individuals presented the highest CHGM rates together with a low hematocrit.
Global leukocyte count was positively correlated with hemoglobin rate (R 2 = 0.50, p < 0.05), CHGM percentage (R 2 = 0.60, p < 0.05) and weight (R 2 = 0.47, p < 0.05). No correlations between other parameters and differential leukocytes count were detected.
One of the birds captured in the breeding season had a very red wound on one the wings. The stress rate (H, L) of that bird was the highest found for both seasons (0.657, while the sampled population average was 0.168). It also had the highest hematocrit rate (56%).
Of the total 31 T. leucomelas individuals examined, 18 presented Plasmodium parasites, a 58% prevalence as detected in parallel by microscopy and PCR. The breeding season had the highest occurrence of Plasmodium infection, with 61.1% of the T. leucomelas infected, whereas the molting season presented an infection rate of 53.8%. No significant statistical difference was noticed between the periods. According to results of the statistical analysis, the presence of T. leucomelas does not influence other hematological parameters.
DISCUSSION
Hemoglobin and CHGM levels were significantly higher during breeding season. This probably occurs because there is an increase in energy demand during the reproductive period (oviposition, incubation and parental care energy costs) and consequently an increase of oxygen consumption and the need for hemoglobin and CHGM to transport it. OTS & HÕRAK (1996), HÕRAK et al. (1998) and KILGAS et al. (2006) also noticed that in the breeding season the extra efforts result in an health aggravation.
During the breeding season, females were the heaviest individuals because their eggs were in the formation stage. Weight had a positive correlation with global leukocyte rates. Females under rising reproductive effort stress can have their health conditions decreased and their H, L rates raised (MORENO et al. 2002, WILLIAMS 2005). In the current study, an increase in H, L ratio was not found for the breeding season, but higher global leukocyte rates can also be an indication of female stress in this season (CLINCHY et al. 2004). MACHADO-FILHO et al. (2010) found a significant global leukocyte rate increase for Elaenia spp. (Tyrannidae) in the breeding season. One of the individuals we analyzed can serve as an example of the relationship between stress and high global leukocyte rates: the highest leukocyte rate from our sample population was measured in a bird that had a wounded wing and tarsus, probably the result of a predator attack.
Females have a tendency to lower hematocrit values since estrogen inhibits erythropoiesis (red blood cell synthesis). In contrast, androgen, the male hormone, stimulates this process (DAWSON & BARTOLOTTI 1997, PERRY et al. 2000). This probably explains why the hematocrit rates decrease with an increase in weight, because the heaviest individuals were females. It has been previously reported that lower hematocrit values can occur due to a decrease in the number of erythrocytes (e.g. in case of anemia or blood loss) (MATOS & MATOS 1988); but it can also be related to hemoparasite infection, body injuries, stress, intoxication (MATOS & MATOS 1988) or poor health conditions caused by molting or migration (YOUNG et al. 1993). However, we did not find any correlation between these parameters.
The prevalence of Plasmodium in the individuals studied was considered high when compared with the results of studies conducted in other tropical regions. FECCHIO et al. (2007), sampling in the Brazilian Cerrado, found a prevalence of 1.4%. In three different regions of Colombia, MATTA et al. (2004) also found low Plasmodium parasitism rates in wild birds (2%). VALKIUNAS et al. (2004) reported a similar result of 0.6% for wild birds in a Puerto Rican conservation area. In these studies, only optic microscopy was used. The use of microscopy to diagnose chronic infections with low level of parasitemia can underestimate parasite presence (JARVI et al. 2002). Therefore, one of the factors that might have contributed to the high infection rates found in the Turdus leucomelas population in PERPRETO is the greater sensibility and accuracy of the method that associates PCR and microscopic observation. In agreement with our results, RIBEIRO et al. (2005) evaluated the Plasmodium prevalence in several wild bird species from the Brazilian Atlantic Forest in the state of Minas Gerais using microscopy and PCR, and found hemoparasite occurrences of 7.3% and 34.3%, respectively.
SEBAIO et al. (2010) found 23.1% of T. leucomelas, and 44.4% of T. rufiventris birds infected with Plasmodium. Their results are similar to ours. Differences may be due to species-specific susceptibility to infection associated with genetics, behavior, exposure to parasites (MARINI et al. 1996) and environmental (LEE et al. 2006) factors. Species of Turdidae have a tendency to be more susceptible to Plasmodium infection. According to VALKIUNAS (2005) Turdidae is one of the families with the highest prevalence of infection among Holartic Passeriformes. One of the reasons mentioned by the author is the size of individuals in this family, which makes them more attractive and more convenient for hematophagous vector feeding.
During the reproductive season, the prevalence rate tended to be higher than in the molt season. If infection causes a decrease in health a decrease in all hematological parameters is to be expected in the breeding season. It has been postulated that seasonal variation in hemoparasite prevalence is related to intrinsic factors of the reproductive cycle (BENNETT et al. 1982, DEVICHE et al. 2001, GARVIN & GREINER 2003). This occurs because the greater effort and energy demands of the reproductive period conflicts with the energy necessary to maintain the organism, compromising the immune system and individual health. Therefore, a rise in hemoparasitic infection is expected. HÕRAK et al. (1998) noticed that an increase of brood size results in more energy expenditure and, as a consequence, brings an immune system deficit. Similarly, VALKIUNAS (2005) suggested that nesting females are less active on the nest and can be more easily infected by vectors.
Our study does not confirm that hemoparasite infection can cause changes in the hematological parameters of T. leucomelas birds. The pathogenicity of blood parasites in wild birds has been difficult to demonstrate because of the ability of hosts to maintain infections below a threshold at which effects become apparent (MERINO et al. 2000, MARZAL et al. 2005). However, due to the small sample size of the experiment and large residual variation in some of the parameters, our results must be interpreted cautiously. Knowing the extent to which hemoparasite infection can cause changes in the hematological parameters birds is important to fully understand the ecological relationships and the impact of these infections on natural bird populations in tropical environments.
ACKNOWLEDGEMENTS
We are grateful to Marcos Callisto, Miguel Marini and Nelson Martins for their comments on earlier versions of the manuscript. Júlio Fontenelle helped us with statistical analysis and Jonathan Macedo was our English native reviewer. Felipi Cristóvão and Alexandre Enout helped us in the field, and Márcia Campos assisted us in the laboratory. We also thank IEF, Antônio Tonhão de Almeida (park manager) and his staff. Rogério P. Martins for allowing the use of the dependencies of his laboratory at UFMG. This research was undertaken during the master dissertation of DNCL at the Graduate Program in Ecology, Conservation and Management of Wildlife at the Universidade Federal de Minas Gerais, Brazil. This program is supported by Capes, US Fish, CNPq and FAPEMIG.
LITERATURE CITED
Submitted: 03.II.2011; Accepted: 13.X.2011.
Editorial responsibility: Carolina Arruda Freire
- ATKINSON, C.T.; R.J. DUSEK; K.L. WOODS & W.M. IKO. 2000. Pathogenicity of avian malaria in experimentally-infected Hawaii Amakihi. Journal of Wildlife Diseases 36: 197-204.
- BENNETT, G.F. & H.J.I. BORRERO. 1976. Blood parasite of some birds from Colombia. Journal of Wildlife Diseases 12: 454-458.
- BENNETT, G.F.; M.A. WHITEWAY & C.B. WOODWORTH-LYNAS. 1982. Host-Parasite Catalogue of the Avian Haematozoa. Memorial University of Newfoundland, Occasional Papers in Biology, vol. 5, 243p.
- BUSH, A.O.; K.D. LAFFERTY; J.M. LOTZ & A.W. SHOSTAK. 1997. Parasitology meets ecology on its own terms, Margolis et al. revisited. Journal of Parasitolology 83: 575-583.
- CAMPBELL, T.W. 1994. Hematology, p. 176-199. In: B. W. Ritchie, G.J. Harrison & L.R. Harrison (Eds). Avian medicine, principles and applications. Florida Lake Worth, Wingers Publishing, 424p.
- CAMPBELL, T.W. 1995. Avian hematology and cytology. Iowa, Iowa State University Press, 369p.
- CLINCHY, M.; L. ZANETTE; R. BOONSTRA; J.C. WINGFIELD & J.N.M. SMITH. 2004. Balancing food and predator pressure induces chronic stress in songbirds. Proceedings of the Royal Society of London. Series B: Biological Sciences 271: 2473-2479.
- COLES, E.H. 1984. Patologia clínica veterinária São Paulo, Editora Manole, 566p.
- DAWSON, R.D. & G.R. BORTOLOTTI. 1997. Total plasma protein level as an indicator of conditions in wild American Kestrels (Falco sparvarius). Canadian Journal of Zoology 75: 680-686.
- DEVICHE, P.; E.C. GREINER & X. MANTECA. 2001. Seasonal and age-related changes in blood parasite prevalence in dark-eyed juncos (Junco hyemalis, Aves, Passeriformes). Journal of Experimental Zoology 289: 456-466.
- DUBIEC, A. & M. CICHOÑ. 2001. Seasonal decline in health status of Great Tit (Parus major) nestlings. Canadian Journal of Zoology 79: 1829-1833.
- DYTHAM, C. 2003. Choosing and using statistics - A biologist's guide. Malden, Blackwell Science, 248p.
- EEVA, T.; E. LEHIKOINEN & C. SUNELL. 1997. The quality of pied flycatcher (Ficedula hypoleuca) and great tit (Parus major) females in an air pollution gradient. Annales Zoologici Fennici 34: 61-71.
- FAIR, J.M. & R.E. RICKLEFS. 2002. Physiological, growth, and immune responses of Japanese quail chicks to the multiple stressors of immunological challenge and lead shot. Archives of Environmental Contamination and Toxicology 42: 77-87.
- FAIRBROTHER, A.; M.A. CRAIG; K.WALKER & D. O'LOUGHLIN. 1990. Changes in mallard (Anas platyrhynchos) serum chemistry due to age, sex, and reproductive condition. Journal of Wildlife Diseases 26: 67-76.
- FECCHIO, A.; M.A. MARINI & E.M BRAGA. 2007. Baixa prevalência de hemoparasitos em aves silvestres no Cerrado do Brasil Central. Neotropical Biology and Conservation 2: 127-135.
- GARVIN, M.C. & E.C. GREINER.2003. Epizootiology of haemoproteus danielewskyi in blue-jays (Cyanocitta cristata) in southcentral Florida. Journal of Wildlife Diseases 39: 1-9.
- GARVIN, M.C.; J.V. REMSEN; M.A.BISHOP & J.F.BENNETT. 1993. Hematozoa from passeriform birds in Louisiana. Journal of. Parasitology 79: 318-321.
- GROSS, W.B. & H.S. SIEGEL. 1983. Evaluation of the heterophil/lymphocyte ratio as a measure of stress in chickens. Avian Diseases 27: 972-979.
- HOLMES, J.C. 1996. Parasitism and threats to biodiversity in shrinking ecosystems. Biodiversity and Conservation 5: 975-983.
- HOLMES, J.C. & P. PRICE. 1986. Communities of parasites, p. 975-983. In: D.J. ANDERSON & J. KIKKAWA (Eds). Community ecology, patterns and processes. Oxford, Blackwell Scientific Publications, 1142p.
- HOPINKS, B.A.; J.K. SKEELES; G.E. HOUGHTEN; D. SLAGLE & K. GARDNER. 1990. A survey of infectious diseases in wild turkeys (Meleagridis gallopavo silvestris) from Arkansas. Journal of Wildlife Diseases 26: 468-472.
- HÕRAK, P.; I. OTS & A. MURUMAGI. 1998. Haematological healt state indices of reproducing Great Tits, a response to brood size manipulation. Ecology 12: 750-756.
- IBAMA. 1994. Manual de anilhamento de aves silvestres. Brasília, Instituto Brasileiro do Meio Ambiente e dos Recursos Renováveis, Ministério do Meio Ambiente e da Amazônia Legal, 146p.
- JARVI, S.L.; J.J SCHULTZ & C.T. ATKINSON. 2002. PCR diagnostics underestimate the prevalence of avian malaria (Plasmodium relictum) in experimentally-infected passerines. Journal of Parasitology 88: 153-158.
- KILGAS, P.; R. MAND; M. MAGI & V.TILGAR. 2006. Hematological parameters in brood-rearing great tits in relation to habitat, multiple breeding and sex. Comparative Biochemistry and Physiology 114: 224-231.
- LEE, K.A.; L.B. MARTIN; D. HASSELQUIST; R.E. RICKLFS & M. WIKELSKI. 2006. Contrasting adaptive immune defenses and blood parasite prevalence in closely related Passer sparrows Oecologia 150: 383-392.
- LUCAS, A.M. & C. JAMROZ. 1961. Atlas of avian hematology. Washington, DC, United States Department of Agriculture, 271p.
- MACHADO-FILHO, R.A.N.; G.M. BALSAMÃO & M.Â. MARINI. 2010. Seasonal differences in immune profiles and body conditions of migratory and permanent resident Neotropical flycatchers. Condor 112: 579-590.
- MARINI, M.A.; B.L. REINERT; M. BORNSCHEIN; J.C. PINTO & M. PICHORIM. 1996. Ecological correlates of ectoparasitism in birds from the Atlantic Forest, Brazil. Ararajuba 4: 93-102.
- MARZAL, A.; F. DE LOPE; C. NAVARRO & A.P. MOLLER. 2005. Malarial parasites decrease reproductive success, an experimental study in a passerine bird. Oecologia 142: 541-545.
- MATOS, M.S. & P.F. MATOS. 1988. Laboratório clínico médico-veterinário. São Paulo, Editora Atheneu, 342p.
- MATTA, N.E.; N. BASTO; R. GUTIERREZ; O.A. RODRÍGUES & E.C. GREINER. 2004. Prevalence of blood parasites in Tyrannidae (Flycatchers) in the Eastern Plains of Colombia. Memórias do Instituto Oswaldo Cruz 99: 271-274.
- MAXWELL, M.H. 1993. Avian blood leukocyte responses to stress. World Poultry Science 49: 34-43.
- MERINO, S.; J. MORENO; J.J. SANZ & E. ARRIERO. 2000. Are avian blood parasites pathogenic in the wild? A medication experiment in Blue Tits (Parus caeruleus). Proceedings of the Royal Society of London 267: 2507-2510.
- MORENO, J.; A. LEON; J.A. FARGALHO & E. MORENO. 1998. Breeding time, health and immune response in tne chinstrap penguin Pygoscelis antarctica Oecologia 115: 312-319.
- MORENO, J.; S. MERINO; J.J. SAZ & E. ARRIERO. 2002. An indicator of maternal stress immunity in Magellanic penguins Spheniscus magellanicus Annales Zoologici Fennici 38: 111-116.
- NORTE, A.C.; M.M. ARAÚJO; H.L. SAMPAIO; J.P. SOUSA & J.A. RAMOS. 2009. Haematozoa infections in a great tit Parus major population in Central Portugal, relationship with breeding effort and health. Ibis 151: 677-688.
- OTS, I. & P. HORAK. 1996. Great tits Parus Major trade for reproduction. Proceedings of the Royal Society of London 263: 1443-1447.
- PERRY, M.J.; A. SAMUELS; D. BIRD & J.H. TOBIAS. 2000. Effects of high-dose estrogen on murine hematopoietic bone marrow precede those on osteogenesis. American Journal of Physiology, Endocrinology and Metabolism 279: 1159-1165.
- POST, J.; J.M.J. REBEL & A.A.H.M. TER HUURNE. 2003. Automated blood cell count: a sensiteve and reliable method to study corticosterone-related stress in broilers. Poutry Science 82: 591-595.
- QUILLFELDT, P.; J.F. MASELO & E. MOSTL. 2004. Blood chemistry in relation to nutrition and ectoparasite load in Wilson's storm-petrels Oceanites oceanicus Polar Biology 27: 168-176.
- RIBEIRO, S.F.; F. SEBAIO; F.C.S. BRANQUINHO; M.A. MARINI; A.R. VAGO & E.M. BRAGA. 2005. Avian malaria in Brazilian passerine birds, parasitism detected by nested PCR using DNA from stained blood smears. Parasitology 130: 261-267.
- SEBAIO, F.; E.M. BRAGA; F. BRANQUINHO; L.T. MANICA & M.A. MARINI. 2010. Blood parasites in Brazilian Atlantic Forest birds, effects of fragment size and habitat dependency. Bird Conservation International 20 (4): 432-439. doi:10.1017/S0959270910000110.
- SHUTLER D.; A. MULLIE & R.G. CLARK. 2004. Tree swallow reproductive investment, stress, and parasites. Journal of Zoology 82: 442-448.
- SICK, H. 1997. Ornitologia brasileira. Rio de Janeiro, Editora Nova Fronteira, 912p.
- SMITH, F.M.; N.H. WEST & D.R. JONES. 2000. The cardiovascular system, p. 141-231. In: G.C. WHITTOW (Ed). Sturkie's avian physiology. San Diego, Academic Press, 592p.
- SUTHERLAND, W.J.; I. NEWTON & R.E. GREEN. 2004. Bird ecology and conservation - A handbook of techniques. Oxford, Oxford University Press, 386p.
- VALKIUNAS, G. 2005. Avian malaria parasites and other Haemosporidia. Boca Raton, CRC Press, 932p.
- VALKIUNAS, G.; T.A. IEZHOVA; D.R. BROOKS; B. HANELT; S.V. BRANT; M.E. SUTHERLIN & D. CAUSEY. 2004. Additional observations on blood parasites of birds in Costa Rica. Journal of Wildlife Diseases 40: 555-561.
- WILLIAMS, T.D. 2005. Immune defence and host life history. American Naturalist 160: 9-22.
- YOUNG, B.E.; M.C. GARVIN & D.B. MCDONALD. 1993. Blood parasites in birds from Monteverde, Costa Rica. Journal of Wildlife Diseases 29: 555-560.
- ZAR, J.H. 1998. Biostatistical analysis. New Jersey, Prentice Hall, 929p.
Publication Dates
-
Publication in this collection
17 Jan 2012 -
Date of issue
Dec 2011
History
-
Received
03 Feb 2011 -
Accepted
13 Oct 2011