Abstract
The objectives of the present study were to identify species of reef fish that use the Rio Formoso estuary (northeastern Brazil) as a refuge and natural nursery and to describe the spatial distribution of the estuary fish fauna in rainy and dry seasons. A total of 5475 specimens, across 78 species and 39 families, were analyzed; 51.3% of the species were of reef origin. Among these, Eucinostomus melanopterus (Bleeker, 1863), E. gula (Cuvier, 1830), and Sphoeroides testudineus (Linnaeus, 1758), in this order, were the most abundant in the upper estuary; S. greeleyi Gilbert, 1900, E. melanopterus, and Lutjanus synagris (Linnaeus, 1758) were the most abundant in the mid estuary; and E. gula and Albula vulpes (Linnaeus, 1758) in the lower estuary. The percentages of reef species were 39.2%, 54.2% and 66.7% for the upper, mid and lower regions, respectively. The greatest diversity was found in the upper estuary and greatest abundance occurred in the mid region. The type of sediment was a strong determinant of the spatial distribution of fish fauna. The most abundant species were found in a mesohaline (5-18) to euhaline (30-40) salinity regimen, suggesting some capacity for osmotic regulation. The fish fauna of the Rio Formoso estuary receives a direct influence from the reefs and coastal region between Sirinhaém and Tamandaré, thereby providing a greater richness of reef fish.
Connectivity; estuary; fish community; reef fishes; spatial variation
ECOLOGY
Spatial distribution of the estuarine ichthyofauna of the Rio Formoso (Pernambuco, Brazil), with emphasis on reef fish
Andréa C. G. de Paiva I; Marina F. V. Lima I; José R. B. de SouzaII; Maria E. de AraújoI
ILaboratório Nécton e Aqüicultura, Departamento de Oceanografia, Universidade Federal de Pernambuco. Avenida Arquitetura, Cidade Universitária, 50740-550 Recife, Pernambuco, Brasil. E-mail: acg_paiva@yahoo.com.br; betharau08@gmail.com
IILaboratório de Comunidades Marinhas, Departamento de Zoologia, Universidade Federal de Pernambuco. Avenida Professor Moraes Rego, Cidade Universitária, 50670-420 Recife, Pernambuco, Brasil. E-mail: jrbsouza@ufpe.br
ABSTRACT
The objectives of the present study were to identify species of reef fish that use the Rio Formoso estuary (northeastern Brazil) as a refuge and natural nursery and to describe the spatial distribution of the estuary fish fauna in rainy and dry seasons. A total of 5475 specimens, across 78 species and 39 families, were analyzed; 51.3% of the species were of reef origin. Among these, Eucinostomus melanopterus (Bleeker, 1863), E. gula (Cuvier, 1830), and Sphoeroides testudineus (Linnaeus, 1758), in this order, were the most abundant in the upper estuary; S. greeleyi Gilbert, 1900, E. melanopterus, and Lutjanus synagris (Linnaeus, 1758) were the most abundant in the mid estuary; and E. gula and Albula vulpes (Linnaeus, 1758) in the lower estuary. The percentages of reef species were 39.2%, 54.2% and 66.7% for the upper, mid and lower regions, respectively. The greatest diversity was found in the upper estuary and greatest abundance occurred in the mid region. The type of sediment was a strong determinant of the spatial distribution of fish fauna. The most abundant species were found in a mesohaline (5-18) to euhaline (30-40) salinity regimen, suggesting some capacity for osmotic regulation. The fish fauna of the Rio Formoso estuary receives a direct influence from the reefs and coastal region between Sirinhaém and Tamandaré, thereby providing a greater richness of reef fish.
Key words: Connectivity; estuary; fish community; reef fishes; spatial variation.
Fish constitute around 99% of the nektonic species in estuarine environments, where they transform the energetic potential of detritus, carrying energy from the lowest to the highest trophic levels, allowing exchanges between neighboring ecosystems and storing energy by entering estuaries, where they remain throughout part of their lives (ARAÚJO et al. 2004).
The community of estuarine fish is usually composed of resident, marine migrant, and freshwater species (ALBARET & ALBARET 1994). These species use the estuary during one the phases of their lives as a site for feeding, reproduction, and rearing larvae and juveniles (DAY JR et al. 1989, BLABER 2000). When located near reefs, estuarine systems present high abundance of young fish of reef origin (MUMBY et al. 2004, DORENBOSCH et al. 2004, MUMBY 2006), which use shallow habitats in the estuaries, such as marine phanerogam meadows and mangroves, as nurseries (DORENBOSCH et al. 2004) and shelter (SHULMAN 1985). In these environments, these fish find a broad variety of feeding resources during their growth (WERNER & GILLIAM 1984), particularly due to the elevated primary production and consequent increased secondary production (ROBERTSON & BLABER 1992).
The distribution of estuarine ichthyofauna can be defined based on abiotic factors such as salinity, temperature, turbidity, and dissolved oxygen (BLABER 2000). Seasonal oscillations and the physicochemical parameters in the estuaries determine the permanence (or not) of populations during their life cycle (CHAVES & OTTO 1999). Some species migrate to regions closer to the mouth or farther upstream in response to variation in salinity (BLABER & BLABER 1980, BLABER et al. 1989, DAY JR et al. 1989); a few species move from shallow to deeper waters due to chances in temperature, or to the ocean, where the hydrological conditions are more stable (BLABER et al. 1990, ALBERT & BERGSTAD 1993, LEKVE et al. 1999). The type of substrate is one of the main determining factors of ecological characteristics in lake and estuarine systems (WEINSTEIN 1982, ROSS & EPPERLY 1985). The spatial distribution of fish communities in each estuary can vary according to the characteristics of the sediment and the heterogeneity in the substrate, as well as the presence of vegetation, which affects prey availability (BLABER & BLABER 1980, GARCIA & VIEIRA 1997, MARSHALL & ELLIOT 1998, VIEIRA et al. 1998).
Through an understanding of the ecology organisms, one can improve the management and conservation of these renewable resources (LOEBMANN & VIEIRA 2005) and regional faunistic surveys are crucial for establishment coastal monitoring programs (MORGADO & AMARAL 1989). Even though it is located within two Areas of Environmental Protection (AEP), the estuarine complex of the Rio Formoso has experienced severe impacts due to the indiscriminate use of its fisheries and by the environmental pressure from urban centers, agriculture, and shrimp farming (CPRH 2008).
Given this scenario, the goals of the present study are (1) to identify the species of reef fish that use the estuary of the Rio Formoso as an area of natural nursery and shelter, and (2) to describe the spatial distribution of the marine ichthyofauna across two seasons (dry and rainy), correlating the observed patterns with environmental variation.
MATERIAL AND METHODS
The present study was carried out in the Rio Formoso (12 km), which begins in the northwestern region of the municipality of Rio Formoso, in the state of Pernambuco, Brazil (08º37'-08º40'S, 035º04'-035º08'W) (CONDEPE 1992) (Fig. 1). This municipality is part of two AEPs: the State AEP of Guadalupe and the Federal AEP Costa dos Corais (CPRH 2008). LIRA et al. (1979) suggested the division of the estuary of the Rio Formoso into three morphologically distinct regions: upper, mid, and lower. The upper region of formed by tidal creeks and channels, up to 2 m in depth, and its banks are densely colonized by mangrove forests and sandy/muddy deposits. The mid region is broader and less colonized by mangrove forests, with stretches intercalated with coconut palms. The right margin of this region, from the mouth of the Rio Ariquindá and the Rio dos Passos, is shallow (2-3m), with sandy banks that emerge during low tides (CPRH 2008). The lower estuarine region encompasses the broadest and deepest segment of the estuary, being characterized by the absence of mangroves, by the presence of a broad sandy bank that emerges during low tides, and by the proximity to the reefs located in the Praia dos Carneiros (LIRA et al. 1979). The climate in the region, according to the classification of KÖPPEN (1948), is the tropical As', with mean annual precipitation of 2050 mm, with May, June, July being rainiest months and October, November, and December being the driest months (CPRH 2008).
Samples were obtained in each of the three regions in the estuary of the Rio Formoso. Fishing sites and gear that are traditionally used by local fishermen were chosen in each of the zones, as indicated by the fishermen themselves. The sampled areas in the upper, mid, and lower regions included the Região dos Paus (08º40.09'S; 035º07.54'W and 08º40.19'S; 035º07.59'W), the Boca de Camboa (08º40.92'S; 035º06.86'W and 08º41.12'S; 035º06.73'W) and the Praia dos Carneiros (035º05.40'W and 08º41.48'S; 035º05.64'W), respectively (Fig. 1).
Diurnal collections in each estuarine region were carried out bimonthly between October, 2005 and August, 2006. Three consecutive trawls were using a beam trawl net (22 x 2 m) and a mesh size of 8 mm between adjacent knots. The net was trawled manually and perpendicularly to the margin of the estuary, at a depth between 2.0 and 0 m for approximately eight min per trawl.
Each fish was fixed in the field with 10% formaldehyde. In the laboratory, all specimens were identified, counted, weighted, measured, and preserved in 70% ethanol. Species identification followed for the most part the diagnoses of ARAÚJO et al. (2004), CERVIGÓN (1991, 1993, 1994, 1996), FIGUEIREDO & MENEZES (1978; 1980; 2000), MENEZES (1983), MENEZES & FIGUEIREDO (1980, 1985), according to the phylogenetic organization cited in MENEZES et al (2003) and NELSON (2006). Specimens were deposited in the Ichthyological Collection of the Departamento de Oceanografia, Universidade Federal de Pernambuco, under voucher numbers 1390-1476.
Data on water temperature (ºC), salinity, pH and dissolved oxygen (ml.l-1) were obtained at a depth of approximately 60 cm simultaneously to specimen collections. Sediment samples (three replicates) were obtained in each of the estuary regions, during the low tide, using a PVC corer (10 x 10 cm), both in the dry and in the rainy seasons. The granulometric analysis followed the method described by SUGUIO (1973). Sediment classification followed the Oceanographic Atlas of the North Atlantic Ocean (1965) based on the measured mud content (silt and clay). Tides were determined based on the tidal chart of the Porto de Suape (Pernambuco) made available in the website of the Diretoria de Hidrografia e Navegação (DHN).
Species were classified into six groups based on their spatial location: Group I, found only the upper estuary zone; Group II, found only in the mid estuary region; Group III, found only in the lower estuarine region; Group IV, found in both the upper and mid regions; Group V, found in the mid and lower regions; and Group VI, found in all regions.
According to the mode of occupation, species were classified into seven categories: resident, marine-dependent, migrants in trophic ecophase, marine visitors, marine migrants, occasional, and freshwater visitors. These categories were based on the studies by DEEGAN & THOMPSON (1985), VILLARROEL (1994), ELLIOT & DEWAILLY (1995), VASCONCELOS-FILHO & OLIVEIRA (2000), GARCIA & VIEIRA (2001), BARLETTA et al. (2003), and CHAVES & BOUCHEREAU (2004). The classification of reef fish was based mostly on HUMANN & DELOACH (2002). Reef fishes were those that live in association with the reefs (SALE 2002).
To determine the most representative species in each estuarine region, species were considered to be among the most abundant if their density corresponded to at least 10% of all captured fishes. This proportion was maintained for all reef fishes, as long as the absolute number of specimens for the species was equal or larger than 30 individuals.
A similarity analysis was carried out using the software Primer 5.1.2 based on the Bray-Curtis coefficient and the UPGMA algorithm as the clustering method (CLARK & WARWICK 1994). The data matrix for this analysis was build using the values of the Relative Importance Index (RII%), including species with indices above 100 in at least one of the estuary regions. The calculation of the RII% used data on the frequency of occurrence (FO%), numeric percentage (NP%), and biomass percentage (BP%) for each species using the formula IIR = FO% x (NP% + BP%), in which FO% corresponds to the ratio between the number of times a given species occurred in the analyzed samples and the total number of samples per estuary region; NP%, the ratio between the number of individuals of a species and the total number of collected individuals per region; and BP%, the ratio between the biomass of individuals of a species and the total biomass of the collected individuals per estuarine region (VIEIRA et al. 1996).
The species diversity was calculated using the diversity index of Shannon-Wiener (H' = å pi log2 pi), and tested using an ANOVA. The homogeneity of variances of the variables was determined using the Bartlett test. The Tukey test was then applied at a confidence level of 95% (p<0.05) to determine which variables were statistically different.
The Pearson correlation test was used to assess the relationship between the ichthyofauna and environmental variables by comparing species richness, diversity (Shannon and Margalef), and evenness (Pielou).
RESULTS
A total of 5475 specimens, belonging to 78 species and 39 families, were collected in all three regions of the estuary of the Rio Formoso. More than half of those species (51.3%) are of reef origin and are juveniles (Tab. I).
Thirteen species were captured only in the upper estuary region, nine in the mid estuary, and six in the lower estuary; 23 species were found both in the upper and mid regions, 12 in the mid and lower regions, and 15 species occurred in all regions. With respect to the occupation status, there were 15 residents, 15 marine-dependents, 22 marine visitors, 10 marine migrants in trophic ecophase, two occasional, one freshwater visitor, and 12 were unclassified (Fig. 2). The obtained proportions of reef fishes were 39.2%, 54.2%, and 66.7% in the upper, mid, and lower regions, respectively.
The species richness varied from 51 species in the upper, 50 in the mid, and 33 in the lower estuary regions, whereas the number of reef fishes in each region was 20, 32, and 22, respectively. There were no statistically significant differences in (alpha) diversity in the upper region (F(5, 12) = 1.246; p = 0.347), as well as in the mid region (F(5, 12) = 2.107; p = 0.135). On the other hand, there were significant differences among the collection months in the lower region (F(5, 12) = 6.494, p = 0.004). In that region, diversity (H') was significantly higher in December than in July and August; likewise, diversity in February was higher than in June (Tukey test, MS = 0.298, d.f. = 12) (Fig. 3). In addition, the species diversity per sample was highest, on average, in the upper estuary region. One can observe in that figure that the diversity (H') varies the most among regions in the rainy season and in part of the dry season.
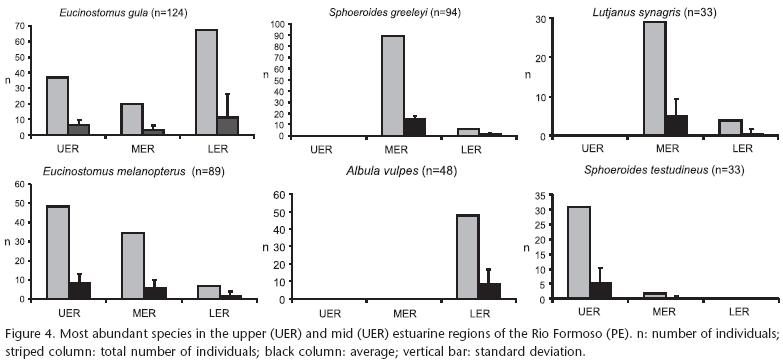
Diapterus rhombeus (Cuvier, 1829) and C. edentulus (Cuvier, 1829) were the most abundant species in the upper estuarine region, as were R. amazonica (Steindachner, 1879), A. clupeoides (Swainson, 1839), and H. clupeola (Cuvier, 1829) in the mid region (Fig. 4). Of the reef species, E. melanopterus (Bleeker, 1863), E. gula (Cuvier, 1830), and S. testudineus (Linnaeus, 1758) are noteworthy, in that order, as the most abundant in the upper estuary region, as were S. greeleyi Gilbert, 1900, E. melanopterus, and L. synagris (Linnaeus, 1758), in the mid region; and E. gula and A. vulpes (Linnaeus, 1758) in the lower region (Fig. 5).
Two main groups could be identified based on the similarity analyses (A and B) (Fig. 6). Group A was composed by species that showed the highest RII% in the upper and mid estuary regions. This group is further divided into two subgroups (A1 and A2), which are formed by species that coexist in zones of similar silt and clay bottoms (upper and mid). In subgroup A1 there is a predominance of resident species, whereas in A2 marine-dependent species are most common. Group B formed two subgroups: B1 and B2. The first was composed of species with the highest RII% for the mid and lower estuary regions, being mostly marine visitors or migrants in trophic ecophase. Subgroup B2 includes species found exclusively in the lower estuary region.
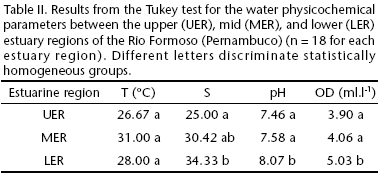
Salinity varied significantly among estuarine regions. Dissolved oxygen and pH were significantly higher in the lower estuary region (Tab. II). Species richness (S) was weakly to moderately correlated with environmental parameters (temperature, salinity, pH, and dissolved oxygen). The highest correlations were found between species richness and mud content (silt and clay) (r = 0.47) (Fig. 7).
With respect to the relationship between the most abundant species in the present study and the salinity regimen in the estuaries proposed by the Veneza System (1958), one could infer that R. amazonica, D. rhombeus, A. clupeoides, C. edentulus, and H. clupeola were collected in salinity regimens from mesohaline (5-18) to euryhaline (30-40), suggesting some capacity for osmoregulation (euryhalinity).
DISCUSSION
Of the 78 species collected in the estuary of the Rio Formoso, 74.4% are marine migrants (dependents, visitors, and in trophic ecophase), 19.2% are residents; 2.5 are occasional, and 1.3% are freshwater visitors. The absence of a large river in this estuary might allow for a higher average salinity and an ichthyofauna that is predominantly marine in origin (LIRA et al. 1979, CPRH 2008). The estuary ichthyofauna is composed of migrant, resident, and occasional species. The former can travel from the ocean or freshwater to the estuary, whereas the resident species remain there for their entire life-cycle (VASCONCELOS-FILHO & OLIVEIRA 2000, CABERTY et al. 2004). Occasional species enter the estuary due to trophic opportunism, some current or wind, or flooding. Migrant marine species can be either visitors or dependants, with the latter being classified according to their ecophase (trophic or genesic, CABERTY et al. 2004).
In Northeastern Brazil, fishes of marine origin occur in elevated proportions in the estuaries (Lowe-McConell 1999), accounting for 84.2% of the species in the Canal de Santa Cruz (Pernambuco) (VASCONCELOS-FILHO et al. 2004). In estuaries in southern Africa, the most prevalent group of fishes was also composed of marine migrants, which reproduce and seek shelter there and where juveniles grow until their gonads begin their maturation (Lowe-McConell 1999). However, in the Baía de Guaratuba (Paraná), marine species were less common, accounting for 43% of the collected fauna, followed by residents, at a frequency of 21% (CHAVES & BOUCHEREAU 2004). That lower proportion of marine species is likely due to the large input of freshwater and the lower evaporation rate in this bay in relation to typically tropical regions.
Resident fish in temperate and tropical estuaries are represented by few species in relation to the migrant species (DAY JR. et al. 1989, BLABER 2000). In the Canal de Santa Cruz (VASCONCELOS-FILHO & OLIVEIRA 2000) and in an estuary in Venezuela (VILLARROEL 1994), resident species accounted for 16.7% and 27% of the collected ichthyofauna, respectively. The proportion of resident species in the Rio Formoso estuary (19%) was within the abovementioned range. The low number of resident species can be due to the complex physiological mechanisms necessary to cope with life in an environment in which considerable physicochemical variations take place (ALBARET et al. 2004, LOEBMANN & VIEIRA 2005). Possibly this is because estuaries are environments with a more recent origin than their neighboring coastal systems. Some fish species carry out ontogenetic migrations among marine meadows, mangroves, and coral reefs, using estuaries are nurseries. These corridors facilitate the interactions between individuals, gene flow, and larval dispersal, reducing population fluctuations (MUMBY 2006). When the species diversities of estuarine fishes in Cuba among the abovementioned ecosystems are compared, reefs and mangroves are most similar (VALDÉS-MUÑOZ 2001). Studies carried out in the Caribbean showed that the mangrove environment strongly influences the structure of the community of reef fish, more than doubling the biomass and increasing the richness and abundance of many adult species, when the habitat is connected to mangroves (NAGELKERKEN et al. 2001, MUMBY et al. 2004).
Of the 78 species recorded across the three estuary regions of the Rio Formoso, more than half is of reef origin, accounting for 51.3% of the total. Reef species predominated in the mid and lower estuary regions (groups III and V), corresponding to 54.2% and 66.7% of the total number of species collected in these regions, respectively. Those are the regions closest to the ocean and, therefore, to the reefs in the Praia de Carneiros. The ichthyofauna of the Rio Formoso estuary is directly influenced by the fauna of reefs and coastal regions, between Sirinhaém and Tamandaré, with a higher richness of reef fishes.
Many species of resident fish in Brazilian reefs are endemic (FLOETER & GASPARINI 2000), being characterized by their diversity (FERREIRA et al. 1995). Approximately 360 fish species are associated with Brazilian reefs (FROESE & PAULY 2008), whereas 103 have been reported for the reefs of Tamandaré (Pernambuco) (FERREIRA et al. 1995). Of those, 40 species (39%) occurred in the Rio Formoso estuary.
The most pronounced variation in diversity among the three estuary areas took place in the rainy season, suggesting that was a period of most marked environmental chance, both due to the higher input of rain water, but also due to the increase frequency of rip currents, with the ensuing responses by the ichthyofauna. On the other hand, during the dry season, the decrease in rain frequency resulted in a more homogeneous estuary, with marine waters entering most of the estuary and more similar diversities among regions.
Fish communities associated with tropical reefs are rich and complex (CAMPOS & OLIVEIRA 2001). Reef fish families include Gerreidae and Lutjanidae (LOWE-MCCONNELl 1999), here represented by E. gula and E. melanopterus (Gerreidae) and L. synagris (Lutjanidae). These species were among the six most abundant, particularly in the upper and mid estuary regions of the Rio Formoso. Lutjanus synagris, one of the most abundant species in the mid estuarine region, is also common in mangrove areas in the Bahamas (LAYMAN & SILLIMAN 2002). Although E. gula is a common species in estuarine waters, adults are most commonly found in shallow marine water (MENEZES & FIGUEIREDO 1980). This fact suggests that juveniles of this species migrate to estuaries to find shelter and food and, when adults, return to the marine environments, where they find protected sites to find shelter, such as in reefs.
In addition to gerreids and lutjanids, the puffer fish, S. testudineus and S. greeleyi (Tetraodontidae), were among the most common reef species in the upper and mid estuary regions. Sphoeroides testudineus inhabits bays and estuaries, with incursions into freshwater (FIGUEIREDO & MENEZES 2000), being common in sandy and rocky substrates and marine phanerogam meadows in an estuary in the Bahamas (LAYMAN & SILLIMAN 2002). The puffer fish occurred in all environments in the Rio Formoso estuary, tolerating many levels of salinity (11.5 to 36). Sphoeroides greeleyi is restricted to areas of higher salinity (20 and 36.5) when compared to S. testudineus (PRODOCIMO & FREIRE 2004), being the most abundant species in the mid estuary region.
Although the mid and upper regions had lower salinities than in the lower region, reef fish were collected in larger numbers in the first two regions. It is possible that the trawls encompassed more habitat heterogeneity upstream than in the lower region, given that the latter was mostly sampled in flooded plains. Moreover, the limit between the euryhaline and the mesohaline regions should be in the mid estuary region, where the highest richness was recorded, indicating that it is a zone of admixture.
On the other hand, Albula vulpes (Albulidae) was only collected in the lower region, being one of the most abundant species living in association with reefs. The sediment in this region is sandy and salinity ranged between 28 and 37.5. This species occurred in similar conditions in Bahia (SANTOS et al. 1999) and in the Bahamas (LAYMAN & SILLIMAN 2002). However, A. vulpes is an amphidromous species, performing regular migrations between marine and freshwater (RIED 2004). In addition, it has a broad array of dietary items, feeding mostly on benthonic invertebrates (CRABTREE et al. 1998).
As in the case of reefs, the ichthyofauna of estuaries and lakes is characterized by a high proportion of predator species (around 80%), although most are not specialists (BLABER 2000). The majority of fish species feed preferentially on benthonic invertebrates (CAMARGO & ISAAC 2003) and is demersal (FROESE & PAULY 2008). In the Rio Formoso estuary, the most abundant reef fish have a demersal habit and are carnivores. Some are first order (A. vulves, E. gula, and S. greeleyi), which feed exclusively on benthonic invertebrates; and others are second-order (L. synagris), which feed on both these invertebrates and actinopterygian fishes (BLABER 2000).
Some reef fish are found in adjacent habitats, such as sand banks, coastal lagoons, estuaries, and marine phanerogam meadows (LOWE-MCCONNELl 1999). This fact was corroborated in the Rio Formoso, where most collected species are found in reef environments. The main difference between the reefs and the remaining coastal habitats is that the abundance of invertebrates is low in reefs, yet with a high turnover. In other words, reef fish live in a highly competitive environment with low food availability. The opposite occurs in the estuaries, where food availability is not the most critical factor, thus increasing the survivorship of the young. Therefore, the definition and/or classification of reef fishes should be reassessed. Although many species occur in reefs, few of them are specialists and competitive in high luminosity, oligotrophic environments such as reefs. It is possible that juvenile fish are distributed in corridors that stretch from the reefs to the estuaries, being therefore more preyed upon in low-complexity environments, such as tidal plains and sandy channels found in the lower region of the estuaries.
The morphological complexity of the tidal channels (BLABER 2000) and the mangrove vegetation, particularly of Rhizophora mangle Linnaeus, provide shelter and protection against predators (MUMBY 2006), as well as feeding resources for several species (SCHAEFFER-NOVELLI 1989), including reef fish. In the Rio Formoso, the upper region of the estuary also showed a significant percentage of fishes also found in reefs, accounting for 39% of the total number of species collected in the upper estuary region.
The ichthyofauna of the Rio Formoso was classified in the present study into six groups, depending on the occurrence of the species across three estuary regions. The species recorded in the upper and mid regions usually had demersal or benthonic habits, and most were either marine-dependent or visitors. These species can enter estuarine systems due to trophic opportunism (CABERTY et al. 2004). Several species, when in their juvenile phase, are dominant in estuarine systems, using them as feeding sites, including representatives of the families Atherinopsidae, Gerreidae, Lutjanidae, and Haemulidae (LAYMANN & SILLIMAN 2002).
The composition of the community of estuarine fishes is also the result of habitat variation and population potential (ALBARET & ALBARET 1994). The most abundant species in the Rio Formoso estuary are distributed among Engraulidae, Clupeidae, and Gerreidae, three of the five most prevalent families in Brazilian estuaries (e.g. ALVES & SOARES-FILHO 1996, ARAÚJO et al. 1998, CASTRO 2001, GARCIA & VIEIRA 2001, BARLETTA et al. 2003). The predominance of these three families is likely to do the fact that species of
Engraulidae and Clupeidae form schools (FIGUEIREDO & MENEZES 1978) and that juvenile specimens of Gerreidae are abundant at some periods in the year in estuarine systems (MENEZES & FIGUEIREDO 1980).
Rhinosardinia amazonica (Clupeidae) was the most numerous species, both in the Rio Formoso estuary and in the Rio Caeté, Pará (BARLETTA et al. 2003). As in the case of the tidal creeks in that river, stratified estuaries, such as in the Rio Formoso, also have a predominance of marine waters in the environments, which could favor the dominance of this species. Harengula clupeola (Clupeidae), Diapterus rhombeus (Gerreidae), Anchovia clupeoides, and Cetengraulis edentulus (Engraulidae) were also among the most abundant species in the studies by CHAVES & OTTO (1999), ARAÚJO et al. (1998), BARLETTA et al. (2003), SPACH et al. (2003, 2004). In the Baía de Guaratuba, the higher abundance of Eucinostomus was associated with more elevated levels of salinity (15 to 35) (CHAVES & OTTO 1999). This pattern was also found in the present study for species in the genus Eucinostomus and Eugerres brasilianus (Cuvier, 1830) (26.5 to 37). In addition, the type of fishing gear used in these studies trawl nets allows for the capture of more school-forming pelagic species (LOPES et al. 1993).
The estuarine ichthyofauna has several degrees of tolerance to variations in water salinity (CAMARGO & ISAAC 2001). The most abundant species in the Rio Formoso estuary were collected between the mesohaline (5 to 18) to the euhaline (30 to 40) regimens. Similar results were obtained in Itamaracá, Pernambuco (ESKINAZI 1972, VASCONCELOS-FILHO et al. 1994, GUEDES et al. 2005), where most species were found in all salinity regimens. Harengula clupeola occurred from freshwater up to the euhaline sector; A. clupeoides, D. rhombeus, and R. amazonica, from the oligo to the euhaline sectors, and C. edentulus from the meso- to the euhaline sectors, allowing for the inference of their relative degree of euryhalinity.
Estuaries are characterized by marked daily and seasonal variations in salinity, which are influenced by the height of the tide, the distance from the ocean, and the river regimen (COELHO et al. 2004). In the Rio Formoso, the limited input of freshwater and the elevated incursion of marine tides (LIRA et al. 1979) cause a large part of the estuary to have high salinity. In the innermost (upper) region, this variable reached 34, which is a salinity characteristic of euhaline (marine) environment. According to the classification system of Veneza, the salinity regimen of the estuarine regions studied in the Rio Formoso, were found to be between mesohaline and euhaline, varying between 11.5 and 37.5. The maximum values of pH and dissolved oxygen are found in areas with the strongest marine influence, whereas the lowest values are found close to the river mouths (MACÊDO et al. 2000).
The kind of regional bottoms was an important determinant for the spatial distribution of the ichthyofauna. In the lower estuary region of the Rio Formoso, the sediments, composed of sand and gravel, were distinct from those in the mid and upper regions, which were classified as muddy/sandy, with higher proportion of mud (silt and clay). The upper estuary region had the highest diversity, whereas the mid region had the highest abundance. Likewise, the highest abundance of fishes in the Baía de Sepetiba (RJ) was found in the innermost region, where the concentration of nutrients from the continental drainage would contribute to a higher productivity (ARAÚJO et al. 1998).
The type of substrate and the organisms that live in it determine the distribution of the fish that feed on them (e.g. GIBSON 1994, LOWE-MCCONNEL 1999, CAMARGO & ISAAC 2003). Several demersal fish species are associated with a particular type of sediment (GIBSON & ROBB 1992). Many estuaries have extensive areas of unconsolidated sediments, such as sand, silt, and clay, which are exposed during low tide and distributed according to the ocean and river currents. Estuarine sediments are rich in organic matter due to the detritus coming from the adjacent vegetation and those carried by the river, with silt harboring the highest amount of organic matter (NOVA SCOTIA MUSEUM OF NATURAL HISTORY 2007). Levels of silt and clay in the substrate are directly proportional to the levels organic matter (PAIVA et al. 2005), indicating that the innermost regions (upper and mid) of the Rio Formoso harbor a higher increment of these components.
ACKNOWLEDGMENTS
We thank Paulo Guilherme de A. Albuquerque (UFRPE) for assistance in manufacturing the trawl nets; José L. FIGUEIREDO (USP) pela ajuda na identificação de Rhinosardinia amazonica, Paulo de T. da C. CHAVES (UFPR) pelas sugestões na metodologia de coleta, Sigrid NEUMANN-LEITÃO (UFPE) and Alessandra C. da Silva for suggestions in some of the statistical analyses. We also thank CPRH for the collection permits, and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, the Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco, and the Conselho Nacional de Pesquisa for providing scholarships to the authors.
LITERATURE CITED
Submitted: 17.VI.2008; Accepted: 12.VI.2009.
Editorial responsibility: Paula da Cunha Lana
- ALBARET. J. & P.S. ALBARET. 1994. Diversité des poisons des lagunes et des estuaries oust-africains. Synthèses géographiques. Annales du Museum république Africanne Central, Zoologie 275: 165-177.
- ALBARET, J.; M. SIMIER; F.S. DARBOE; J. ECOUTIN; J. REFFRAY & L.T. MORAIS. 2004. Fish diversity and distribution in the Gambia Estuary, West Africa, in relation to environmental variables. Aquatic Living Resources 17: 35-46.
- ALBERT, O.T. & O.A. BERGSTAD. 1993. Temporal and spatial variation in the species composition of trawl samples from a demersal fish community. Journal of Fish Biology 43: 209-222.
- ALVES, M.I.M. & A.A. SOARES-FILHO. 1996. Peixes do estuário do rio Jaguaribe (Ceará-Brasil): aspectos fisioecológicos. Ciência Agronômica 27 (1/2): 5-16.
- ARAÚJO, F.G.; A.G. CRUZ-FILHO; M.C.C. AZEVÊDO & A.C.A. SANTOS. 1998. Estrutura da comunidade de peixes demersais da Baía de Sepetiba, RJ. Revista Brasileira de Biologia 58 (3): 417-430.
- ARAÚJO, M.E.; J.M.C. TEIXEIRA & A.M.E. OLIVEIRA. 2004. Peixes estuarinos marinhos do nordeste brasileiro. Guia Ilustrado. Recife, Editora Universitária EFPE e EFC, 260p.
- BARLETTA, M.; A. BARLETTA-BERGAN; U. SAINT-PAUL & G. HUBOLD. 2003. Seasonal changes in density, biomass, and diversity o estuarine ishes in tidal mangrove creeks of the lower Caeté Estuary (northern Brazilian coast, east Amazon). Marine Ecology Progress Series 256: 217-228.
- BLABER, S.J.M. 2000. Tropical estuarine fishes: ecology, exploitation and conservation. Queensland, Australia BLACKWELL Science, 372p.
- BLABER, S.S.J.; D.T. BREWER; J.P. SALINI & J. KERR. 1990. Biomass, catch rates and abundances of demersal fishes, particularly predators of prawns, in a tropical bay in the Gulf of Carpentaria, Australia. Marine Biology 107: 397-408.
- BLABER, S.M.J. & T.G. BLABER. 1980. Factors affecting the distribution of juvenile estuarine and inshore fish. Journal of Fish Biology 17: 143-162.
- BLABER, S.M.J.; D.T. BREWER & J.P. SALINI. 1989. Species composition and biomass of fishes in different habitats of a tropical norther Australia estuary: their occurrence in the adjoining sea and estuarine dependence. Estuarine and Coastal Shelf Science 29: 509-531.
- CABERTY, S.; J. BOUCHEREAU & P.T. CHAVES. 2004. Organisation et fonctionnement trophiques de l'assemblage ichtyque d'um écosystème lagunaire à mangrove antillais au moyen de l'indice trophique de contribution. Cahier de Biologie Marine 45: 243-254.
- CRABTREE, R.E; C. STEVENS; D. SNODGRASS & F.J. STENGARD. 1998. Feeding habits of bonefish, Albula vulpes, from the waters of the Florida Keys. Fish bulletin 96: 754-766.
- CAMARGO, M. & V.J. ISAAC. 2001. Os peixes estuarinas da região norte do Brasil: lista de espécies e considerações sobre sua distribuição geográfica. Boletim do Museu Paraense Emilio Goeldi,Serie Zoologia, 17 (2): 133-157.
- CAMARGO, M. & V.J. ISAAC. 2003. Ictiofauna estuarina, p. 105-142. In: M.E.B. Fernandes (Ed.). Os manguezais da costa Norte do Brasil. São Luís, Fundação Rio Bacanga, vol. 1, 257p.
- CAMPOS, C.E.C. & J.E.L. OLIVEIRA. 2001. Caracterização biométrica e merística do saramunete, Pseudupeneus maculatus (Osteichthys: Mullidae), em Ponta de Pedras, Pernambuco. Boletim do Instituto de Pesca 27 (2): 185-189.
- CASTRO, A.C.L. 2001. Diversidade da assembléia de peixes em igarapés do estuário do rio Paciência (MA Brasil). Atlântica 23: 61-72.
- CERVIGÓN, F. 1991. Los peces marinos de Venezuela. Caracas, Fundación Científica Los Roques, vol. 1, 425p.
- CERVIGÓN, F. 1993. Los peces marinos de Venezuela. Caracas, Fundación Científica Los Roques, vol. 2, 497p.
- CERVIGÓN, F. 1994. Los peces marinos de Venezuela. Caracas, Fundación Científica Los Roques, vol. 3, 295p.
- CERVIGÓN, F. 1996. Los peces marinos de Venezuela. Caracas, Fundación Científica Los Roques, vol. 4, 2nd ed., 295p.
- CHAVES, P. & J.L. BOUCHEREAU. 2004. Trophic organization and functioning of fish populations in the Bay of Guaratuba, Brazil, on the basis of a trophic contribution factor. Acta Adriatica 45 (1): 83-94.
- CHAVES, P.T.C. & G. OTTO. 1999. The mangrove as a temporary habitat for fish: the Eucinostomus species at Guaratuba Bay, Brazil (25ş52'S; 48ş39'W). Brazilian Archives of Biology and Technology 42 (1): 61-68.
- CLARK, K.R. & R.M. WARWICK. 1994. Change in marine communities: an approach to statistical analysis and interpretation. United Kingdom Natural Environment Research Council, 144p.
- COELHO, P.A.; L.M.A. BATISTA-LEITE; M.A.C. SANTOS & M.F.A. TORRES. 2004. O Manguezal, p.641-688. In: E. ESKINAZI-LEÇA; S. NEUMANN-LEITÃO & M.F. COSTA (Eds). Oceanografia um cenário tropical. Recife, Bagaço, 761p.
- CPRH. 2008. Diagnóstico Sócio-Ambiental APA de Guadalupe, Litoral sul de Pernambuco, Brasil. Recife, Companhia Pernambucana do Meio Ambiente, available online at: http://www.cprh.pe.gov.br/downloads/socio-ambiental1. doc [Accessed: 28.I.2008]
- CONDEPE. 1992. Monografias Municipais: Rio Formoso. Recife, Condepe, vol. 2, 173p.
- DAY JR, J.W.; C.A.S. HALL; W.M. KEMP & A. YÁÑEZ-ARANCIBIA. 1989. Estuarine Ecology. New York, Wiley, 558p.
- DEEGAN, L.A. & B.A. THOMPSON. 1985. The ecology of fish communities in the Mississipi River deltaic palin. p.35-56. In: A. YÁNEZ-ARANCIBIA (Ed.) Fish community ecology in estuaries and coastal lagoons: towards an ecosystem integration. Mexico, UNAM, 654p.
- DORENBOSCH, M.; M.C. VAN RIEL; I. NAGELKERKEN & G. VAN DER VELGE. 2004. The relationship of reef fish densities to the proximity of mangrove and seagrass nurseries. Estuarine and Coast Shelf Science 60: 37-48.
- ELLIOT, M. & F. DEWAILLY. 1995. The structure and components of European estuarine fish assemblages. Netherlands Journal of Aquatic Ecology 29: 397-417.
- ESKINAZI, A.M. 1972. Peixes do Canal de Santa Cruz, Pernambuco, Brasil. Trabalhos Oceanográficos da Universidade Federal de Pernambuco 13: 283-302.
- FERREIRA, B.P.; M. MAIDA & A.E.T. SOUZA. 1995. Levantamento inicial das comunidades de peixes recifais na região de Tamandaré, Pernambuco. Boletim Técnico Científico do CEPENE 3 (1): 213-230.
- FIGUEIREDO, J.L. & N.A. MENEZES. 1978. Manual de peixes marinhos do sudeste do Brasil. II Teleostei. São Paulo, Museu de Zoologia, Universidade de São Paulo, 110p.
- FIGUEIREDO, J.L. & N.A. MENEZES. 1980. Manual de peixes marinhos do sudeste do Brasil. III Teleostei. São Paulo, Museu de Zoologia, Universidade de São Paulo, 90p.
- FIGUEIREDO, J.L. & N.A. MENEZES. 2000. Manual de peixes marinhos do sudeste do Brasil. VI Teleostei. São Paulo, Museu de Zoologia, Universidade de São Paulo, 116p.
- FLOETER, S.R. & J.L. GASPARINI. 2000. The southwestern Atlantic reef fish fauna: composition and zoogeographic patterns. Journal of Fish Biology 56: 1099-1114.
- FROESE, R. & D. PAULY. 2007. FishBase. Available online at: http://www.fishbase.org [Accessed: X/2007]
- GARCIA, A.M. & J.P. VIEIRA. 1997. Abundância e diversidade da assembléia de peixes dentro e fora de uma pradaria de Ruppia marítima L., no estuário da lagoa dos Patos (RS Brasil). Atlântica 19: 161-181.
- GARCIA, A.M. & J.P. VIEIRA. 2001. O aumento da diversidade de peixes no estuário da Lagoa dos Patos durante o episódio el niño. Atlântica 23: 85-96.
- GIBSON, R.N. & L. ROBB. 1992. The relationship between body size, sediment grain size and the burying ability of juvenile plaice, Pleuroneces platessa L. Journal of Fish Biology 40: 771-778.
- GIBSON, R.N. 1994. Impact of habitat quality and quantity on the recruitment of juvenile flatfishes. Netherlands Journal of Sea Research 32: 191-206.
- GUEDES, D.S.G.; A.L. VASCONCELOS-FILHO, & R.M. MACEDO. 2005. Ictiofauna do infralitoral adjacente às margens do Canal de Santa Cruz Itapissuma, Pernambuco. Boletim Técnico-Científico do CEPENE 13 (2): 65-75.
- HUMANN, P. & N. DELOACH. 2002. Reef Fish Identification: Florida Caribbean Bahamas. Florida, New World Publications, Inc. 481p.
- KÖPPEN, W. 1948. Climatologia: con un estudo de los climas de la tierra. Mexico, Fondo de Cultura Economica. 478p.
- LAYMAN, C.A. & B.R. SILLIMAN. 2002. Preliminary survey and diet analysis of juvenile fishes of an estuarine Creek on Andros Island. Bahamas. Bulletin of Marine Science 70 (l): 199-210.
- LEKVE, K.; N.C. STENSETH; J.G. GJOSAETER; J.M. FROMENTIN & J. GRAY. 1999. Spatiotemporal patterns in diversity of a fish assemblage along the Norwegian Kagerrak coast. Marine Ecology Progress Series 178: 17-27.
- LIRA, L.; M.C. ZAPATA, & V.G. FONSECA. 1979. Aspectos da dinâmica do estuário do Rio Formoso, PE. Cadernos Omega da Universidade Federal de Pernambuco 3 (1/2): 133-156.
- LOEBMANN, D. & J.P. VIEIRA. 2005. Distribuição espacial e abundância das assembléias de peixes no Parque Nacional da Lagoa do Peixe, Rio Grande do Sul, Brasil. Revista Brasileira de Zoologia 22 (3): 667-675.
- LOPES, R.G.; E.S. RODRIGUEZ; PUZZI, A.; J.B. PITA; J.A.P. COELHO & M.L. Freitas. 1993. Levantamento ictiofaunístico em um ponto fixo na baía de Santos, estado de São Paulo, Brasil. Boletim do Instituto de Pesca 20: 7-20.
- LOWE-MCCONNEL, R.H. 1999. Estudos ecológicos de comunidades de peixes tropicais. São Paulo, EDUSP, 534p.
- MACÊDO, S.J.; M.J. FLORES-MONTES & I.C. LINS. 2000. Características Abióticas da Área, p. 7-25. In: H.M. BARROS; E. ESKINAZI-LEÇA; S.J. MACÊDO & T. LIMA (Eds). Gerenciamento participativo de estuários e manguezais. Recife, Editora Universitária da UFPE, 252p.
- MARSHALL, S. & M. ELLIOT. 1998. Environmental influences on the fish assemblage of the Humber Estuary, U.K. Estuarine & Coastal Shelf Science 46: 175-184.
- MENEZES, N.A. 1983. Guia prático para conhecimento e identificação das tainhas e paratis (Pisces, Mugilidae) do litoral brasileiro. Revista Brasileira de Zoologia 2 (1): 1-12.
- MENEZES, N.A. & J.L. FIGUEIREDO. 1980. Manual de peixes marinhos do sudeste do Brasil. III. Teleostei. São Paulo, Museu de Zoologia, Universidade de São Paulo, 90p.
- MENEZES, N.A. & J.L. FIGUEIREDO. 1985. Manual de peixes marinhos do sudeste do Brasil. V. Teleostei. São Paulo, Museu de Zoologia, Universidade de São Paulo, 90p.
- MENEZES, N.A.; P.A. BUCKUP; J.L. FIGUEIREDO & R.L. MOURA. 2003. Catálogo das espécies de peixes marinhos do Brasil. São Paulo, Museu de Zoologia da Universidade de São Paulo, 160p.
- MORGADO, E.H. & A.C.Z. AMARAL. 1989. Anelídeos poliquetos da região de Ubatuba (SP): Padrões de distribuição geográfica. Revista Brasileira de Zoologia 6 (3): 535-568.
- MUMBY, P.J. 2006. Connectivity of reef fish between mangroves and coral reefs: Connectivity of reef fish between mangroves and coral reefs: algorithms for the design of marine reserves at seascape scales. Biological Conservation 128: 215-222.
- MUMBY, P.J.; A.J. EDWARDS; J.E. ARIAS-GONZÁLEZ; K.C. LINDEMAN; P.G. BLACKWELL; A. GALL; M.I. GORCZYNSKA; A.R. HARBONE; C.L. PESCOD; H. RENKEN; C.C.C. WABNITZ & G. LEWELLYN. 2004. Mangroves enhance of coral reef fish communities in the Caribbean. Nature 427: 533-536.
- NAGELKERKEN, I.; S. KLEIJNEN; T. KLOP; R.A.C.J. VAN DEN BRAND; E. COCHERET DE LA MORINIÈRE & G. VAN DER VELDE, 2001. Dependence of Caribbean reef fishes on mangroves and seagrass beds as nursery habitats: a comparison of fish faunas between bays with and without mangroves/seagrass beds. Marine Ecology Progress Series 214: 225-235.
- NELSON, J.S. 2006. Fishes of the world. New York, John Wiley & Sons, 601p.
- NOVA SCOTIA MUSEUM OF NATURAL HISTORY. 2007. Estuaries. Available online at: http://museum.gov.ns.ca/mnh/nature/nhns [Accessed: X/2007]
- PAIVA, A.C.G.; P.A. COELHO & M.F.A. TORRES. 2005. Influência dos fatores abióticos sobre a macrofauna de substratos inconsolidados da zona entremarés em duas áreas do Canal de Santa Cruz, Pernambuco, Brasil. Arquivos de Ciências do Mar 38: 85-92.
- PRODOCIMO, V. & A.C. FREIRE, 2004. Estuarine pufferfishes (Sphoeroides testudineus and S. greeleyi) submitted to sea water dilution during ebb tide: a field experiment. Marine and Freshwater Behaviour and Physiology 37 (1): 1-5.
- RIED, K. 2004. Global register of migratory species from global to regional scales. Bonn, Germany, Federal Agency for Nature Conservation, Final Report of the R&D-Project 808 05 081, 329p.
- ROBERTSON, A.I. & S.J.M. BLABER. 1992. Plankton, epibenthos and fish communities, p. 63-100. In: A.I. ROBERTSON & D.M ALONGI (Eds). Tropical mangrove ecosystems (coastal and estuarine studies). Washinhton, American Geophysical Union, 236p.
- ROSS, S.W. & S.P. EPPERLY. 1985. Utilization of shallow estuarine nursery areas by fishes in Pamlico Sound and adjacent tributaries, p. 207-232. In: A. YÁÑEZ-ARANCIBIA (Ed.). Fish community ecology in estuaries and coastal lagoons: towards an ecosystem integration. Mexico, UNAM, 654p.
- SALE, P.F. 2002. Coral reef fishes. Dynamics and diversity in a complex ecosystem. London, Academic Press, 549p.
- SANTOS, A.C.A; F.R.C. CASTELUCCI; E.P. SANTOS; M.P. SENA & C.F. NEPOMUCENO. 1999. Distribuição e recrutamento do peixe-rei Xenomelaniris brasiliensis (Osteichthyes, Atherinidae) na margem continental oeste da Baía de todos os Santos, Bahia, Brasil. Acta Biológica Leopoldensia 1 (21): 107-118.
- SCHAEFFER-NOVELLI, Y. 1989. Perfil dos ecossistemas litorâneos brasileiros, com especial ênfase sobre o ecossistema manguezal. Publicação especial Instituto Oceanográfico São Paulo 7: 1-16.
- SHULMAN, M.J. 1985. Recruitment of coral reef fishes: effects of distribution of predators and shelter. Ecology 66: 1056-1066.
- SPACH, H.L.; C. SANTOS & R.S. GODEFROID. 2003. Padrões temporais na assembléia de peixes na gamboa do Sucuriú, Baía de Paranaguá, Brasil. Revista Brasileira de Zoologia 20 (4): 591-600.
- SPACH, H.L.; C. SANTOS; R.S. GODEFROID; M. NARDI, & F. CUNHA, 2004. A study of the fish community structure in a tidal creek. Brazilian Journal Biology 64 (2): 337-351.
- SUGUIO, K. Introdução à sedimentologia. 1973. São Paulo, Blucher/EDUSP, 312p.
- VALDÉS-MUÑOZ & A.D. MOCHEK. 2001. Behavior of Marine Fishes of the Cuban Shelf, p. 58-72. In: R. Claro; K.C. LINDEMAN & L.R. Parenti (Eds). Ecology of Marine fishes of the Cuban shelf. Washington, Smithsonian Institution Press, 253p.
- VASCONCELOS-FILHO, A.L.; E.F. CAVALCANTI & S.T. SOUZA. 1994. Composição e distribuição da fauna ictiológica no Canal de Santa cruz. Revista Nordestina de Zoologia 1 (1): 247-262.
- VASCONCELOS-FILHO, A. & A.M.E. OLIVEIRA. 2000. Ictiofauna. In: H.M. BARROS; E. ESKINAZI-LEÇA; S.J. MACÊDO & T. LIMA (Eds). Gerenciamento participativo de estuários e manguezais. Recife, Ed. Universitária, 252p.
- VASCONCELOS-FILHO, A.; D.S. GUEDES; S.F. TEIXEIRA & A.M.E. OLIVEIRA. 2004. Peixes marinhos costeiros e estuarinos. p. 555-570. In: E. ESKINAZI-LEÇA; S. NEUMANN-LEITÃO & M.F. COSTA (Eds). Oceanografia, um cenário tropical. Bagaço, 761p.
- SISTEMA DE VENEZA. 1958. Symposium on the of brackish waters, Venice, april 8-14, 1958. Archives Oceanography and Limnology 11 (Suppl.): 1-248. Available online at: http://www.aslo.org/lo/toc/vol_3/issue_3/0346.pdf [Accessed: X/2007]
- U.S. NAVAL OCEANOGRAPHIC OFFICE. 1965. Oceanographic atlas of the North Atlantic Ocean. Washington, D.C.U.S. Naval Oceanographic Office, Section 5, Marine Geology, 79p.
- VIEIRA, J.P.; J.P. CASTELLO & L.E. PEREIRA. 1998. Ictiofauna, p. 60-68. In: U. Seeliger; C. Odebrecht & J.P. CASTELLO (Eds). Os ecossistemas costeiro e marinho do extremo sul do Brasil. Rio Grande, Ecoscientia, 332p.
- VIEIRA, J.P.; M.C. VASCONCELOS; R.E. SILVA & L.C. FISHER. 1996. A rejeição da pesca do camarão-rosa (Penaeus paulensis) no estuário da lagoa dos Patos, RS, Brasil. Atlântica 18: 123-142.
- VILLARROEL, P.R. 1994. Estrutura de las comunidades de peces de la Laguna de Raya, isla de Margrita, Venezuela. Ciencias Marinas 20 (1): 1-16.
- WEINSTEIN, M.P. 1982. Commentary: a need for more experimental work in estuarine fisheries ecology. Northeast Gulf Science 5 (2): 59-64.
- WERNER, E.E. & J.F. GILLIAM. 1984. The ontogenetic niche and species interactions in size-structured populations. Annual Review of Ecology and Systematics 15: 393-425.
Publication Dates
-
Publication in this collection
27 July 2009 -
Date of issue
June 2009
History
-
Accepted
12 June 2009 -
Received
17 June 2008