Abstract
Despite the great diversity of Brazilian Atlantic forest small mammals, natural history of most species is unknown due to their cryptic and nocturnal habits, but also due to the inadequacy of methods to capture some species, especially those of arboreal habits. A new technique, based on the use of artificial nests (AN) to record arboreal marsupials, is presented. Artificial nests were combined with traditional live traps to study the population ecology of four didelphid marsupial species. After 62 months of monitoring, 119 individuals were recorded 243 times (total success = 5.2%). Only 26 individuals (22%) were recorded by both AN and live trap methods, and two of the four species were never captured by live traps, only by AN. Live traps alone would have provided biased data of the structure of small mammal assemblages, creating artificial tendencies in population dynamics of many species. Detectability estimates based on mark-recapture data could correct bias resulting from the use only live traps, but these estimates require that at least some individuals of each age class or stage are captured. Only the combination of AN and live traps can produce more accurate data on population dynamics and assemblage structure. This study demonstrates that artificial nests represent a new method that should be combined with live traps in studies of small mammal assemblages and populations.
Didelphidae; mark-recapture; live trapping; population dynamics; marsupials
ECOLOGY
Artificial nests as an alternative to studies of arboreal small mammal populations: a five-year study in the Atlantic Forest, Brazil
Diogo Loretto* * Corresponding author, E-mail: diogoloretto@yahoo.com.br ; Marcus Vinícius Vieira
Departamento de Ecologia, Instituto de Biologia, Universidade Federal do Rio de Janeiro. Avenida Brigadeiro Trompovsky, Caixa Postal 68020, 21941-590 Rio de Janeiro, RJ, Brazil
ABSTRACT
Despite the great diversity of Brazilian Atlantic forest small mammals, natural history of most species is unknown due to their cryptic and nocturnal habits, but also due to the inadequacy of methods to capture some species, especially those of arboreal habits. A new technique, based on the use of artificial nests (AN) to record arboreal marsupials, is presented. Artificial nests were combined with traditional live traps to study the population ecology of four didelphid marsupial species. After 62 months of monitoring, 119 individuals were recorded 243 times (total success = 5.2%). Only 26 individuals (22%) were recorded by both AN and live trap methods, and two of the four species were never captured by live traps, only by AN. Live traps alone would have provided biased data of the structure of small mammal assemblages, creating artificial tendencies in population dynamics of many species. Detectability estimates based on mark-recapture data could correct bias resulting from the use only live traps, but these estimates require that at least some individuals of each age class or stage are captured. Only the combination of AN and live traps can produce more accurate data on population dynamics and assemblage structure. This study demonstrates that artificial nests represent a new method that should be combined with live traps in studies of small mammal assemblages and populations.
Key words: Didelphidae; mark-recapture; live trapping; population dynamics; marsupials.
doi: 10.1590/S1984-46702011000300013
Currently, at least 230 terrestrial mammal species are recognized in the Atlantic Forest biome (MACHADO et al. 2008), 55 of which are considered endemic species. These endemic species are restricted to 11-16% of the biome's original coverage (RIBEIRO et al. 2009). Habitat loss and the cryptic habits of most medium to large or small mammal species make them difficult to study (BEKER & DALPONTE 1991, DELCIELLOS et al. 2006, PREVEDELLO et al. 2008). About 10% of the Atlantic forest mammal species are small marsupials, opossums and mouse opossums (REIS et al. 2006, Didelphimorphia: Didelphidae). The difficulty in detecting the small marsupials is perhaps the cause of recognizing just one species as threatened in Brazil (MACHADO et al. 2008), thus reflecting the lack of knowledge of their biology and even geographic distribution.
Studies of all aspects of small mammal biology depend on the capture of an adequate number of individuals, which generally are obtained using live traps. Less abundant or less trappable species are difficult to study. Consequently, the use of live traps has largely determined the species of small mammals which have been studied, not only in population studies using mark-recapture (e.g. MALCOLM 1991, GRAIPEL et al. 2003, MACEDO et al. 2006), but also in studies of diet (ATRAMENTOWICZ 1988, CARVALHO et al. 1999), vertical stratification (CUNHA & VIEIRA 2002, LORETTO & VIEIRA 2005), locomotion (CANT 1992), and use of space (MORAES JR & CHIARELLO 2005a,b, PREVEDELLO et al. 2008).
Efforts have been made to develop techniques capable of recording rare species, such as spreading traps in a wide range of sites and forest strata (e.g. CHARLES-DOMINIQUE et al. 1981, MALCOLM 1991, 1995, VIEIRA & MONTEIRO-FILHO 2003), and using pitfall traps (e.g. PARDINI et al. 2005, UMETSU et al. 2006). Arboreal small mammals, however, remain difficult to capture and study (MALCOLM 2004). In humid forests, the more arboreal the species, the less recorded it is. This is the case of arboreal marsupials such as the woolly opossum Caluromys philander (Linnaeus, 1758), and the gracile mouse opossum Gracilinanus microtarsus (Wagner, 1842) (MALCOLM 2004, MACEDO et al. 2007).
Artificial nests (AN) were less used to record and study small mammals, but have the potential to detect species that never or seldom enter a live trap, particularly arboreal small mammals. The first use of AN to study marsupials possibly dates back to ENDERS (1935 apud HUNSAKER II 1977) in Panama. In Brazil, it was first used by MONTEIRO-FILHO & MARCONDES-MACHADO (1996) to record small arboreal marsupials, using nesting boxes made of wood. TUBELIS (2000) changed the nest model to a bamboo-made shelter and was successful in the study of a G. microtarsus population.
Herein, the number of captures of four of Atlantic Forest marsupials species using traditional live trapping and AN are compared. The same sites were surveyed using AN and live traps concomitantly, during a five-year period.
MATERIAL AND METHODS
The study was conducted in the Serra dos Órgãos National Park, a ca. 20,000 ha protected area, contiguous to other protected areas along the Serra do Mar, forming one of the largest remaining continuous stretch of Atlantic Forest (LINO & ALBUQUERQUE 2007) The field site was in the Guapimirim municipality, Rio de Janeiro State, in a site locally known as Garrafão (22º28'S, 42º59'W). The forest is part of the Montane rainforest complex (RIZZINI 1979), in an old-growth successional stage (details in MACEDO et al. 2007). Currently, the area is surrounded by vacation homes which can have some influence on the structure and composition of the forest (MACEDO et al. 2007). The weather is mesothermic (NIMER 1989), super-humid from October to March and humid otherwise. June, July, and August were the less humid months, but periods of real hydric deficit, as defined by WALTER (1986), are unlikely. During the study, the minimum and maximum mean monthly temperature varied from 15.7 to 24.9ºC, respectively, and monthly rainfall varied from 0.2 to 508 mm (INMET, Teresópolis, RJ).
Since April 1997, the Laboratório de Vertebrados of the Universidade Federal do Rio de Janeiro (UFRJ) develops a small mammal mark-recapture monitoring program using live traps. Animals were captured using Sherman XLK (30.5 x 9.8 x 8 cm), Tomahawk 210 (41 x 14 x 14 cm) and Tomahawk 105 (50.8 x
17.8 x 17.8 cm) traps. Live traps were set in three 0.64 ha trapping grids, established at different altitudes [ca. 750, 650, 520 m asl, details in MACEDO et al. (2007) and KAJIN et al. (2008)]. Each grid had 25 trap stations 20 m apart, two traps per station, one Tomahawk 210 and one Sherman, both on the ground. Additionally, half of the trap stations included a canopy platform (6-15 m high), each one with a XLK Sherman and a 210 Tomahawk. A total of 81 traps were used per night, per grid, which were set in bimonthly trapping sessions of five consecutive nights each. A mixture of peanut butter, banana, oats and bacon was used as bait. Traps were checked and rebaited every day to maximize attraction.
Captured individuals were first marked with numbered ear tags (National Band and Tag Co., Newport, Kentucky). Afterwards, their body mass, body and tail length were measured, and their sex, breeding status, and developmental stage by teeth eruption ( following MACEDO et al. 2006) was registered. Pouch young were marked by toe clipping (details in GENTILE et al. 2004 and KAJIN et al. 2008), a method considered harmless to early immature stage marsupials (FISHER & BLOMBERG 2009).
Artificial nests were set in three 1.44 ha grids, overlaying the trapping grids. Artificial nest grids had 21 nest stations, 3040 m apart (details in LORETTO 2005 and DELCIELLOS et al. 2006). Artificial nests were made of painted bamboo culms (Bambusa vulgaris var. vittata Schrad), with few changes from the design of TUBELIS (2000). We used circular 51 mm entrances to limit the use of AN to the smaller marsupials. The animals were removed from the nest through the inspection window which consisted of a light weighted square piece of wood fixed on the top of the culm by strings in order to prevent water and other animals to enter.
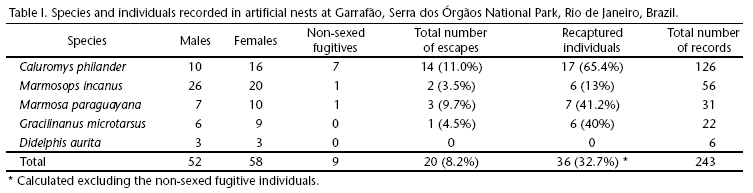
Each nest station had AN at three heights, 0, 2.5 and 5 m (for a total of 189 AN). Wood ladders fixed on tree trunks gave us access to nests. We did not use baits or any attractive devices in AN. Individuals were free to enter and leave nests before researchers arrive. Nests were inspected during the day time, and most individuals were awakened when the AN were picked up by the researcher. During a small percentage of inspections (8.2%, see Tab. I) the animals noticed the researcher's approach and escaped. Once an individual was recognized as present inside the AN, the entrance was closed, and the nest was carried to the ground where animals were handled, marked, and measured following the same protocol used for those captured in live traps. Animals were released inside the nest in which they were found, after the nest was fixed back at the same position in the tree (Figs 1-4). Efficiency of AN was evaluated by the ratio of records divided by total checking effort. To evaluate population dynamics patterns, AN records were combined every two months to coincide with the live trap study. When comparing both methods, we only present data from the same time span of both studies. This study was carried out using the ICMBIO/IBAMA collecting permits 02001, 004671/98-51 and 16704-1.
RESULTS
Artificial nests were monitored during 62 months, from June 2003 to July 2008, with a 9,798 nest-checking effort, whereas live trapping, during the same period, had a total effort of 37,665 trap-nights. We recorded 119 individuals in AN, of seven species, in 243 occasions, a 2.48% capture success. We also recorded 267 abandoned or temporarily unoccupied nests (55.7% of the records), resulting in a 5.2% total success (TS) when all the used nests are considered. Live trapping success, in the study area, was about 3.5% (MACEDO et al. 2007).
The most recorded species in AN was Caluromys philander, considering the total number of captures (Tab. I). This was also the species which escaped the most. Marmosops incanus (Lund, 1840) was the species with the largest number of individuals recorded (N = 46). Marmosa paraguayana (Thomas, 1905), G. microtarsus, and Didelphis aurita Wied-Neuwied, 1826 were captured less. The four-eyed gray opossum Philander frenatus (Olfers, 1818), and the climbing mouse Rhipidomys sp. were also recorded, but only one individual each, hence were not considered in these analyses. Nine unmarked individuals escaped before the researcher could reach the nest and were also not considered in this analysis (Tab. I).
Only 26 (23.6%) of the 110 marked individuals were also captured in live traps, and just eight (7.3%) were captured in live traps before recorded in AN. Individuals of C. philander and G. microtarsus were never captured in live traps during this study. For M. incanus and M. paraguayana, the addition of AN to live traps provided an increase of 25% and 50%, respectively, in the number of individuals known to be alive in the area. Adults were the most frequently recorded age class in AN (54%, N = 131), compared to sub-adults (24.2%, N = 59), and juveniles (11.1% = 27). In twenty-six records (10.7%) the individuals escaped before their development stage was evaluated.
The number of pregnant and lactating females in nests was low, but a high number of weaned juveniles and sub-adults (35% of the total) was recorded. During the last three breeding seasons (from August to February), females with pouch young were recorded 12 times in AN: five C. philander, one G. microtarsus, and one M. paraguayana. One litter of C. philander had five young, and four litters had four young (total of 21: 10 males and 11 females), the single litter recorded for G. microtarsus had four young (one male, two females and a fugitive non-sexed individual), and the single litter of M. paraguayana had 10 pouch-young (six males and four females).
On average, 60% of the individuals were recorded just once in AN, but for C. 65.4% were recorded more than once. The other three species were seldom recaptured (Tab. I). This pattern was the opposite found for the same species with live trapping captures (MACEDO et al. 2007).
Seasonal trends of AN and live traps records showed remarkable differences for the two species recorded by both methods. For M. incanus, live traps showed peaks in number of individuals during July, and depressions in February and April, but the opposite pattern was obtained by AN (Fig. 5). When using just live trap data, the seasonality effect was overestimated as just one individual was captured during the end of the breeding season, February. There were more individuals present in the study area during this month, mostly juveniles (R.B. Almeida unpubl. data), but they were detectable only with AN (Fig. 5). For M. paraguayana the seasonal abundance pattern was similar using live traps or AN, but the sum of records from both methods suggests that population abundances are less variable than would be inferred based on only one of the methods (Fig. 6).
The presence and abundance of C. philander and G. microtarsus could only be evaluated using AN as the majority of individuals were not captured by live traps (Figs 7 and 8). Thus, by only using AN it was possible to recognize these two species as permanently present in the study area and to determine that their records peaked in February and March.
DISCUSSION
The populations of C. philander and G. microtarsus could only be detected using AN, as well as part of the M. incanus and M. paraguayana populations. The 26 individuals of C. philander and 15 of G. microtarsus recorded by AN not only increased the number of records in the study area, but also indicated permanent populations (Figs 7 and 8), which could not be detected using only live trap data. Additionally, for M. incanus and M. paraguayana it is clear that their fluctuations in the number of individuals captured are less extreme than indicated by live trapping. Live traps mainly captured more individuals during the beginning of the breeding season (from June to August), whereas AN mainly recorded individuals in February and March, the end of breeding season. For M. incanus the capture peaks were caused by a live trapping limitation in capturing the juveniles. Conversely, AN succeeded in recording juveniles when they were not susceptible to capture using live traps (R.B. Almeida, unpubl. data). If M. incanus is indeed semelparous (MARTINS et al. 2006, LEINER et al. 2008), adult males die just after reproduction, but there are already weaned and independent juveniles present in the area.
Detectability varied between sampling methods, a fact occasionally recognized in small mammal studies such as VOSS & EMMONS (1996) and VOSS et al. (2001), which indicates that no single methodology is effective for recording the presence of all the species in an area. A mark-recapture program could solve this limitation, permitting the use of statistical estimates of detectability or capture probability of a method (WILLIAMS et al. 2002). Thus, why not simply use detectability estimates to corrected abundance estimates based on mark-recapture with live traps? The problem is that estimates of detectability require that a minimum number of individuals of all stages or age classes are captured within a trapping session, the primary period of a double sampling design (WILLIAMS et al. 2002). Detectability cannot be estimated if most or all the individuals of a species are never captured in live traps, such as C. philander, G. agilis, and M. incanus in the present study. Thus, accurate estimates of population parameters would benefit from the combination of live traps and AN.
This study demonstrates that artificial nests represent a new method that should be combined with live traps in studies of small mammal assemblages and populations. The recording of different species using alternative methods has been demonstrated, mostly related to the use of live traps to capture arboreal small mammals in canopy platforms (MALCOLM 1991, 1995, GRAIPEL 2003, GRAIPEL et al. 2003, VIEIRA et al. 2004, ASTÚA et al. 2006), and pitfall traps to capture animals that are rarely or never captured in live traps (PARDINI & UMETSU 2006, FOURNIERCHAMBRILLON et al. 2000). Artificial nests now become another particularly appropriate method which can be used to study arboreal small mammals.
As reported previously by TUBELIS (2000), most individuals were recorded just once in AN, which is a limitation of the method. This limitation may be related to the disturbance of the nest which is necessary for the observer to detect the animal, but it is also possible that animals use AN only as temporary shelters. This effect is difficult to circumvent as the presence of the researcher and animal handling are necessary. Nevertheless, AN have a low cost/benefit ratio, an important practical consideration when choosing a method. Artificial nests require only low cost materials, and reveal unique aspects of a population, or even whole populations which would not otherwise be detected.
It is known that the exclusive use of live traps, when sampling small mammals, generates a result bias in population and community parameters (e.g. O'FARRELL 1994, WOODMAN et al. 1996). Paradoxically, the ecology of small mammals is still mostly based on live trap data. Here, the use of artificial nests is presented, as a relatively low cost method, and as another alternative to achieve a more accurate picture of small mammal assemblages and population dynamics, particularly of arboreal small mammals. This picture is taken from another view point, when individuals are involved in activities other than foraging, possibly providing insights on unique aspects of their natural history. Combined with live traps, artificial nests would allow recaptures of at least a portion of the population and species of the assemblage, which would counteract the weakness of each method, and combine the advantages of both. Mid to long-term studies of population and community ecology of small arboreal mammals should consider the use of artificial nests.
ACKNOWLEDGMENTS
We thank all the students of the Laboratório de Vertebrados who helped in the fieldwork, and the administrative staff of the Serra dos Órgãos National Park. Special thanks to André Cunha, Vanina Antunes, Emiliano Ramalho, Joana Macedo, Vitor Rademaker, Maja Kajin, Paulo Almeida and Lívia Mariano who helped us gather the present dataset. Ana Delciellos, Bernardo Papi, Eduardo Fox, Jayme Prevedello, Míriam Pinto, and anonymous referees made invaluable comments and suggestions on earlier versions of the manuscript. FUJB, CNPq, PIBIC/CNPq, PROBIO (MMA - GEF), PRONEX, and FAPERJ provided financial support throught grants to Carlos E.V. Grelle, Marcus V. Vieira, and Rui Cerqueira.
LITERATURE CITED
Submitted: 23.III.2010;
Accepted: 27.II.2011.
Editorial responsibility: Heraldo L. de Vasconcelos
- Ástua, D.; R.T. Moura; C.E.V. Grelle& M.T. Fonseca. 2006. Influence of baits, trap type and position for small mammals capture in a Brazilian lowland Atlantic Forest. Boletim do Museu de Biologia Mello Leitão 19: 31-44.
- Atramentowicz, M. 1988. La frugivorie opportuniste de trois marsupiaux didelphidés de Guyane. Revue d'Ecologie (La Terre et la Vie) 43: 47-57.
- Becker, M. & J.C. Dalponte. 1991. Rastros de mamíferos silves tres brasileiros. Brasília, Editora da Universidade de Brasília, 180p.
- Cant, G.H. 1992. Positional behavior and body size of arboreal primates: a theoretical framework for field studies as an illustration of its application. American Journal of Physical Anthropology 88: 273-283.
- Carvalho, F.M.V.; P.S. Pinheiro; F.A.S. Fernandez & J.L. Nessimian. 1999. Diet of small mammals in Atlantic Forest fragments in southeastern Brazil. Revista Brasileira de Zoociências 1: 91-101.
- Charles-Dominique, P.; M. Atramentowicz; M. Charles-dominique; H. Gerard; A. Hladik; C.M. Hladik & M.F. Prévost. 1981. Les mammifères frugivores arboricoles nocturnes d'une foret guyanaise: inter-relations plantes-animaux. Revue d'Ecologie (La Terre et la Vie) 35: 341-345.
- Cunha, A.A. & M.V. Vieira. 2002. Support diameter, incline, and vertical movements of four didelphid marsupials in the Atlantic forest of Brazil. Journal of Zoology, London 258: 419-426.
- Delciellos, A.C.; D. Loretto & M.V. Vieira. 2006. Novos métodos no estudo da estratificação vertical de marsupiais neotropicais. Oecologia Brasiliensis 2 (10): 135-153.
- Fisher, D.O. & S.P. Blomberg. 2009. Toe-bud clipping of juveniles small marsupials for ecological field research: No detectable negative effects on growth or survival. Austral Ecology 34: 858-865.
- Fournier-Chambrillon C.; P. Fournier; J.M. Gaillard; C. Genty; E. Hansen & J.C. Viê. 2000. Mammal trap efficiency during the fragmentation by flooding of a neotropical rain forest in French Guiana. Journal of Tropical Ecology 16: 841-851.
- Gentile, R.; R. Finotti; V. Rademaker & R. Cerqueira. 2004. Population dynamics of four marsupials and its relations to resource production in the Atlantic Forest in Southeastern Brazil. Mammalia 68 (2-3): 109-119.
- Graipel, M.E. 2003. A simple ground-based method for trapping small mammals in the forest canopy. Mastozoología Neotropical 10 (1): 177-181.
- Graipel, M.E.; J.J. CHEREM; P.R.M. MILLER & L. GLOCK. 2003. Trapping small mammals in the forest understory: a comparison of three methods. Mammalia 67 (4): 551-558.
- Hunsaker II, D. 1977. Ecology of new world marsupials, p. 95-156. In: D. HUNSAKER II. (Ed.). The Biology of Marsupials. New York, Academic Press, 537p.
- Kajin, M.; R. Cerqueira; M.V. Vieira & R. Gentile. 2008. Nine-year demography of the black-eared opossum Didelphis aurita (Didelphimorphia: Didelphidae) using life tables. Revista Brasileira de Zoologia 25 (2): 206-213.
- Leiner, N.O.; E.Z.F. Setz & W.R. Silva. 2008. Semelparity and factors affecting the reproductive activity of the Brazilian slender opossum (Marmosops paulensis) in southeastern Brazil. Journal of Mammalogy 89: 153-158.
- Lino, C.F. & J.L. Alburquerque. 2007. Mosaicos de Unidades de Conservação no Corredor da Serra do Mar. São Paulo, Conselho Nacional da Reserva da Biosfera da Mata Atlântica, 96p.
- Loretto, D. 2005. O uso de ninhos artificiais no estudo comportamental de pequenos marsupiais arborícolas. Boletim da Sociedade Brasileira de Mastozoologia 44: 3-5.
- Loretto, D. & M.V. Vieira. 2005. The effects of reproductive and climatic seasons on moviments in the black-eared opossum (Didelphis aurita Wied-Neuwied, 1826). Journal of Mamma-logy 86 (2): 188-194.
- Macedo, J.; D. Loretto; M.V. Vieira & R. Cerqueira. 2006. Classes de desenvolvimento em marsupiais: um método para animais vivos. Mastozoología Neotropical 13 (1): 133-136.
- Macedo, J.; D. Loretto; M.C.S. Mello; S.R. Freitas; M.V. Vieira & R. Cerqueira. 2007. História Natural dos mamíferos de uma área perturbada do Parque Nacional da Serra dos Órgãos, Rio de Janeiro, Brasil, p. 165-182. In: C. Cronemberger & E.B. Viveiros de castro (Eds). Ciência e Conservação da Serra dos Órgãos. Brasília, IBAMA, 298p.
- Malcolm, J.R. 1991. Comparative abundances of Neotropical small mammals by trap height. Journal of Mammalogy 72 (1): 188-192.
- Malcolm, J.R. 1995. Forest structure and the abundance and diversity of neotropical small mammals, p. 179-197. In: M.D. LOWMAN & N.M. NADKARNI (Eds). Forest Canopies. New York, Academic Press, 626p.
- Malcolm, J.R. 2004. Ecology and conservation of canopy mammals, p. 297-331. In: M.D. LOWMAN & H.B. RINKER (Eds.). Forest canopies. New York, Elsevier Academic Press, 2nd ed., 517p.
- Martins, E.G.; V. Bonato; C. Queiroz Da-Silva & S.F. Reis. 2006. Seasonality in reproduction, age structure and density of the gracile mouse opossum Gracilinanus microtarsus (Marsupialia: Didelphidae) in a Brazilian cerrado. Journal of Tropical Ecology 22: 461-468.
- Machado, A.B.M.; G.M. Drummond& A.P. Paglia. 2008. Livro vermelho da fauna brasileira ameaçada de extinção. Brasília, Ministério do Meio Ambiente, vol. 2, 1420 p.
- Monteiro-Filho, E.L.A. & L.O.M. Marcondes-Machado. 1996. The utilization of nest-boxes by small mammals. Ciência e Cultura 48 (4): 221-224.
- Moraes Jr, E.A. & A.G. Chiarello. 2005a. Sleeping sites of woolly mouse opossum Micoureus demerarae (Thomas) (Didelphimorphia, Didelphidae) in the Atlantic Forest of south-eastern Brazil. Revista Brasileira de Zoologia 22 (4): 1-5.
- Moraes Jr, E.A. & A.G. Chiarello. 2005b. A radio tracking study of home range and movements of the marsupial Micoureus demerarae (Thomas) (Mammalia, Didelphidae) in the Atlantic forest of south-eastern Brazil. Revista Brasileira de Zoologia 22 (1): 85-91.
- Nimer, E. 1989. Climatologia do Brasil. Rio de Janeiro, IBGE, Departamento de Recursos Naturais e Estudos Ambientais, 422p.
- O'farrell, M.J.; W.A. Clark; F.H. Emerson; S.M. Juarez; F.R. KAY; T.M. O'farrell; T.Y. Goodlett. 1994. Use of a mesh live trap for small mammals: are results from Sherman live traps deceptive? Journal of Mammalogy 75: 692-699.
- Pardini, R. & F. Umetsu. 2006. Pequenos mamíferos não-voadores da Reserva Florestal do Morro Grande - distribuição das espécies e da diversidade em uma área de Mata Atlântica. Biota Neotropica 6 (2): 1-22.
- Pardini, R.; S.M. Souza; R. Braga-Neto & J.P. Metzger. 2005. The role of forest structure, fragment size and corridors in maintaining small mammal abundance and diversity in a tropical forest landscape. Biological Conservation 124: 253-266.
- Prevedello J.A.; P. Ferreira; B.S. Papi; D. Loretto& M.V. Vieira. 2008. Uso do espaço vertical por pequenos mamíferos no Parque Nacional Serra dos Órgãos, RJ: um estudo de 10 anos utilizando três métodos de amostragem. Espaço & Geografia 11 (1): 95-119.
- Reis, N.R.; A.L. Peracchi; W.A. Pedro & I.P. Lima. 2006. Mamíferos do Brasil. Londrina, Authors' Edition, 437p.
- Ribeiro, M.C.; J.P. Metzger; A.C. Martensen; F.J. Ponzoni & M.M. Hirota. 2009. The Brazilian Atlantic Forest: How much is left, and how is the remaining forest distributed? Implications for conservation. Biological Conservation 142: 1141-1153.
- Rizzini, C.T. 1979. Tratado de fitogeografia do Brasil. São Paulo, Editora da Universidade de São Paulo, vol. 2, 374p.
- Tubelis, D.P. 2000. Aspects of the breeding biology of the gracile mouse opossum Gracilinanus microtarsus in a second growth forest in southeastern Brazil. Papéis Avulsos do Museu de Zoologia da USP 41: 173-185.
- Umetsu, F.; L. Naxara & R. Pardini. 2006. Evaluating the efficiency of pitfall traps for sampling small mammals in the Neotropics. Journal Mammalogy 87: 757-765.
- Vierira, E.M. & E.L.A. Monteiro-Filho. 2003. Vertical stratification of small mammals in the Atlantic rain forest of south-eastern Brazil. Journal of Tropical Ecology 19: 501-507.
- Vieira, M.V.; C.E.V. Grelle & R. Gentile. 2004. Differential trappability of small mammals in three habitats of southeastern Brazil. Brazilian Journal of Biology 64 (4): 895-900.
- Voss, R.S. & L.H. Emmons. 1996. Mammalian diversity in neotropical lowland rainforests: a preliminary assessment. Bulletin of the American Museum of Natural History 230: 3-115.
- Voss, R.S.; D.P. Lunde & N.B. Simmons. 2001. The mammals of Paracou, French Guiana: a Neotropical lowland rainforest fauna - part 2. Nonvolant species. Bulletin of the American Museum of Natural History 263: 3-236.
- Walter, H. 1986. Vegetação e zonas climáticas: tratado de ecologia global. São Paulo, Editora Pedagógica e Universitária, 313p.
- Williams, B.K.;, J.D. Nichols & M.J. Conroy. 2002. Analysis and management of animal populations. San Diego, Academic Press, 817p.
- Woodman, N.; R.M. Timm; N.A. Slade& T.J. Doonan. 1996. Comparison of traps and baits for censusing small mammals in neotropical lowlands. Journal of Mammalogy 77: 274-281.
Publication Dates
-
Publication in this collection
25 July 2011 -
Date of issue
June 2011
History
-
Accepted
27 Feb 2011 -
Received
23 Mar 2010