Abstract
Fourteen Trichoderma isolates were evaluated for their tolerance to two heavy metals, nickel and cadmium. Three isolates, MT-4, UBT-18, and IBT-I, showed high levels of nickel tolerance, whereas MT-4, UBT-18, and IBT-II showed better tolerance of cadmium than the other isolates. Under nickel stress, biomass production increased up to a Ni concentration of 60 ppm in all strains but then decreased as the concentrations of nickel were further increased. Among the nickel-tolerant isolates, UBT-18 produced significantly higher biomass upon exposure to nickel (up to 150 ppm); however, the minimum concentration of nickel required to inhibit 50% of growth (MIC50) was highest in IBT-I. Among the cadmium-tolerant isolates, IBT-II showed both maximum biomass production and a maximum MIC50 value in cadmium stress. As the biomass of the Trichoderma isolates increased, a higher percentage of nickel removal was observed up to a concentration of 40 ppm, followed by an increase in residual nickel and a decrease in biomass production at higher nickel concentrations in the medium. The increase in cadmium concentrations resulted in a decrease in biomass production and positively correlated with an increase in residual cadmium in the culture broth. Nickel and cadmium stress also influenced the sensitivity of the Trichoderma isolates to soil fungistasis. Isolates IBT-I and UBT-18 were most tolerant to fungistasis under nickel and cadmium stress, respectively.
Keywords
Heavy metals; Biosorption; Trichoderma; MIC50; Fungistasis
Introduction
Trichoderma species are imperfect filamentous fungi with teleomorphs and belong to the order Hypocreales in the Ascomycete division. Trichoderma spp. are among the most frequently isolated soil fungi and are well known for their biocontrol ability against a wide range of plant pathogenic fungi,11 Howell CR. Mechanisms employed by Trichoderma species in the biological control of plant disease; the history and evolution of current concept. Plant Dis. 2003;87:4-10. induction of localized and systemic defense responses in plants,22 Yedidia I, Benhamou N, Chet I. Induction of defense responses in cucumber (Cucumis sativus L.) by the biocontrol agent Trichoderma harzianum. Appl Environ Microbiol. 1999;65:1061-1070. and plant growth enhancement.33 Harman GE, Howell CR, Viterbo A, Chet I, Lorito M. Trichoderma species-opportunistic, avirulent plant symbionts. Nat Rev. 2004;2:43-56.,44 Hoyos-Carvajal L, Orduz S, Bissett J. Growth stimulation in bean (Phaseolus vulgaris L.) by Trichoderma. Biol Control. 2009;51:409-416. They play an important role in ecology by taking part in decomposition of plant residues, as well as in biodegradation of man-made chemicals and bioaccumulation of high amounts of various metals from wastewater and soil.55 Ezzi MI, Lynch JM. Biodegradation of cyanide by Trichoderma spp. and Fusarium spp. Enzyme Microb Technol. 2005;36:849-854.,66 Anand P, Isar J, Savan S, Saxena PK. Bioaccumulation of copper by Trichoderma viride. Bioresour Technol. 2006;97:1018-1025. Metal-containing pollutants are increasingly released into soil from industrial wastewater, as well as from wastes derived from chemical fertilizers and pesticides used in agriculture.77 Ting ASY, Choong CC. Bioaccumulation and biosorption efficacy of Trichoderma isolates SP2F1 in removing Copper (Cu II) from aqueous solutions. World J Microbiol Biotechnol. 2009;25:1431-1437. Some metal-containing pollutants are not biodegradable; they enter the food chain and lead to bioaccumulation.88 Errasquin LE, Vazquez C. Tolerance and uptake of heavy metals by Trichoderma atroviride isolated from sludge. Chemosphere. 2003;50(1):137-143. Minute amounts of metals, except those that are non-essential for biological functions, such as mercury, arsenic, lead and cadmium, influence vital metabolic processes and are required by all forms of life. However, metals may be toxic at concentrations higher than nutritional requirements. Evidence has suggested that Trichoderma spp. exhibit considerable tolerance for metals and accumulate high amounts of metals from polluted habitats.11 Howell CR. Mechanisms employed by Trichoderma species in the biological control of plant disease; the history and evolution of current concept. Plant Dis. 2003;87:4-10.,88 Errasquin LE, Vazquez C. Tolerance and uptake of heavy metals by Trichoderma atroviride isolated from sludge. Chemosphere. 2003;50(1):137-143. Therefore, metal-tolerant Trichoderma spp. may become dominant organisms in some polluted environments and may play an important role in eco-friendly metal removal technology.77 Ting ASY, Choong CC. Bioaccumulation and biosorption efficacy of Trichoderma isolates SP2F1 in removing Copper (Cu II) from aqueous solutions. World J Microbiol Biotechnol. 2009;25:1431-1437. As a component of soil fungistasis, metal ions may influence the growth, sporulation and enzymatic activities of Trichoderma.99 Papavizas GC. Trichoderma and Gliocladium: biology, ecology and potential for biocontrol. Ann Rev Phytopathol. 1985;23:23-54.,1010 Jaworska M, Dluzniewska J. The effect of manganese ions on development and antagonism of Trichoderma isolates. Pollish J Environ Stud. 2007;16(4):549-553. This can cause changes in the quantities of extracellular enzymes and metabolites,1111 Kredics L, Antal Z, Doczi I, Manczinger L. Effect of heavy metal on growth and extracellular enzyme activity of mycoparasitic Trichoderma strains. Bull Environ Contam Toxicol. 2001;66:249-354.,1212 Kredics L, Antal Z, Manczinger L, Nagy E. Beading of mycoparasitic Trichoderma strains for heavy metal resistance. Lett Appl Microbiol. 2001;33:112-116. as well as in overall biocontrol activities against plant pathogenic fungi and in plant growth-stimulating activities. Katayama and Matsumura1313 Katayama A, Matsumura F. Photochemically enhanced microbial degradation of environmental pollutants. Environ Sci Technol. 1991;25:1329-1333. demonstrated a degradation potential of a rhizosphere-competent Trichoderma sp. for several synthetic dyes, pentachlorophenol, endosulfan, and dichlorodiphenyltrichloroethane (DDT). Thus, Trichoderma spp. have acquired an exceptional role as part of a sustainable approach to bioremediation of herbicide/pesticide-laden soils.
However, the microhabitat behavior of Trichoderma spp. upon exposure to metal-containing compounds may differ, depending on the type of the metal and the Trichoderma isolate, and very little information in this regard is available. Hence, an attempt was made to screen Trichoderma isolates for nickel and cadmium tolerance and identify strains that can potentially be used for bioremediation of soils polluted with these metals.
Materials and methods
Isolation of Trichoderma spp. from soil
Trichoderma spp. were isolated from soil on a modified Trichoderma -specific medium (TSM)1414 Saha DK, Pan S. Qualitative evaluation of some specific media of Trichoderma and Gliocladium and their possible modifications. J Mycopath Res. 1997;34:7-13. using a dilution plate method.1515 Dhingra OD, Sinclair JB. Basic Plant Pathology Methods. 2nd edition London: CRC Lewis Publishers; 1995:434. Soil samples were collected from different sugarcane growing areas of Manipur and from tea industry areas in northern districts of West Bengal, where indiscriminate use of chemicals and effluents has caused heavy-metal soil toxicity. The samples were air-dried and ground to powder using a mortar and a pestle. Soil suspensions (1 mL of 10–3, 10–4, and 10–5 dilutions) were plated in Petri plates containing 20 mL of modified TSM. The suspensions were distributed uniformly over the medium surface by horizontal shaking and incubated at 28 ± 1 °C for seven days. Green colonies of the antagonist usually appeared after four or five days of incubation. Each colony was observed under a microscope using lactophenol cotton blue stain and identified to the genus level based on the available taxonomic literature.1616 Gams W, Bissett J. Morphology and identification of Trichoderma. In: Kubicek CP, Harman GE, eds. Trichoderma and Gliocladium – Basic Biology, Taxonomy and Genetics. vol. 1. London C.P: Taylor & Francis; 2002:3–31. The shape, size and aggregation of phialospores and phialides were used as main identification criteria, along with cultural characteristics on potato dextrose agar (PDA). The colonies identified as Trichoderma spp. were transferred onto PDA slants and kept at 4 °C for further use. The isolates from Manipur were designated as MT, and the isolates from the tea plantation areas were designated as IBT, followed by a number. One isolate, namely, UBT-18, was obtained from the culture collection of the Department of Plant Pathology, Uttar Banga Krishi Viswavidyalaya.
Selection of nickel- and cadmium-tolerant isolates of Trichoderma spp.
In vitro tolerance of Trichoderma spp. to different concentrations of nickel and cadmium was determined by the poisoned food technique.1515 Dhingra OD, Sinclair JB. Basic Plant Pathology Methods. 2nd edition London: CRC Lewis Publishers; 1995:434. PDA medium (100 mL) was prepared in 250-mL conical flasks, then appropriate quantities of nickel and cadmium stock solutions were added to molten PDA to get the required concentrations (40, 60, 100, 150, and 200 mg/L), and the resulting media were poured into Petri plates after gentle shaking. The non-amended medium served as a control. The plates were inoculated by placing 6-mm mycelial discs of 4-day-old cultures of the Trichoderma isolates on the agar surface and incubated at 28 ± 1 °C for 2–3 days. Isolates showing maximum radial growth on the media, irrespective of the metal concentration, were selected for further studies.
Biomass production by Trichoderma isolates and determination of minimum inhibitory concentration of the metals
For biomass preparation, selected Trichoderma isolates were inoculated on PDA plates and incubated at room temperature (27 ± 1 °C). After five days, a small portion (0.5 mm) from the fungal mass was cut, transferred into a 250-mL conical flask containing 50 mL of potato dextrose (PD) broth supplemented with different concentrations of a metal (0, 40, 60, 100, 150, and 200 ppm), and incubated in triplicates at 27 ± 1 °C for seven days. The biomass was harvested by filtering through Whatman no. 1 filter paper and then washed thoroughly with deionized water to remove the growth medium. The harvested mycelia were oven-dried at 60 °C for 48 h and the dry weight was measured using a Sartorius LA8200S digital weight balance with an accuracy of 0.1 mg. The inhibition of biomass production was calculated based on the dry weight using the following formula:
where PI is the percentage of inhibition; X is biomass in the control (0 ppm) broth; and Y is biomass in the metal-containing broth.
The minimum inhibitory concentration of each metal, causing 50% of growth inhibition (MIC50) of the selected Trichoderma isolates, was calculated from the growth inhibition results.
Estimation of residual metals in culture broth
After harvesting the biomass of each isolate grown in the PD broth amended with different concentrations of nickel or cadmium (0, 10, 25, 50 and 100 ppm), the culture broth was assayed for residual metal, following the method described by Tandon.1717 Tandon HLS. Methods of Analysis of Soils, Plants, Waters, Fertilisers and Organic Manures. New Delhi, India: Fertilisers Development and Consultation Organisation; 2005:86–87. Five milliliters of the culture broth was placed in a 100-mL clean beaker, followed by the addition of 10 mL of a triacid mixture (HNO3:H2SO4:HClO4, 9:4:1, v/v/v), and the content was mixed by swirling and kept overnight. The mixture was digested in a digestion chamber at 60 °C followed by heating at 90 °C until the production of red NO2 fumes ceased. The content was further evaporated until the volume was reduced to about 2–3 mL. The completion of digestion was confirmed when the liquid became colorless. After cooling the beaker, its content was transferred quantitatively to a 50-mL capacity volumetric flask, diluted to 50 mL with distilled water, and kept overnight. On the next day, it was filtered through Whatman no. 44 filter paper. The filtrates were analyzed for cadmium or nickel using a Perkin Elmer Analyst 200AA flame spectrophotometer. Each sample was analyzed two or three times at a wavelength of 445 nm for nickel or 229 nm for cadmium. Residual metal concentrations were expressed in µg/mL of culture broth.
Tolerance of Trichoderma isolates to soil fungistasis under heavy metal stress
Fungistatic effects of soil were studied using the soil cellophane agar disk method1818 Jackson RM. An investigation of fungistasis in Nigerian soils. J Gen Microbiol. 1958;18:248-258. with a slight modification. In this experiment, soil samples were amended with different concentrations of nickel or cadmium to adjust the metal contamination levels to 50, 100 or 150 ppm and kept for one month for stabilization. Well-saturated, metal-contaminated soil (100 g) was filled in a plastic cup, and the upper surface was smoothed using thumb pressure. Cellophane paper was cut to the diameter of the cup and boiled to eliminate plasticizer effects. A single piece of cellophane was placed on the smooth soil surface, and a disc (1 cm in diameter) of 2% water agar was placed on the cellophane paper. The entire system was refrigerated for 24 h to activate the agar disc. On the next day, a conidial suspension (103 cfu/mL) of the selected metal-tolerant Trichoderma isolate was applied to the agar disc and incubated at 28 ± 1 °C for 20 h. After the incubation, the disc was transferred to a glass slide and stained with 0.1% lactophenol cotton blue to examine conidial germination under a light microscope at 20× magnification. The percentage of germinated conidia was recorded, and the percent of germination inhibition was calculated.
Statistical analysis
The experiments were conducted using a factorial, completely randomized design with three replications, considering the isolates as factor A and the metal concentrations as factor B. An analysis of variance (ANOVA) was performed for all parameters using the INDOSTAT package. Comparison of means was done by Duncan's multiple range test at the p < 0.05 level of significance.1919 Steel RGD, Torrie JH. Principles and Procedures of Statistics. New York: McGraw Hill; 1980:672. The identical letters in the results denote non-significant differences among the treatments within each isolate.
Results and discussion
Trichoderma spp. are ubiquitous microorganisms distributed in almost all types of crop rhizosphere2020 Chet I, Inbar J, Hadar Y. Fungal antagonists and mycoparasites. In: Wicklow FS., ed. The Mycota IV: Environmental and Microbial Relationships. Heidelberg: Springer Verlag; 1997:165–184.,2121 Klein D, Eveleigh DE. Ecology of Trichoderma. In: Kubicek CP, Harman GE, eds. Trichoderma and Gliocladium – Basic Biology, Taxonomy and Genetics. vol. 1. London: Taylor & Francis;1998:57–73. and have even been found in metal-polluted ecosystems.2222 Hussein H, Farag S, Moawad H. Isolation and characterisation of Pseudomonas resistant to heavy metals contaminants. Arab J Biotechnol. 2003;7:13-22.,2323 Paremeswari E, Lakshamanan A, Thilagavathi T. Biosorption and metal tolerance potential of filamentous fungi isolated from metal polluted ecosystem. Elec J Environ Agric Food Chem. 2010;9(4):664-671. In this study, 14 Trichoderma isolates, namely, MT-1, MT-4, MT-7, MT-8, MT-11, MT-13, MT-18, MT-21, MT-23, MT-24, MT-25, IBT-I, IBT-II, and UBT-18, were identified and selected for further study, based on their cultural variability and growth rates. Cultural variability existed among the isolates with respect to their mycelial growth pattern, color of sporulation, and pigmentation of the medium (Table 1), indicating that the isolates might be able to produce secondary metabolites. The maximum growth rate was demonstrated by UBT-18, followed by MT-13, MT-8, MT-4, and MT-23.
The effects of different concentrations of Ni on mycelial growth of the 14 Trichoderma isolates were tested (Table 2), and the maximum radial growth was shown by UBT-18 (70.8 mm) at a Ni concentration of 40 ppm, followed by isolate MT-4 (66.0 mm), with a significant difference between the two isolates. Six other isolates, namely, IBT-I, IBT-II, MT-1, MT-23, and MT-24, exhibited significantly higher mycelial growth at the same Ni concentration compared with their corresponding non-amended cultures. With a further increase of Ni concentration in the medium to 60 ppm, only four isolates, IBT-I, IBT-II, MT-21, and MT-23, showed significantly higher mycelial growth versus their respective controls. At higher concentrations of Ni, from 100 to 200 ppm, there was a significant reduction in mycelial growth of all isolates, although three of them, viz., MT-4, IBT-I, and UBT-18, consistently showed higher mycelial growth at concentrations of up to 150 ppm. Although MT-21 showed a higher level of tolerance to Ni toxicity at 200 ppm, its growth was comparatively low at concentrations from 60 to 150 ppm compared with the other Trichoderma isolates.
The effects of nickel on radial growth of the 14 Trichoderma isolates are presented in Figs. 1 and 2. It was noticed that the growth of the Trichoderma isolates was significantly influenced by the heavy metal; in particular, at 40 ppm of Ni in the amended medium the growth was even higher than that observed in the non-amended medium. At higher concentrations of the heavy metal, from 60 to 200 mg/L, there was a significant reduction in radial mycelial growth of all the Trichoderma isolates. Isolate MT-4 showed the highest tolerance to nickel. Taken together, isolates MT-4, IBT-I, and UBT-18 were considered to be resistant since their radial growth decreased at a slower rate than that of the other isolates, while isolates MT-7, MT-18, MT-11, MT-13, and MT-25 were most sensitive and no mycelial growth was detected at 200 mg/L. Hence, three isolates, namely, MT-4, IBT-I and UBT-18, were finally screened due to their high Ni tolerance.
Screening of the Trichoderma isolates for their cadmium tolerance revealed that the isolates varied significantly in their levels of tolerance, irrespective of the cadmium concentrations tested (Table 3). There was a significant reduction in the mycelial growth of the Trichoderma isolates upon exposure to cadmium (Fig. 3). At a cadmium concentration of 40 ppm, the maximum mycelial growth was shown by IBT-II (73.4 mm), which was significantly higher than that of UBT-18 (66.6 mm), followed by MT-4 and MT-24 (62.6 and 62.2 mm, respectively). The degree of tolerance of the Trichoderma isolates, irrespective of the cadmium level, is presented in Fig. 3. The data indicated that MT-4, UBT-18, and IBT-II were tolerant, IBT-I, MT-7, MT-11, MT-18, MT-21, and MT-23 were moderately tolerant, and MT-1, MT-8, and MT-13 were susceptible to cadmium toxicity. In Fig. 4, the effects of cadmium concentrations on the Trichoderma isolates are depicted, and a trend of a decreasing growth rate of the isolates was observed with increasing concentrations of cadmium.
In the absence of a rational method for an a priori prediction of a biosorption potential of a microorganism, the only method for identifying and developing newer and efficient biosorbents is sustained screening of microbes.2424 Muraleedharan TR, Iyengar L, Venkobachar C, Biosorption:.an attractive alternative for metal removal and recovery. Curr Sci. 1991;61:379–385. Variations in metal tolerance among different species of a genus or within the same species might be due to the presence of one or more resistance mechanisms exhibited by different fungi.2525 Zafar S, Aqil F, Ahmad I. Metal tolerance and biosorption potential of filamentous fungi isolated from metal contaminated agricultural soil. Bioresour Technol. 2007;98:2557-2561. Sarkar et al.2626 Sarkar S, Satheshkumar A, Jayanthi R, Premkumar R. Biosorption of nickel by live biomass of Trichoderma harzianum. Res J Agric Sci. 2010;1(2):69-74. reported Trichoderma harzianum to be moderately tolerant to up to 60 ppm of Ni, at that concentration the level of inhibition of mycelial growth was 33.3%. A further increase in the Ni concentration reduced the growth, and total inhibition was observed at 200 mg/L. Lima et al.2727 de Lima AF, de Gabrielle FM, de Lima MAB, et al. Role of morphology and polyphosphate in Trichoderma harzianum related to cadmium removal. Molecules. 2011;16:2486-2500. also observed influence of cadmium on radial growth of T. harzianum. The results of the present investigation are in line with these earlier observations.
When the effects of different nickel concentrations on biomass production of the three promising Trichoderma isolates (in order of ranking) were studied, the maximum biomass weight was recorded for UBT-18 (359 mg), which was significantly higher than the values obtained for MT-4 (256.67 mg) and IBT-I (225 mg), at a Ni concentration of 40 ppm. With a further increase of the nickel concentration in the medium to 60 ppm, biomass production by all the isolates screened was insignificant compared with their respective controls. At higher concentrations of nickel, from 100 to 200 ppm, there was a significant reduction in biomass of all three isolates. Although isolate UBT-18 showed significantly higher biomass production at Ni concentrations of up to 150 ppm, IBT-I showed somewhat higher biomass production compared to the other two isolates at 200 ppm (Table 4).
Effects of different cadmium concentrations on biomass production were studied using the three most tolerant Trichoderma isolates. The maximum biomass value was recorded for IBT-II (523.70 mg), and it was significantly higher than the values obtained for UBT-18 (147.03 mg) and MT-4 (147.01 mg) at a Cd concentration of 40 ppm (Table 5). With a further increase in the cadmium concentration, biomass production was significantly reduced, except that IBT-II showed insignificant variations in biomass production at cadmium concentrations of up to 150 ppm. Isolate MT-4 (165.33 mg) and UBT-18 (166.67 mg) exhibited insignificant variations between each other in biomass production upon exposure to cadmium toxicity (up to 200 ppm).
Significant reductions in microbial biomass and soil respiration have been found in metal-contaminated soils compared to uncontaminated ones.2828 Doelman P. Resistance of soil microbial communities to heavy metals. In: Jensen V, Kjoller A, Sorensen CH, eds. Microbial Communities in Soil. London, UK: Elsevier Applied Science Publishers; 1986.
29 Hattori H. Influence of heavy metals on soil microbial activities. Soil Sci Plant Nutr. 1992;38:93-100.-3030 Konopka A, Zakharova T, Bischoff M, Oliver L, Nakatsu C, Turco RF. Microbial biomass and activity in lead-contaminated soil. Appl Environ Microbiol. 1999;65:2256-2260. Optimum biosorption conditions depend on pH, biomass of the microorganism, contact time and temperature. The Langmuir, Freundlich and Dubinin–Radushkevich model, which describes the biosorption isotherm of a metal ion, has indicated that biosorption of cadmium by Hylocomium splendens biomass occurs through chemical ion exchange.3131 Sari A, Mendil D, Tuzen M, Soylak M. Biosorption of Cd(II) and Cr(III) from aqueous solution by moss (Hyloconium splendens) biomass: equilibrium, kinetic and thermodynamic studies. Chem Eng J. 2008;144(1):1-9. The main functional groups responsible for a biosorption process are hydroxyls, carbonyls, carboxyls, sulfonates, amides, imidazoles, phosphonates, and phosphodiester groups as established by Pradhan et al.3232 Pradhan S, Singh S, Rai LC. Characterization of various functional groups present in the capsule of Microcystis and study of their role in biosorption of Fe, Ni and Cr. Bioresour Technol. 2007;98:595-601. and Volesky.3333 Volesky B. Biosorption and me. Water Res. 2007;41:4017-4029. Some of these groups are present in Trichoderma sp. biomass and may interact with the metal ions. It has also been reported that binding of Ni(II) to biopolymers occurs mainly in the peptidoglycan layer of the cell surface.3434 Lin Z, Wu J, Xue R, Yang Y. Spectroscopic characterization of Au3+ biosorption by waste biomass of Saccharomyces cerevisiae. Spectrochim Acta. 2005;61:761-765.
The minimum inhibitory concentrations of nickel and cadmium required for 50% of growth inhibition (MIC50) of the isolates were computed, and the results are presented in Table 6. The highest minimum inhibitory concentration of nickel was calculated for IBT-I (1884.93 ppm), followed by MT-4 (638.90 ppm), whereas the highest MIC50 of cadmium was calculated for IBT-II (227.92 ppm), followed by MT-4 (71.16 ppm).
Determination of residual nickel in the culture broth after harvesting mycelial biomass from the metal-amended media revealed total removal of nickel by the increased biomass of the Trichoderma isolates at Ni concentrations of up to 40 ppm, followed by an increase in residual nickel and a decrease in biomass production at higher nickel concentrations in the medium. Among the isolates, IBT-I was most potent in biosorption of nickel, followed by UBT-18 and MT-4 (Fig. 5). The results are in agreement with the findings of Sarkar et al., 2626 Sarkar S, Satheshkumar A, Jayanthi R, Premkumar R. Biosorption of nickel by live biomass of Trichoderma harzianum. Res J Agric Sci. 2010;1(2):69-74. who recorded 90.2% removal of Ni from a 50 ppm-amended culture broth by T. harzianum after seven days of growth, beyond that, there was no increase in metal uptake. The use of a solid-phase extraction process to determine biosorption of heavy metals showed that 0.59 µg of nickel could be removed by Aspergillus fumigatus from a liter of polluted water.3535 Soylak M, Tuzen M, Mendil D, Turkekul I. Biosorption of heavy metals on Aspergillus fumigatus immobilized Dianon HP-2MG resin for their atomic absorption spectrometric determinations. Talanta. 2006;70(5):1129-1135.
The trend of cadmium biosorption was quite different, so that the increasing cadmium concentrations resulted in decreasing biomass production by all of the test isolates and positively correlated with increased residual cadmium in the culture broth (Fig. 6).
It has been suggested that metal uptake by T. harzianum is highly pH- and temperature-dependent and the maximum metal uptake takes place at pH 4.3636 Tsezos M, Volesky B. Biosorption of uranium and thorium. Biotechnol Bioeng. 1981;23:583-604.,3737 Tobin JM, Cooper DG, Neufeld RJ. Uptake of metal ions by Rhizopus arrhizus. Appl Environ Microbiol. 1984;47:821-824. At pH values above 7, metal uptake is reduced as metals exist as hydroxide colloids and precipitate at alkaline pH due to osmotic changes and a hydrolyzing effect,3838 Filipovic-Kovacevic Z, Sipos L, Briski F. Biosorption of chromium, copper, nickel and zinc ions onto fungal pellets of Aspergillus niger 405 from aqueous solutions. Food Tech Biotech. 2000;38:211-216.,3939 Nasseri S, Mazaheri AM, Noori SM, Rostami KH, Shariat M, Nadafi K. Chromium removal from tanning effluent using biomass of Aspergillus oryzae. Pak J Biol Sci. 2002;5:1056-1059. thus resulting in a decrease in the sorption rate.4040 Liu N, Luo S, Yang Y, Zhang T, Jin J, Liao J. Biosorption of americium-241 by Saccharomyces cerevisiae. J Radio Anal Nuclear Chem. 2002;252:187-191. As biomass of T. harzianum increased, the pH of the medium was shown to become more acidic4141 Benitez T. Increased antifungal and chitinase specific activates of Trichoderma harzianum CECT 2413 by addition of a cellulose binding-domain. Appl Microbiol Biotechnol. 2004;64:675-685.; however, below pH 4 biomass production was reduced and subsequently the residual metal concentration increased. Low absorption of heavy metals at low pH is attributed to the competition between the hydrogen ion and the metal ion at the sorption site.4242 Congeevaram S, Dhanarani S, Park J, Dexilin M, Thamaraselvi K. Biosorption of chromium and nickel by heavy metal resistant fungal and bacterial isolates. J Hazard Mater. 2007;146:270-277.
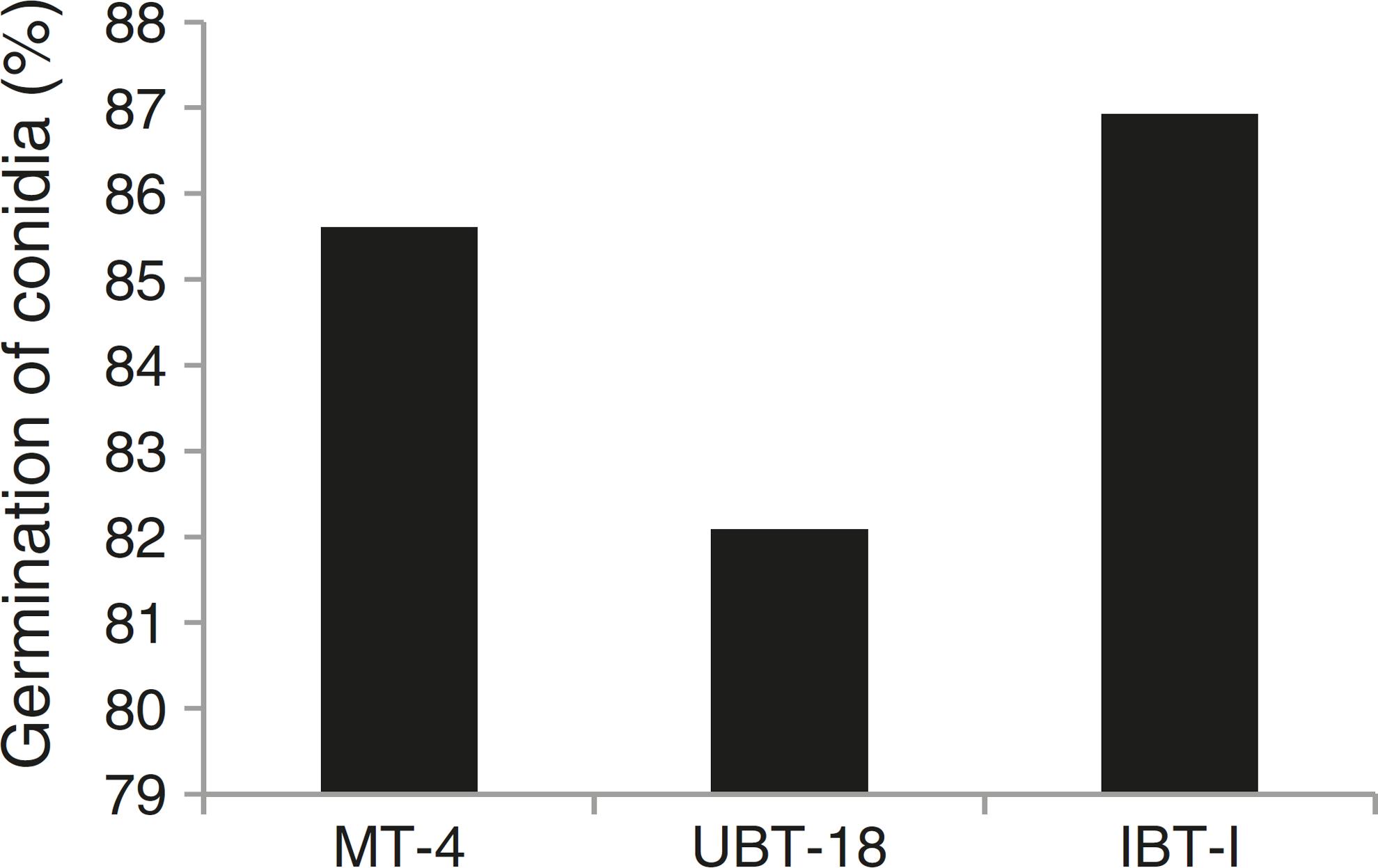
The results obtained while studying germination of conidia of the Trichoderma isolates under nickel and cadmium stress conditions revealed that the isolates differed significantly in their responses to fungistatic effects (Table 7). Under non-amended conditions, a significantly lower germination rate was shown by UBT-18 (88.23%) compared to IBT-I and MT-4 (93.73 and 91.73%, respectively). The different levels of nickel stress also had significant effects on spore germination; however, the variation in the interaction effect with the Trichoderma isolates was non-significant. Irrespective of the nickel concentration, IBT-I was most resistant to fungistatic effects (86.93% conidial germination), followed by MT-4 and UBT-18 (85.61 and 82.09% conidial germination, respectively) (Fig. 7). In the absence of cadmium, IBT-II exhibited the highest germination rate (97.30%), which significantly differed from that of MT-4 (88.37%). With the increase in the cadmium concentration, germination was significantly affected, particularly in the case of IBT-II, indicating that the isolate was very sensitive to the fungistatic effect. UBT-18 was found to be most tolerant to the fungistatic effect, even at a higher cadmium stress level (66.83% conidial germination), and its germination rate was significantly different from that of MT-4 (57.04%). Irrespective of the cadmium concentration, UBT-18 showed the highest resistance to the fungistatic effect (76.50% conidial germination), followed by IBT-II and MT-4 (73.06 and 69.24% conidial germination, respectively) (Fig. 8). Partial annulment of soil fungistasis has a significant impact on survival and population dynamics of Trichoderma and Gliocladium in soil.4343 Papavizas GC, Lumsden RD. Biological control of soil borne fungal propagules. Ann Rev Phytopathol. 1980;18:389-412. Roy and Pan4444 Roy A, Pan S. Effect of fungistasis on germinability of wild and mutant isolates of Trichoderma harzianum and Gliocladium virens. J Mycol Plant Pathol. 2005;35(2):319-323. reported that gamma irradiation significantly increased phialospore and chlamydospore germination rates in mutants of T. harzianum and Gliocladium virens compared to their wild types.
Effect of fungistasis on conidial germination of the Trichoderma isolates under nickel and cadmium stressed condition.
In conclusion, the present investigation highlights the significance of Trichoderma spp. as potential metal biosorbents. Four isolates obtained in this study, viz., MT-4, UBT-18, IBT-I, and IBT-II, can be exploited as potent bioremediation agents in nickel- and cadmium-polluted agricultural fields.
-
Associate Editor: Cynthia Canêdo da Silva
References
-
1Howell CR. Mechanisms employed by Trichoderma species in the biological control of plant disease; the history and evolution of current concept. Plant Dis 2003;87:4-10.
-
2Yedidia I, Benhamou N, Chet I. Induction of defense responses in cucumber (Cucumis sativus L.) by the biocontrol agent Trichoderma harzianum. Appl Environ Microbiol 1999;65:1061-1070.
-
3Harman GE, Howell CR, Viterbo A, Chet I, Lorito M. Trichoderma species-opportunistic, avirulent plant symbionts. Nat Rev 2004;2:43-56.
-
4Hoyos-Carvajal L, Orduz S, Bissett J. Growth stimulation in bean (Phaseolus vulgaris L.) by Trichoderma. Biol Control 2009;51:409-416.
-
5Ezzi MI, Lynch JM. Biodegradation of cyanide by Trichoderma spp. and Fusarium spp. Enzyme Microb Technol 2005;36:849-854.
-
6Anand P, Isar J, Savan S, Saxena PK. Bioaccumulation of copper by Trichoderma viride. Bioresour Technol 2006;97:1018-1025.
-
7Ting ASY, Choong CC. Bioaccumulation and biosorption efficacy of Trichoderma isolates SP2F1 in removing Copper (Cu II) from aqueous solutions. World J Microbiol Biotechnol 2009;25:1431-1437.
-
8Errasquin LE, Vazquez C. Tolerance and uptake of heavy metals by Trichoderma atroviride isolated from sludge. Chemosphere 2003;50(1):137-143.
-
9Papavizas GC. Trichoderma and Gliocladium: biology, ecology and potential for biocontrol. Ann Rev Phytopathol 1985;23:23-54.
-
10Jaworska M, Dluzniewska J. The effect of manganese ions on development and antagonism of Trichoderma isolates. Pollish J Environ Stud 2007;16(4):549-553.
-
11Kredics L, Antal Z, Doczi I, Manczinger L. Effect of heavy metal on growth and extracellular enzyme activity of mycoparasitic Trichoderma strains. Bull Environ Contam Toxicol 2001;66:249-354.
-
12Kredics L, Antal Z, Manczinger L, Nagy E. Beading of mycoparasitic Trichoderma strains for heavy metal resistance. Lett Appl Microbiol 2001;33:112-116.
-
13Katayama A, Matsumura F. Photochemically enhanced microbial degradation of environmental pollutants. Environ Sci Technol. 1991;25:1329-1333.
-
14Saha DK, Pan S. Qualitative evaluation of some specific media of Trichoderma and Gliocladium and their possible modifications. J Mycopath Res 1997;34:7-13.
-
15Dhingra OD, Sinclair JB. Basic Plant Pathology Methods 2nd edition London: CRC Lewis Publishers; 1995:434.
-
16Gams W, Bissett J. Morphology and identification of Trichoderma In: Kubicek CP, Harman GE, eds. Trichoderma and Gliocladium – Basic Biology, Taxonomy and Genetics vol. 1. London C.P: Taylor & Francis; 2002:3–31.
-
17Tandon HLS. Methods of Analysis of Soils, Plants, Waters, Fertilisers and Organic Manures New Delhi, India: Fertilisers Development and Consultation Organisation; 2005:86–87.
-
18Jackson RM. An investigation of fungistasis in Nigerian soils. J Gen Microbiol 1958;18:248-258.
-
19Steel RGD, Torrie JH. Principles and Procedures of Statistics New York: McGraw Hill; 1980:672.
-
20Chet I, Inbar J, Hadar Y. Fungal antagonists and mycoparasites In: Wicklow FS., ed. The Mycota IV: Environmental and Microbial Relationships Heidelberg: Springer Verlag; 1997:165–184.
-
21Klein D, Eveleigh DE. Ecology of Trichoderma In: Kubicek CP, Harman GE, eds. Trichoderma and Gliocladium – Basic Biology, Taxonomy and Genetics vol. 1. London: Taylor & Francis;1998:57–73.
-
22Hussein H, Farag S, Moawad H. Isolation and characterisation of Pseudomonas resistant to heavy metals contaminants. Arab J Biotechnol 2003;7:13-22.
-
23Paremeswari E, Lakshamanan A, Thilagavathi T. Biosorption and metal tolerance potential of filamentous fungi isolated from metal polluted ecosystem. Elec J Environ Agric Food Chem 2010;9(4):664-671.
-
24Muraleedharan TR, Iyengar L, Venkobachar C, Biosorption:.an attractive alternative for metal removal and recovery. Curr Sci 1991;61:379–385.
-
25Zafar S, Aqil F, Ahmad I. Metal tolerance and biosorption potential of filamentous fungi isolated from metal contaminated agricultural soil. Bioresour Technol 2007;98:2557-2561.
-
26Sarkar S, Satheshkumar A, Jayanthi R, Premkumar R. Biosorption of nickel by live biomass of Trichoderma harzianum. Res J Agric Sci 2010;1(2):69-74.
-
27de Lima AF, de Gabrielle FM, de Lima MAB, et al. Role of morphology and polyphosphate in Trichoderma harzianum related to cadmium removal. Molecules 2011;16:2486-2500.
-
28Doelman P. Resistance of soil microbial communities to heavy metals. In: Jensen V, Kjoller A, Sorensen CH, eds. Microbial Communities in Soil London, UK: Elsevier Applied Science Publishers; 1986.
-
29Hattori H. Influence of heavy metals on soil microbial activities. Soil Sci Plant Nutr 1992;38:93-100.
-
30Konopka A, Zakharova T, Bischoff M, Oliver L, Nakatsu C, Turco RF. Microbial biomass and activity in lead-contaminated soil. Appl Environ Microbiol 1999;65:2256-2260.
-
31Sari A, Mendil D, Tuzen M, Soylak M. Biosorption of Cd(II) and Cr(III) from aqueous solution by moss (Hyloconium splendens) biomass: equilibrium, kinetic and thermodynamic studies. Chem Eng J 2008;144(1):1-9.
-
32Pradhan S, Singh S, Rai LC. Characterization of various functional groups present in the capsule of Microcystis and study of their role in biosorption of Fe, Ni and Cr. Bioresour Technol 2007;98:595-601.
-
33Volesky B. Biosorption and me. Water Res 2007;41:4017-4029.
-
34Lin Z, Wu J, Xue R, Yang Y. Spectroscopic characterization of Au3+ biosorption by waste biomass of Saccharomyces cerevisiae. Spectrochim Acta 2005;61:761-765.
-
35Soylak M, Tuzen M, Mendil D, Turkekul I. Biosorption of heavy metals on Aspergillus fumigatus immobilized Dianon HP-2MG resin for their atomic absorption spectrometric determinations. Talanta 2006;70(5):1129-1135.
-
36Tsezos M, Volesky B. Biosorption of uranium and thorium. Biotechnol Bioeng 1981;23:583-604.
-
37Tobin JM, Cooper DG, Neufeld RJ. Uptake of metal ions by Rhizopus arrhizus. Appl Environ Microbiol 1984;47:821-824.
-
38Filipovic-Kovacevic Z, Sipos L, Briski F. Biosorption of chromium, copper, nickel and zinc ions onto fungal pellets of Aspergillus niger 405 from aqueous solutions. Food Tech Biotech 2000;38:211-216.
-
39Nasseri S, Mazaheri AM, Noori SM, Rostami KH, Shariat M, Nadafi K. Chromium removal from tanning effluent using biomass of Aspergillus oryzae. Pak J Biol Sci 2002;5:1056-1059.
-
40Liu N, Luo S, Yang Y, Zhang T, Jin J, Liao J. Biosorption of americium-241 by Saccharomyces cerevisiae. J Radio Anal Nuclear Chem 2002;252:187-191.
-
41Benitez T. Increased antifungal and chitinase specific activates of Trichoderma harzianum CECT 2413 by addition of a cellulose binding-domain. Appl Microbiol Biotechnol 2004;64:675-685.
-
42Congeevaram S, Dhanarani S, Park J, Dexilin M, Thamaraselvi K. Biosorption of chromium and nickel by heavy metal resistant fungal and bacterial isolates. J Hazard Mater 2007;146:270-277.
-
43Papavizas GC, Lumsden RD. Biological control of soil borne fungal propagules. Ann Rev Phytopathol 1980;18:389-412.
-
44Roy A, Pan S. Effect of fungistasis on germinability of wild and mutant isolates of Trichoderma harzianum and Gliocladium virens. J Mycol Plant Pathol 2005;35(2):319-323.
Publication Dates
-
Publication in this collection
Apr-Jun 2016
History
-
Received
16 Feb 2014 -
Accepted
10 Aug 2015