ABSTRACT
Twelve known compounds, including eight alkaloids, three lignans and one gossypol derivative, were isolated from the branches of Polyalthia rumphii (Blume ex Hensch.) Merr., Annonaceae. The chemical structures were determined by spectroscopic methods and comparison with literature data. All the isolates were evaluated the cytotoxicity against three human cancer cell lines: Hela, MCF-7 and A549, the results showed that partial of isolates displayed weak cytotoxicities with the IC50 values ranging from 25 to 40 µg/ml.
Keywords:
Alkaloids; Lignan; Gossypol derivative; Cytotoxic activity; Polyalthia rumphii
Introduction
Polyalthia rumphii (Blume ex Hensch.) Merr., Annonaceae, distributes widely in tropic and subtropic area (Editorial Board of FOC, 2011Editorial Board of FOC, 2011. Flora of China, vol. 19. Science Press, Beijing, pp. 696.). Chinese used many species of this genus as traditional medicinal plants to treat intractable diseases (Yuan et al., 2011Yuan, Y., Huang, G.J., Wang, T.S., Chen, G.Y., 2011. In vitro screening of five Hainan plants of Polyalthia (Annonaceae) against human cancer cell lines with MTT assay. J. Med. Plants Res. 15, 837-841.). Especially Li ethnic minority, located in Hainan Island, used the extracts of P. rumphii for prevention of fever, hypertension and inhibition of cancer cells (Yuan et al., 2011Yuan, Y., Huang, G.J., Wang, T.S., Chen, G.Y., 2011. In vitro screening of five Hainan plants of Polyalthia (Annonaceae) against human cancer cell lines with MTT assay. J. Med. Plants Res. 15, 837-841.; Machana et al., 2012Machana, S., Weerapreeyakul, N., Barusrux, S., Thumanu, K., Tanchanuch, W., 2012. Synergistic anticancer effect of the extracts from Polyalthia evecta caused apoptosis in human hepatoma (HepG2) cells. Asian Pac. J. Trop. Biomed. 2, 589-596.). Previous phytochemical research reported that alkaloids and lignans, isolated from P. rumphii, were with anticancer activities (Wang et al., 2012aWang, D.F., Chou, G.X., Zhao, N.Y., Zhang, T., Xu, H., 2012a. Study on chemical constituents in stems of Dendrobium nobile. Chin. Trad. Herb. Drugs 43, 1492-1945.,bWang, T.S., Yuan, Y., Wang, J., Han, C.R., Chen, G.Y., 2012b. Anticancer activities of constituents from the stem of Polyalthia rumphii. Pak. J. Pharm. Sci. 25, 353-356., 2013Wang, T.S., Luo, Y.P., Wang, J., He, M.X., Zhong, M.G., Li, Y., Song, X.P., 2013. (+)-Rumphiin and polyalthurea, new compounds from the stems of Polyalthia rumphii. Nat. Prod. Commun. 8, 1427-1429.). As part of our ongoing purpose of screening the bioactive constituents from P. rumphii, this present investigation described the isolation, structure identification, and all isolates were evaluated the cytotoxic activities against three human cancer cell lines by MTT assay. By chromatographic methods, twelve know compounds were isolated, the chemical structures of them were mainly determined by physical and spectroscopic methods including [α]D, NMR and MS as well as by comparing with literature data.
Materials and methods
General
NMR spectra were recorded on a Bruker AV-400, deuterated reagents were CDCl3 (TMS as internal standard) and CD3OD (residual solvent as internal standard). ESI-MS was measured on a Bruker amaZon SL mass spectrometer, and HR-ESI-TOF-MS were measured on an AB Sciex TripleTOF 5600+ high-resolution mass spectrometer. Preparative HPLC were performed on a Waters 2545-2767-2489 HPLC system. The single-crystal was diffracted on an X-Ray Diffractometer of Bruker Smart Apex-II CCD.
Plant material
Fresh branches of Polyalthia rumphii (Blume ex Hensch.) Merr., Annonaceae, were collected in September 2013, from Bawang Mountain of Hainan Island, China, and authenticated by Prof. Qiongxin Zhong (Hainan Normal University). The voucher specimen (No. 201309XHAL) was deposited at our key laboratory.
Extraction and isolation
The branches of P. rumphii were air-dried at room temperature, and then reduced to coarse powder by crushing machine. This coarse powder (6.78 kg) was extracted in July 2014, with 95% EtOH (15 l × 3) at room temperature, and the solvents were evaporated under reduced pressure to get crude EtOH gum, which was continuously suspended in 0.1% H2SO4 (4 l). The liposoluble components were removed by PE (petroleum ether) from acidic solution, and the acidic aqueous solution was extracted by EtOAc (4 l × 3) to get part I (94.8 g), the remaining aqueous layer was alkalinized (pH 9–10) by ammonia and extracted with CHCl3 (4 l × 3) to get part II (48.1 g).
Part I was fractioned on a silica gel column (200–300 mesh, Qingdao Haiyang Chem. Co., Ltd.) using gradient PE-EtOAc solvent system to afford forty fractions (XMZ-1 to XMZ-40). XMZ-10 (0.3 g) was isolated by silica gel column (400 mesh, Qingdao Haiyang Chem. Co., Ltd.) with gradient PE-EtOAc solvent system, then followed by HPLC on an Agilent C-8 column (Agilent Zorbax Eclipse XDB C-8, 250 × 9.4 mm, 5 µm, MeOH:H2O 80:20, 4 ml/min) to provide 7 (44.4 mg). XMZ-12 (4.2 g) was purified by repeated silica gel column (400 mesh) with gradient light petroleum and EtOAc to give 3 (5.9 mg). XMZ-30 (0.7 g) was similarly purified by repeated silica gel column and followed by HPLC on Agilent C-8 column (MeOH:H2O 80:20, 4 ml/min) to give 8 (37.9 mg). Combined fractions (1.6 g) of XMZ-22 to XMZ-29 was introduced to a silica gel column (400 mesh) and eluted by gradient PE-EtOAc system (3:1 to pure EtOAc) to give 9 (23.2 mg). 10 (95.7 mg), 11 (40 mg) and 12 (8.9 mg) were isolated and purified from XMZ-33 (2.5 g) by repeated silica gel column (400 mesh) with gradient CHCl3-CH3OH system. XMZ-35 (3.1 g) was separated by HPLC on YMC-Pack ODS-A column (250 × 20 mm, 5 µm, MeOH:H2O 35:65, 10 ml/min) to obtain 1 (4.4 mg). XMZ36 was re-chromatographed on a silica gel column (gradient PE-EtOAc 10:1 to 2:8), followed by HPLC on YMC-Pack ODS-A column (MeOH:H2O 2:8, 10 ml/min) to obtain 6 (12 mg).
Part II was chromatographed on a silica gel column (200–300 mesh) using PE-EtOAc gradient (50:1 to 1:5) to afford 31 fractions (SWJ-1 to SWJ-31). SWJ-12 (4.2 g) was subjected to a silica gel column (400 mesh) with gradient PE-EtOAc solvent system (5:1 to 1:4) to yield 2 (9.6 mg), 4 (9.2 mg) and 5 (8.7 mg).
Cytotoxicity assay
The cytotoxic activities of all compounds were evaluated by MTT method to against three human cancer cell lines: Hela, MCF-7 and A549, using Doxorubicin as positive control. Cells were cultured in DMEM medium, supplemented with 10% fetal bovine serum in 5% CO2 at 37 ºC. Cells (100 µl) were distributed evenly into 96-well microplates, containing 5 × 103 cells per well, and incubated for 12 h. Designated wells were treated with DMSO solutions of tested compound at various concentrations (0.0625, 0.32, 1.6, 8 and 40 µg/ml) in triplicate. After 48 h incubation, MTT was added to each well to incubate an additional 4 h. After treatment, the absorbance of each well was read at 490 nm by an enzyme-labeled detector (BioTek ELx800). IC50 values to different cell lines were determined by the dose–response curve (Yu et al., 2016Yu, Z.X., Fu, Y.H., Chen, G.Y., Song, X.P., Han, C.R., Li, X.B., Song, X.M., Wu, A.Z., Chen, S.C., 2016. New clerodane diterpenoids from the roots of Polyalthia laui. Fitoterrapia 111, 36-41.).
Statistical analysis
Statistical significance was determined with the standard deviation generally less than 10%, and all values were carried out using the t-test (SPSS 12.0).
Results and discussion
In present phytochemical investigation on searching for bioactive agents from this plant, the extracting method was optimized to afford two parts, part I was from acidic solution of EtOH extracts, part II was from basic solution. Chromatographic separation of these two parts, twelve compounds were obtained, containing nine isolates from part I, and three isolates from part II.
The chemical structures were identified mainly by spectroscopic analysis to be (−)-8-oxodiscretamine (1) (Shono et al., 2016Shono, T., Ishikawa, N., Toume, K., Arai, M.A., Masu, H., Koyano, T., Kowithayakorn, T., Ishibashi, M., 2016. Cerasoidine, a bis-aporphine alkaloid isolated from Polyalthia cerasoides during screening for Wnt signal inhibitors. J. Nat. Prod. 79, 2083-2088.), N-formylannonaine (2) (Yusoff et al., 2014Yusoff, M., Hamid, H., Houghton, P., 2014. Anticholinesterase inhibitory activity of quaternary alkaloids from Tinospora crispa. Molecules 19, 1201-1211.), isooncine (3) (Wu et al., 1990Wu, Y.C., Duh, C.Y., Wang, S.K., Chen, K.S., Yang, T.H., 1990. Two new natural azafluorene alkaloids and a cytotoxic aporphine alkaloid from Polyalthia longifolia. J. Nat. Prod. 53, 1327-1331.), isoursuline (4) (Chen et al., 2000Chen, C.Y., Chang, F.R., Shih, Y.C., Hsieh, T.J., Chia, Y.C., Tseng, H.Y., Chen, H.C., Chen, S.J., Hsu, M.C., Wu, Y.C., 2000. Cytotoxic constituents of Polyalthia longifolia var. pendula. J. Nat. Prod. 63, 1475-1478.), cleistopholine (5) (Wang et al., 2011Wang, L., Chen, G.Y., Han, C.R., Yuan, Y., Yang, B., Zhang, Y., Wang, J., Zhong, X.Q., Huang, X., 2011. Two novel alkaloids from the stem of Saprosma hainanense and their cytotoxic activities in vitro. Chem. Pharm. Bull. 59, 338-340.), northalifoline (6) (Lee et al., 2010Lee, J., Kim, N.H., Nam, J.W., Lee, Y.M., Jang, D.S., Kim, Y.S., Nam, S.H., Seo, E.K., Yang, M.S., Kim, J.S., 2010. Scopoletin from the flower buds of Magnolia fargesii inhibits protein glycation, aldose reductase, and cataractogenesis ex vivo. Arch. Pharm. Res. 33, 1317-1323.), N-2-phenylethylcinnamamide (7) (Gu et al., 2013Gu, Q., Cai, Y.N., Yang, G.M., Cai, B.C., Pan, Y., 2013. Study on chemical constituents and antitumor activity of Oxytropic falcata. Chin. J. Exp. Trad. Med. Form. 19, 72-75.), N-trans-cinnamoyltyramine (8) (Wang et al., 2012aWang, D.F., Chou, G.X., Zhao, N.Y., Zhang, T., Xu, H., 2012a. Study on chemical constituents in stems of Dendrobium nobile. Chin. Trad. Herb. Drugs 43, 1492-1945.,bWang, T.S., Yuan, Y., Wang, J., Han, C.R., Chen, G.Y., 2012b. Anticancer activities of constituents from the stem of Polyalthia rumphii. Pak. J. Pharm. Sci. 25, 353-356.), binaphthalene-2-phenol-3-aldehyde (9) (Wu et al., 1989Wu, G.P., Ying, H.Q., Yan, Z.L., Guo, Z.M., Hou, J.Y., Zhu, C.Q., Shi, D.X., Bian, J., 1989. Synthesis and antifertility actions of gossypol derivatives and phenol aldehydes. Acta Pharm. Sin. 24, 502-511.), arborone (10) (Tulake et al., 2012Tulake, A., Jiang, Y., Tu, P.F., 2012. Nine lignans from Artemisia absinthium L.. J. Chin. Pharm. Sci. 21, 360-364.), syringaresinol (11) (Mo and Mai, 2012Mo, X.Y., Mai, J.B., 2012. Chemical constituents of ethyl acetate extract part of Stelmatocrypton khasianum. Chin. J. Exp. Trad. Med. Form. 18, 61-63.), balanophonin (12) (Wang et al., 2010Wang, Q.H., Peng, K., Tan, L.H., Dai, H.F., 2010. Aquilarin A, a new benzenoid derivative from the fresh stem of Aquilaria sinensis. Molecules 15, 4011-4016.), including eight alkaloids (1–8), three lignans (10–12) and one gossypol derivative (9).
It should be noted that all isolates were reported from this plant for the first time. Among them, binaphthalene-2-phenol-3-aldehyde (9) was firstly isolated from the natural resources, and its complete 1H NMR and 13C NMR were assigned by 2D NMR. N-formylannonaine (2) exists as a mixture of two conformational isomers (Z/E = 1/2.5, calculated by the integration of 1H NMR) in deuterated methanol. A single crystal of isoursuline (4) was firstly recrystalized, and the crystal structure had been determined by single-crystal X-ray diffraction method (Niu et al., 2015Niu, Z.G., Chen, H.H., Chen, T.T., Xia, Q., Li, G.N., 2015. Synthesis, crystal structure and IR spectra of binuclear Cr (III) Complex with 2-(1H-Tetrazol-5-yl) pyridine ligand. J. Hainan Norm. Univ. (Nat. Sci.) 28, 176-179.). The X-ray data were deposited in the Cambridge Crystallographic Data Centre with number of CCDC-1494107.
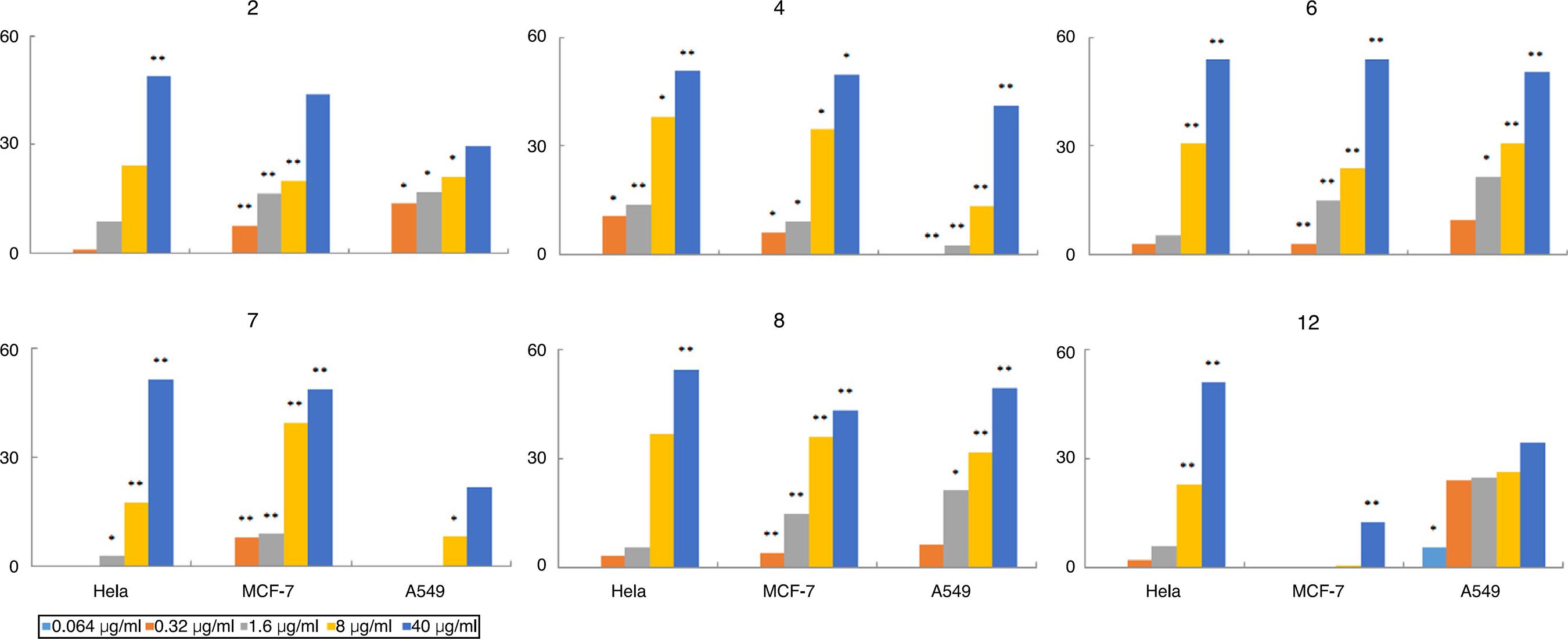
Partial tested compounds showed cytotoxicities inhibiting three human cancer cell lines in vitro (Fig. 1): A considerable increase of inhibition ratio (IR, %) data was demonstrated with the increase in the concentrations. IR values more than 50% were only at high dose of 40 µg/ml, those who achieved more than 50% of IR were 4 to Hela of 50.6% (p < 0.01), 6 to Hela of 53.7% (p < 0.01), 7 to Hela of 51.5% (p < 0.01), 8 to Hela of 54.7% (p < 0.01), 12 to Hela of 51.1% (p < 0.01), 6 to MCF-7 of 53.8% (p < 0.01), 6 to A549 of 50.3% (p < 0.01). While all of compounds at low dose of 0.0625 µg/ml were failed to inhibit the proliferation of the tested cell lines.
The inhibition ratio (IR, %) of six cytotoxical compounds against three cancer cell lines at various concentrations. IR values were calculated using the formula: IR (%) = [OD (control group) − OD (administration group)]/OD (control group) × 100%. *p < 0.05, **p < 0.01.
In order to evaluate the cytotoxicities, the IR data were used to calculate the IC50 values by the dose–response curves, which were presented in Table 1. Among the tested isolates, five alkaloids (2, 4, 6, 7 and 8) and one lignan (12) demonstrated full or partial activities against these tested cancer cell lines ranging from 25 to 40 µg/ml. Among these active alkaloids, 6 and 8 demonstrated broad-spectrum active to all these tested cell lines, both of which were with amide functional group. Furthermore, all active compounds showed antiproliferative action to Hela cell lines.
(−)-8-Oxodiscretamine (1) Brown, amorphous powder (4.4 mg). [α]D: −37.3 (c 0.15, MeOH). ESI-MS m/z: 364.2 [M+Na]+. HRMS-ESI-TOF m/z: [M+H]+ calcd for C19H20NO5: 342.1336, found: 342.1350. 1H NMR (CDCl3, 400 MHz) δ: 6.67 (H-1, s), 6.77 (H-4, s), 2.73 (H-5α, m), 2.91 (H-5β, m), 2.91 (H-6α, m), 4.96 (H-6β, d, J 10.4 Hz), 7.08 (H-11, d, J 8.0 Hz), 6.92 (H-12, d, J 8.0 Hz), 3.02 (H-13α, m), 2.86 (H-13β, m), 4.77 (H-14, d, J 12.8 Hz), 3.91 (2-OCH3, s), 4.00 (9-OCH3, s). 13C NMR (CDCl3, 100 MHz) δ: 108.3 (C-1), 145.7 (C-2), 144.5 (C-3), 114.4 (C-4), 128.2 (C-4a), 29.22 (C-5), 38.4 (C-6), 162.3 (C-8), 121.6 (C-8a), 147.4 (C-9), 149.0 (C-10), 118.2 (C-11), 122.8 (C-12), 130.7 (C-12a), 39.2 (C-13a), 55.2 (C-14), 127.1 (C-14a), 56.2 (2-OCH3), 62.4 (9-OCH3).
N-formylannonaine (2) Yellow solid (9.6 mg). ESI-MS m/z (+): 294.1 [M+H]+, 316.1 [M+Na]+. IR (KBr) cm−1: 2926, 2860, 1645, 1585, 1470, 1408, 1260, 1203, 1116, 1044, 972. Z-isomer: 1H NMR (CDCl3, 400 MHz) δ: 6.59 (H-3), 2.90 (H-4ax), 2.69 (H-4eq), 3.41 (H-5ax), 3.85 (H-5eq), 5.05 (H-6a), 2.84 (H-7ax), 3.24 (H-7eq), 7.23–7.34 (H-8,9,10), 8.10 (H-11), 5.99/6.11 (OCH2O), 8.27 (CHO). 13C NMR (CD3OD, 100 MHz) δ: 143.3 (C-1), 147.1 (C-2), 107.5 (C-3), 126.6 (C-3a), 31.0 (C-4), 42.2 (C-5), 49.5 (C-6a), 33.6 (C-7), 135.2 (C-7a), 128.1 (C-8), 127.6 (C-9), 126.6 (C-10), 128.9 (C-11), 130.5 (C-11a), 117.5 (C-11b), 124.7 (C-11c), 101.0 (OCH2O), 162.1 (CHO); E-isomer: 1H NMR (CDCl3, 400 MHz) δ: 6.62 (H-3), 2.75 (H-4ax), 2.75 (H-4eq), 3.10 (H-5ax), 4.47 (H-5eq), 4.63 (H-6a), 3.16 (H-7ax), 2.90 (H-7eq), 7.23–7.34 (H-8,9,10), 8.11 (H-11), 5.99/6.11 (OCH2O), 8.40 (CHO). 13C NMR (CD3OD, 100 MHz) δ: 143.1 (C-1), 147.6 (C-2), 107.9 (C-3), 126.6 (C-3a), 29.7 (C-4), 36.2 (C-5), 53.3 (C-6a), 37.8 (C-7), 134.5 (C-7a), 128.9 (C-8), 128.0 (C-9), 127.1 (C-10), 128.5 (C-11), 130.3 (C-11a), 117.0 (C-11b), 124.1 (C-11c), 101.1 (OCH2O), 162.1 (CHO).
Isooncine (3) Yellow amorphous solid (5.9 mg). ESI-MS m/z (+): 241.1 [M+H]+. IR (KBr) cm−1: 3435, 2925, 2825, 1740, 1640, 1463, 1383, 1252, 1119. 1H NMR (CDCl3, 400 MHz) δ: 8.31 (H-3, d, J 5.2 Hz), 7.36 (H-5, s), 7.22 (H-8, s), 6.87 (H-2, d, J 5.2 Hz), 3.99 (6-OMe, s), 2.58 (1-Me, s).
Isoursuline (4) Yellow single crystal (9.2 mg). ESI-MS m/z (+): 242.2 [M+H]+, 264.2 [M+Na]+. IR (KBr) cm−1: 3434.6, 2925.6, 1625.3, 1570.6, 1466.5. 1H NMR (CD3OD, 400 MHz) δ: 2.95 (1-Me, s), 7.05 (H-2, J 5.6 Hz), 8.32 (H-3, J 5.6 Hz), 3.97 (6-OMe, s), 6.94 (H-7, J 8 Hz), 7.22 (H-8, J 8 Hz). 13C NMR (CD3OD, 100 MHz) δ: 149.4 (C-1), 17.3 (1-Me), 126.3 (C-2), 152.0 (C-3), 165.8 (C-4a), 126.5 (C-4b), 144.7 (C-5), 156.0 (C-6), 57.2 (6-OMe), 113.6 (C-7), 118.2 (C-8), 129.2 (C-8a), 192.8 (C-9), 127.7 (C-9a).
Cleistopholine (5) Yellow amorphous solid (8.7 mg). ESI-MS m/z (+): 224.1 [M+H]+, 246.1 [M+Na]+, 469.2 [2M+Na]+. IR (KBr) cm−1: 2930, 1682, 1591, 1460, 1414, 1334, 1301, 1125. 1H NMR (CD3OD, 400 MHz) δ: 8.90 (H-2, d, J 4.8 Hz), 7.50 (H-3, dd, J 4.8, 0.8 Hz), 8.37 (H-5, m), 7.83 (H-6, m), 7.83 (H-7, m), 8.27 (H-8, m), 2.92 (22.8-Me, s). 13C NMR (CD3OD, 100 MHz) δ: 153.3 (C-2), 131.4 (C-3), 151.5 (C-4), 129.2 (C-4a), 127.5 (C-5), 134.6 (C-6), 134.2 (C-7), 127.3 (C-8), 129.0 (C-8a), 185.0 (C-9), 150.1 (C-9a), 181.9 (C-10), 132.0 (C-10a).
Northalifoline (6) Amorphous solid (12 mg). ESI-MS: m/z 216.0 [M+Na]+, 194.0 [M+H]+. 1HNMR (400 MHz, CD3OD) δH (ppm): 7.43 (1H, s, H-8), 6.65 (1H, s, H-5), 3.84 (3H, s, 4-OCH3), 3.41 (2H, t, J 6.8, H-3), 2.80 (2H, t, J 6.8, H-4). 13CNMR (100 MHz, CD3OD) δC (ppm): 168.8 (C-1), 152.1 (C-6), 148.2 (C-7), 135.3 (C-4a), 121.1 (C-8a), 115.0 (C-8), 111.5 (C-5), 56.5 (6-OCH3), 41.1 (C-3), 28.5 (C-4).
N-2-phenylethylcinnamamide (7) Amorphous solid (44.4 mg). ESI-MS: m/z 252.4 [M+H]+, 274.3 [M+Na]+. 1H NMR (400 MHz, CDCl3) δH (ppm): 7.62 (1H, d, J 15.6 Hz, H-7'), 7.49(2H, dd, J 8.0, 3.2 Hz, H-2', 6'), 7.36 (3H, m, H-3',4',5'), 7.24 (5H, m, H-2,3,4,5,6), 6.32 (1H, d, J 15.6 Hz, H-8'), 3.66 (2H, q, J 6.8 Hz, H-8), 2.90 (2H, t, J 6.8 Hz, H-7), 5.64 (1H, brs, === Inserir caracter correspondente ao PDF === NH === Inserir caracter correspondente ao PDF === ). 13C NMR (CDCl3, 100 MHz) δC (ppm): 165.8 (C-9), 141.1 (C-7'), 138.9 (C-1'), 134.8 (C-1), 129.9 (C-4'), 128.79 (C-3',5'), 128.81 (C-2,6), 128.7 (C-2',6'), 127.8 (C-3,5), 126.6 (C-4), 120.5 (C-8'), 40.8 (C-8), 35.6 (C-7).
N-trans-cinnamoyltyramine (8) Amorphous solid (37.9 mg). ESI-MS: m/z 268.3 [M+H]+, 290.2 [M+Na]+. 1H NMR (400 MHz, CD3OD) δ H (ppm): 7.54 (1H, d, J 15.8 Hz, H-7'), 7.51 (2H, m, H-2', 6'), 7.37 (3H, m, H-3', 4', 5'), 7.06 (2H, d, J 8.3 Hz, H-2, 6), 6.72 (2H, d, J 8.3 Hz, H-3, 5), 6.56 (1H, d, J 16.0 Hz, H-8'), 3.48 (2H, t, J 7.8 Hz, H-8), 2.77 (2H, t, J 7.6 Hz, H-7), 4.60 (1H, brs, === Inserir caracter correspondente ao PDF === NH === Inserir caracter correspondente ao PDF === ). 13C NMR (100 MHz, CD3OD) δC (ppm): 131.3 (C-1), 130.8 (C-2, 6), 116.3 (C-3, 5), 157.0 (C-4), 35.8 (C-7), 42.6 (C-8), 136.4 (C-1'), 128.9 (C-2', 6'), 130.7 (C-3', 5'), 130.8 (C-4'),141.7 (C-7'), 122.0 (C-8'), 168.7 (C-9').
Binaphthalene-2-phenol-3-aldehyde (9) Yellow amorphous solid (23.2 mg). ESI-MS: m/z 365.0 [M+Na]+, HRMS-ESI-TOF (m/z 365.0806 [M+Na]+, calcd. for 365.0784). 1H NMR (400 MHz, CDCl3) δH (ppm): 8.38 (2H, s, H-4,4'), 8.05 (2H, d, J 7.2,2.0 Hz, H-5,5'), 7.45 (4H, m, H-6,6',7,7'), 7.24 (2H, dd, J 7.2,2.0 Hz, H-8,8'), 10.23 (2H, s, H-9,9'), 10.61 (2H, s, -OH); 13C NMR (100 MHz, CDCl3) δC (ppm): 115.6 (C-1,1'), 152.7 (C-2,2'), 121.1 (C-3,3'), 137.4 (C-4,4'), 137.4 (C-4a,4a'), 129.0 (C-5,5'), 129.6 (C-6,6'), 123.9 (C-7,7'), 123.5 (C-8,8'), 126.7 (C-8a, 8a'), 195.7 (C-9,9').
Arborone (10) Amorphous solid (95.7 mg). ESI-MS: m/z 485.2 [M+Na]+, 947.3 [2M+Na]+. 1H NMR (400 MHz, CDCl3) δH (ppm): 7.46 (2H, s, H-2,6), 6.55 (2H, s, H-2',6'), 5.08 (1H, d, J 5.6 Hz, H-7'), 4.42 (1H, m, Hβ-9), 4.35 (1H, m, H-8), 4.28 (1H, dd, J 7.6, 2.4 Hz, Hα-9), 3.93 (3H, s, 4-OCH3), 3.92 (6H, s, 3,5-OCH3), 3.84 (6H, s, 3',5'-OCH3), 3.83 (3H, s, 4'-OCH3), 3.42 (2H, d, J 6.8 Hz, H-9'), 2.98 (1H, m, H-8'); 13C NMR (100 MHz, CDCl3) δC (ppm): 198.5 (C-7), 153.2 (C-3,5), 153.0 (C-3',5'), 142.8 (C-4), 136.9 (C-4'), 133.6 (C-1'), 131.1 (C-1), 106.4 (C-2,6), 102.5 (C-2',6'), 81.3 (C-7'), 68.7 (C-9), 61.7 (C-9'), 60.8 (4-OCH3), 60.7 (4'-OCH3), 56.2 (3,5-OCH3), 56.0 (3',5'-OCH3), 49.7 (C-8'), 48.6 (C-8).
Syringaresinol (11) Amorphous solid (40 mg). ESI-MS: m/z 441.1 [M+Na]+, 417.1 [M−H]−. 1H NMR (400 MHz, CDCl3) δH (ppm): 6.56 (4H, s, H-2,2',6,6'), 4.74 (2H, d, J 3.5 Hz, H-7,7'), 3.35 (2H, m, H-8,8'), 3.58 (4H, d, J 8 Hz, H-9,9'), 3.88 (12H, s, 3,3',5,5'-OCH3). 13C NMR (100 MHz, CDCl3) δC (ppm): 131.1 (C-1,1'), 101.0 (C-2,2',6,6'), 146.2 (C-3,3',5,5'), 133.4 (C-4,4'), 146.2 (C-3,3',5,5'), 85.1 (C-7,7'), 53.3 (C-8,8'), 70.8 (C-9,9'), 55.4 (3,3',5,5'-OCH3).
Balanophonin (12) Amorphous solid (8.9 mg). ESI-MS: m/z 379.1 [M+Na]+, 355.1 [M−H]−. 1H NMR (400 MHz, CDCl3) δH (ppm): 9.64 (1H, d, J 7.6 Hz, H-9'), 7.41 (1H, d, J 15.6 Hz, H-7'), 7.13 (1H, brs, H-6'), 7.04 (1H, brs, H-2'), 6.89 (1H, brs, H-2), 6.88 (2H, m, H-5,6), 6.60 (1H, dd, J 15.6,7.6 Hz, H-8'), 5.64 (1H, d, J 6.8 Hz, H-7), 3.97 (2H, m, H-9), 3.93 (3H, s, 3'-OCH3), 3.87 (1H, s, 3-OCH3), 3.68 (1H, q, J 6.0 Hz, H-8). 13C NMR (100 MHz, CDCl3) δC (ppm): 193.7 (C-9'), 153.1 (C-7'), 151.1 (C-4'), 146.7 (C-3), 145.9 (C-4), 144.8 (C-3'), 132.2 (C-5'), 129.1 (C-1), 128.1 (C-1'), 126.4 (C-8'), 119.4 (C-6), 118.2 (C-6'), 114.5 (C-5), 112.2 (C-2'), 108.7 (C-2), 89.0 (C-7), 63.9 (C-9), 56.1 (3'-OCH3), 56.0 (3-OCH3), 53.0 (C-8).
-
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.Confidentiality of data. The authors declare that no patient data appear in this article.Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Acknowledgements
The authors thank the Program for Innovative Research Team in University (IRT-16R19) for financial support.
References
- Chen, C.Y., Chang, F.R., Shih, Y.C., Hsieh, T.J., Chia, Y.C., Tseng, H.Y., Chen, H.C., Chen, S.J., Hsu, M.C., Wu, Y.C., 2000. Cytotoxic constituents of Polyalthia longifolia var. pendula J. Nat. Prod. 63, 1475-1478.
- Editorial Board of FOC, 2011. Flora of China, vol. 19. Science Press, Beijing, pp. 696.
- Gu, Q., Cai, Y.N., Yang, G.M., Cai, B.C., Pan, Y., 2013. Study on chemical constituents and antitumor activity of Oxytropic falcata Chin. J. Exp. Trad. Med. Form. 19, 72-75.
- Lee, J., Kim, N.H., Nam, J.W., Lee, Y.M., Jang, D.S., Kim, Y.S., Nam, S.H., Seo, E.K., Yang, M.S., Kim, J.S., 2010. Scopoletin from the flower buds of Magnolia fargesii inhibits protein glycation, aldose reductase, and cataractogenesis ex vivo Arch. Pharm. Res. 33, 1317-1323.
- Machana, S., Weerapreeyakul, N., Barusrux, S., Thumanu, K., Tanchanuch, W., 2012. Synergistic anticancer effect of the extracts from Polyalthia evecta caused apoptosis in human hepatoma (HepG2) cells. Asian Pac. J. Trop. Biomed. 2, 589-596.
- Mo, X.Y., Mai, J.B., 2012. Chemical constituents of ethyl acetate extract part of Stelmatocrypton khasianum Chin. J. Exp. Trad. Med. Form. 18, 61-63.
- Niu, Z.G., Chen, H.H., Chen, T.T., Xia, Q., Li, G.N., 2015. Synthesis, crystal structure and IR spectra of binuclear Cr (III) Complex with 2-(1H-Tetrazol-5-yl) pyridine ligand. J. Hainan Norm. Univ. (Nat. Sci.) 28, 176-179.
- Shono, T., Ishikawa, N., Toume, K., Arai, M.A., Masu, H., Koyano, T., Kowithayakorn, T., Ishibashi, M., 2016. Cerasoidine, a bis-aporphine alkaloid isolated from Polyalthia cerasoides during screening for Wnt signal inhibitors. J. Nat. Prod. 79, 2083-2088.
- Tulake, A., Jiang, Y., Tu, P.F., 2012. Nine lignans from Artemisia absinthium L.. J. Chin. Pharm. Sci. 21, 360-364.
- Wang, D.F., Chou, G.X., Zhao, N.Y., Zhang, T., Xu, H., 2012a. Study on chemical constituents in stems of Dendrobium nobile Chin. Trad. Herb. Drugs 43, 1492-1945.
- Wang, L., Chen, G.Y., Han, C.R., Yuan, Y., Yang, B., Zhang, Y., Wang, J., Zhong, X.Q., Huang, X., 2011. Two novel alkaloids from the stem of Saprosma hainanense and their cytotoxic activities in vitro Chem. Pharm. Bull. 59, 338-340.
- Wang, Q.H., Peng, K., Tan, L.H., Dai, H.F., 2010. Aquilarin A, a new benzenoid derivative from the fresh stem of Aquilaria sinensis Molecules 15, 4011-4016.
- Wang, T.S., Luo, Y.P., Wang, J., He, M.X., Zhong, M.G., Li, Y., Song, X.P., 2013. (+)-Rumphiin and polyalthurea, new compounds from the stems of Polyalthia rumphii Nat. Prod. Commun. 8, 1427-1429.
- Wang, T.S., Yuan, Y., Wang, J., Han, C.R., Chen, G.Y., 2012b. Anticancer activities of constituents from the stem of Polyalthia rumphii Pak. J. Pharm. Sci. 25, 353-356.
- Wu, G.P., Ying, H.Q., Yan, Z.L., Guo, Z.M., Hou, J.Y., Zhu, C.Q., Shi, D.X., Bian, J., 1989. Synthesis and antifertility actions of gossypol derivatives and phenol aldehydes. Acta Pharm. Sin. 24, 502-511.
- Wu, Y.C., Duh, C.Y., Wang, S.K., Chen, K.S., Yang, T.H., 1990. Two new natural azafluorene alkaloids and a cytotoxic aporphine alkaloid from Polyalthia longifolia J. Nat. Prod. 53, 1327-1331.
- Yu, Z.X., Fu, Y.H., Chen, G.Y., Song, X.P., Han, C.R., Li, X.B., Song, X.M., Wu, A.Z., Chen, S.C., 2016. New clerodane diterpenoids from the roots of Polyalthia laui Fitoterrapia 111, 36-41.
- Yuan, Y., Huang, G.J., Wang, T.S., Chen, G.Y., 2011. In vitro screening of five Hainan plants of Polyalthia (Annonaceae) against human cancer cell lines with MTT assay. J. Med. Plants Res. 15, 837-841.
- Yusoff, M., Hamid, H., Houghton, P., 2014. Anticholinesterase inhibitory activity of quaternary alkaloids from Tinospora crispa Molecules 19, 1201-1211.
Publication Dates
-
Publication in this collection
Mar-Apr 2018
History
-
Received
12 July 2017 -
Accepted
20 Mar 2018 -
Accepted
5 Apr 2018