Abstracts
BACKGROUND: Patients with metabolic syndrome (MetS) are at high coronary risk and beta-cell dysfunction or insulin resistance might predict an additional risk for early cardiovascular events. OBJECTIVE: This study aimed to evaluate early glucometabolic alterations in patients with MetS, but without previously known type 2 diabetes, after acute coronary syndrome. METHODS: A total of 114 patients were submitted to an oral glucose tolerance test (OGTT) 1-3 days after hospital discharge due to myocardial infarction or unstable angina. Based on the OGTT, we defined three groups of patients: normal glucose tolerance (NGT; n=26), impaired glucose tolerance (IGT; n=39), or diabetes (DM; n=49). The homeostasis model assessment (HOMA-IR) was used to measure insulin resistance; beta-cell responsiveness was assessed by the insulinogenic index at 30 min (ΔI30/ΔG30). RESULTS: Based on the HOMA-IR, patients with DM were more insulin-resistant than those with NGT or IGT (p<0.001). According to the insulinogenic index, the beta-cell responsiveness was also impaired in subjects with DM (p<0.001 vs NGT or IGT). CONCLUSION: High rates of glucometabolic alterations were found after acute coronary syndrome in patients with MetS. As these abnormalities markedly increase the risk for adverse outcomes, early OGTT among MetS patients might be used to identify those at the highest coronary risk.
Insulin resistance; metabolic syndrome; acute coronary syndrome; diabetes mellitus, type 2; insulin-secreting cells
FUNDAMENTO: Pacientes com síndrome metabólica (SM) têm alto risco coronariano e a disfunção da célula beta ou resistência à insulina pode prever um risco adicional de eventos cardiovasculares precoces. OBJETIVO: Avaliar as alterações glicometabólicas precoces em pacientes com SM, mas sem diagnóstico de diabete tipo 2, após síndrome coronariana aguda. MÉTODOS: Um total de 114 pacientes foi submetido ao teste oral de tolerância à glicose (TOTG), 1-3 dias da alta hospitalar, após infarto agudo do miocárdio ou angina instável. Baseado no TOTG, definimos três grupos de pacientes: tolerância normal à glicose (TNG; n=26), tolerância alterada à glicose (TAG; n=39) ou diabetes mellitus (DM; n=49). O Modelo de Avaliação da Homeostase (HOMA-IR) foi usado para estimar a resistência à insulina; a responsividade da célula beta foi avaliada através do índice insulinogênico de 30 minutos (ΔI30/ΔG30). RESULTADOS: Baseado no HOMA-IR, os pacientes com DM eram mais insulino-resistentes do que aqueles com TNG ou TAG (p<0,001). De acordo com o índice insulinogênico, a responsividade da célula beta também estava alterada em indivíduos com DM (p<0,001 vs TNG ou TAG). CONCLUSÃO: Altas taxas de alterações glicometabólicas foram encontradas após síndrome coronariana aguda em pacientes com SM. Como essas anormalidades acentuadamente aumentam o risco de desfechos adversos, o TOTG precoce pode ser utilizado em pacientes com SM para identificar aqueles que apresentam maior risco coronariano.
Resistência à insulina; síndrome metabólica; síndrome coronariana aguda; diabetes mellitus tipo 2; células secretoras de insulina
FUNDAMENTO: Pacientes con síndrome metabólico (SM) tienen alto riesgo coronario y la disfunción de la célula beta o la resistencia a la insulina puede prever un riesgo adicional de eventos cardiovasculares precoces. OBJETIVO: Evaluar las alteraciones glucometabólicas precoces en pacientes con SM, pero sin diagnóstico de diabetes tipo 2, tras el síndrome coronario agudo. MÉTODOS: Un total de 114 pacientes fue sometido a la prueba oral de tolerancia a la glucosa (POTG), de un a tres días tras el alta hospitalaria, y luego de infarto agudo de miocardio o angina inestable. Basado en el POTG, definimos tres grupos de pacientes: tolerancia normal a la glucosa (TNG; n=26), tolerancia alterada a la glucosa (TAG; n=39) o diabetes mellitus (DM; n=49). Se utilizó el Modelo de Evaluación de la Homeostasis (HOMA-IR) para estimarse la resistencia a la insulina; se evaluó la responsividad de la célula beta a través del índice insulinogénico de 30 minutos (ΔI30/ΔG30). RESULTADOS: Basado en el HOMA-IR, los pacientes con DM se mostraban más insulinoresistentes que los individuos con TNG o TAG (p<0,001). De acuerdo con el índice insulinogénico, la responsividad de la célula beta también estaba alterada en individuos con DM (p<0,001 vs. TNG o TAG). CONCLUSIONES: Se encontraron altas tasas de alteraciones glucometabólicas tras el síndrome coronario agudo en pacientes con SM. Como esas anormalidades incrementan acentuadamente el riesgo de desenlaces adversos, el POTG precoz se puede utilizar en pacientes con SM para identificar a los que presentan mayor riesgo coronario.
Resistencia a la insulina; síndrome metabólico; síndrome coronario agudo; diabetes mellitus tipo 2; células secretoras de insulina
ORIGINAL ARTICLE
Universidade Federal de São Paulo, São Paulo, SP - Brazil
Mailing address
SUMMARY
BACKGROUND: Patients with metabolic syndrome (MetS) are at high coronary risk and beta-cell dysfunction or insulin resistance might predict an additional risk for early cardiovascular events.
OBJECTIVE: This study aimed to evaluate early glucometabolic alterations in patients with MetS, but without previously known type 2 diabetes, after acute coronary syndrome.
METHODS: A total of 114 patients were submitted to an oral glucose tolerance test (OGTT) 1-3 days after hospital discharge due to myocardial infarction or unstable angina. Based on the OGTT, we defined three groups of patients: normal glucose tolerance (NGT; n=26), impaired glucose tolerance (IGT; n=39), or diabetes (DM; n=49). The homeostasis model assessment (HOMA-IR) was used to measure insulin resistance; beta-cell responsiveness was assessed by the insulinogenic index at 30 min (ΔI30/ΔG30).
RESULTS: Based on the HOMA-IR, patients with DM were more insulin-resistant than those with NGT or IGT (p<0.001). According to the insulinogenic index, the beta-cell responsiveness was also impaired in subjects with DM (p<0.001 vs NGT or IGT).
CONCLUSION: High rates of glucometabolic alterations were found after acute coronary syndrome in patients with MetS. As these abnormalities markedly increase the risk for adverse outcomes, early OGTT among MetS patients might be used to identify those at the highest coronary risk.
Key words: Insulin resistance; metabolic syndrome; acute coronary syndrome; diabetes mellitus, type 2; insulin-secreting cells.
Introduction
Recently, closer associations between glucometabolic alterations and coronary artery disease have been established1,2. Glucose abnormalities, observed during the acute phase of coronary syndromes (ACS), increase the risk of adverse outcomes3,4. In addition, those patients with known diabetes mellitus and previous myocardial infarction present the highest risk for cardiovascular mortality5.
Several studies have recognized metabolic syndrome (MetS) as an additional risk factor for cardiovascular events, especially among patients with known diabetes mellitus6-8. All these aspects suggest the need of early identification of patients with occult diabetes or presenting glucometabolic abnormalities. In fact, for these patients, a more aggressive treatment, including lifestyle changes, and early achievement of lipid and blood pressure goals could contribute to better outcomes9,10.
Therefore, the aim of this study is to compare the glucometabolic profile, based on the early responses to OGTT, in patients with MetS and recent ACS.
Methods
Patients selected from the Federal University of Sao Paulo were included in the present study if they met the following inclusion criteria: recent admission due to ACS (acute myocardial infarction or unstable angina pectoris), no previously known diabetes mellitus, age 30-75 years, MetS according to the revised National Cholesterol Education Program/Adult Treatment Panel (NCEP/ATP III)11, and stable hemodynamic conditions in the first 1-3 days after hospital discharge. Patients were excluded if they had used hypolipidemic drugs in the last 30 days or presented LDL-C > 130 mg/dL at hospital admission. A total of 114 participants of both sexes were enrolled and the. Informed consent was obtained from all patients. This study was approved by the local ethics committee.
Fasting plasma lipids (cholesterol, HDL-C, triglycerides) were measured using a standardized kit; LDL-C was estimated by the formula of Friedewald, and apolipoproteins A1 and B were determined by nephelometry. The Oral glucose tolerance test (OGTT, 75 g glucose in 200 ml water) was performed 1-3 days after hospital discharge, after three days of nutrition counseling, based on the NCEP/ATP III guidelines and for appropriate and non-restrictive intake of carbohydrates. The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as insulin (mU/L) X (glucose [mg/dL] X 0.055)/22.512. For this calculation, we used the mean of three consecutive samples of fasting insulin. The insulinogenic index was calculated as the difference between the 30 min and 0 min OGTT plasma insulin values divided by the difference between the corresponding plasma glucose values (ΔI30/ΔG30). The area under the curve (AUC) for glucose and insulin were determined based on the blood samples obtained before (t=0) and 30, 60, 90, and 120 min after oral glucose load. Adiponectin was measured by enzyme-linked immunosorbent assay (ELISA) using a commercial kit (Human Adiponectin/Acrp30 Immunoassay - Quantiquine, R&D Systems). Glycated hemoglobin (HbA1c) was measured using high-performance liquid chromatography.
The severity of coronary atherosclerosis was evaluated through the Gensini score, which depends on the degree of luminal narrowing and importance of the coronary stenosis site13. The score evaluates the severity and effects of multiple obstructions, quality of the coronary arteries and the influence of collateral vessels.
To determine differences between the three groups formed after OGTT (NGT, IGT and DM), continuous variables were compared by ANOVA. When necessary, values were log-transformed. Categorical variables were compared using chi-square tests. All tests were 2-tailed, and a p value < 0.05 defined statistical significance. All analyses were performed with SPSS 11.5 for Windows (SPSS Inc, Chicago, IL).
Results
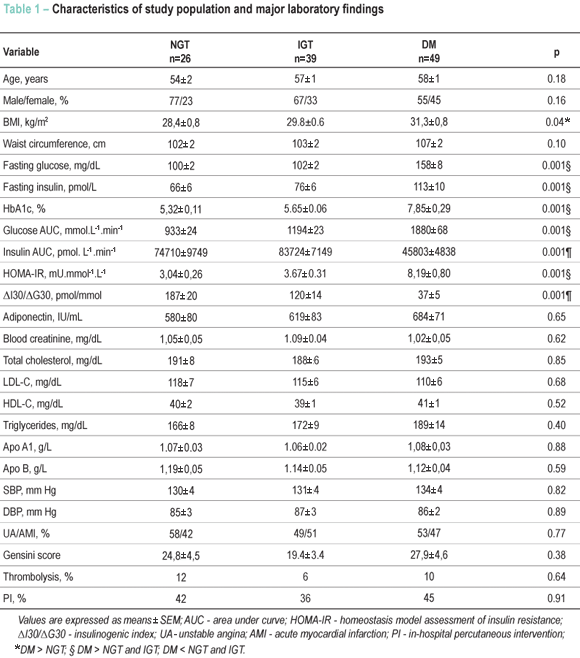
Major characteristics of the study population are shown in Table 1. One to three days after hospital discharge, only 23% (n=26) of the patients had NGT, while 34% (n=39) had IGT and 43% (n=49) were classified as having DM. In-hospital medical treatment and procedures were similar among the groups. Subjects with NGT, IGT and DM were also comparable regarding age and sex distribution, but higher BMI was observed in DM subjects (p=0.036 vs. NGT), with no differences observed regarding waist circumference. The extension and severity of the coronary artery disease, evaluated by the Gensini score, did not differ among the groups. Lipid and apolipoprotein values were similar among the groups. Fasting plasma glucose values were higher in DM patients, as well as 120 minutes after an oral glucose load (p<0.001 vs NGT and IGT). Fasting insulin values were also higher in DM subjects (p<0.001 vs NGT and p<0.004 vs IGT).
Drug therapies at hospital discharge are shown in Table 2. The only difference among the groups was the higher prevalence of hypoglycemic drug use in subjects with DM.
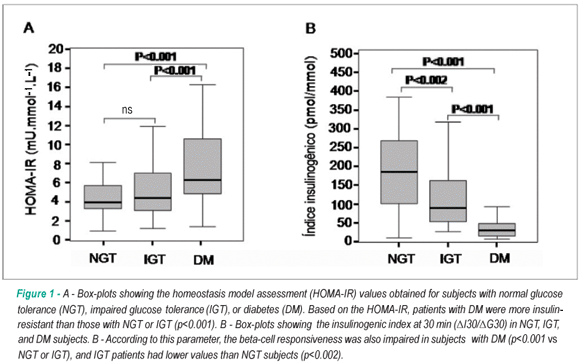
HOMA-IR was higher in DM subjects (p<0.001 vs IGT and NGT), as well as the insulinogenic index (p<0.001 vs NGT and IGT) [Fig 1]. Plasma adiponectin values did not differ among the groups.
Figure 2 shows the AUC for glucose and insulin. Patients with DM presented higher glucose AUC (p<0.001 vs NGT and IGT). Insulin AUC was also higher in subjects with DM (p<0.017 vs NGT, p<0.001 vs IGT).
Discussion
In recent years, MetS has been commonly reported among patients with ACS6,14. This study shows, for the first time, the major glucometabolic abnormalities in patients with MetS, but without previously known type 2 diabetes in the acute phase of coronary syndromes.
The OGTT responses performed 1-3 d after hospital discharge disclosed high rates of IGT (34%) and DM (43%). Based on the HOMA-IR, patients with DM were more insulin-resistant than those with NGT or IGT. Furthermore, according to the insulinogenic index (ΔI30/ΔG30), the beta-cell responsiveness was also impaired in subjects with DM, when compared with NGT or IGT patients.
Even considering that ACS is a stressful situation, which can explain the high rate of glucometabolic alterations on MetS subjects, these abnormalities are associated with adverse outcomes. Feinberg et al5 reported, in 1,060 consecutive patients with no previous diagnosis of diabetes, that MetS is an independent predictor of 30-day and 1-year mortality. However, the authors also observed that among those patients, the ones with fasting glycemia > 140 mg/dL were at the highest mortality risk
In our study, we found that insulin resistance was more frequent in patients with no previous diagnosis of DM. The independent association of insulin resistance with coronary disease is still controversial. Tenenbaum et al15 evaluated the predictive value of HOMA-IR for new major cardiovascular events in 2,938 subjects with preexisting coronary disease. After multivariate analysis, they concluded that insulin resistance is an independent risk factor for cardiovascular events and new-onset diabetes15. In the Multi-Ethnic Study of Atherosclerosis (MESA)2 subclinical atherosclerosis was assessed in 5,810 participants without diabetes and the HOMA-IR was associated with subclinical atherosclerosis; however, the association was not independent after adjustment for non-glucose MetS components.
The present study also showed that DM subjects presented lower beta-cell responsiveness. We used the insulinogenic index to estimate beta-cell function. In comparative studies to evaluate insulin secretion, this index was considered closely related to insulin secretion16. In addition, as part of our DM patients were under use of hypoglycemic drugs at hospital discharge, this method appeared to be more sensitive, considering it measures the early insulin response to oral glucose stimulation.
Both insulin deficiency and insulin resistance can be associated with endothelial dysfunction, as insulin-signaling pathways include the production of nitric oxide (NO) and secretion of endothelin-1. In patients with MetS, inflammatory stimuli can cause an additional imbalance between NO production and endothelin-1 secretion, leading to reduced blood flow and impaired glucose uptake in peripheral muscle17. In addition, endothelial dysfunction has been related to the development of atherosclerosis and cardiovascular events18,19.
In our study, in-hospital procedures (CABG, percutaneous intervention) and the extension of coronary disease evaluated by the Gensini score were similar among the groups. However, our study was not powered to detect significant differences regarding the burden of atherosclerosis or cardiac events. However, Boulon et al20, evaluating the impact of MetS in a follow-up of 480 consecutive patients with ACS, reported an increase in total mortality in the MetS group compared with the non-MetS group20. In the Women's Ischemia Syndrome Evaluation (WISE) study, coronary angiography was obtained in 755 women due to suspected myocardial ischemia. When compared with women that presented normal metabolic status, those with MetS had a lower 4-year survival and event-free survival rate (death, nonfatal myocardial infarction, stroke, or congestive heart failure), and when stratified by the presence of significant coronary disease at the angiography, women with MetS were also at higher risk of cardiovascular events21.
Recently, studies aimed to evaluate the long-term prognosis of IGT or newly-diagnosed DM after myocardial infarction have identified these glucometabolic abnormalities after OGTT as the strongest predictors of death and major cardiovascular events22-24.
In addition, the Euro Heart Survey showed that 20-30% of patients with either acute coronary syndrome or chronic coronary artery disease presented with newly detected glucose intolerance or diabetes and an OGTT was considered the most appropriate method for the clinical assessment of glucometabolic status in these patients25. Finally, the Munich Myocardial Infarction Registry showed the relevance of the in-hospital diagnosis of diabetes mellitus, to promote the early establishment of strategies to reduce cardiovascular mortality26, which is endorsed by both the American College of Cardiology/American Heart Association and American Diabetes Association/American College of Endocrinology for glycemic control in patients with acute coronary syndrome, as a Class IIa recommendation27.
In conclusion, this study describes high rates of impaired glucose metabolism, including insulin resistance and beta-cell dysfunction, in subjects with MetS at the acute phase of coronary syndromes. The study reinforces that an early OGTT can identify subjects with DM or IGT, allowing a more appropriate treatment to be established, as well as the achievement of target lipid, blood pressure and glucose metabolism goals, to improve outcomes in this high-risk population.
Acknowledgments
This study was supported by FAPESP (Research Foundation of the State of São Paulo, Brazil). Dr. Monteiro is also a recipient of a research fellowship from CNPq (Brazilian research foundation).
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Sources of Funding
This study was funded by FAPESP and CAPES.
Study Association
This article is part of the thesis of doctoral submitted by Carlos Manoel de Castro Monteiro, from UNIFESP.
References
- 1. Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events: a meta-regression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999; 22: 233-40.
- 2. Bertoni AG, Wong ND, Shea S, Ma S, Liu K, Preethi S, et al. Insulin resistance, metabolic syndrome, and subclinical atherosclerosis: the multi-ethnic study of atherosclerosis (MESA). Diabetes Care. 2007; 30: 2951-6.
- 3. Hashimoto K, Ikewaki K, Yagi H, Nagasawa H, Imamoto S, Shibata T. Glucose intolerance is common in japanese patients with acute coronary syndrome who were not previously diagnosed with diabetes. Diabetes Care. 2005; 28: 1182-6.
- 4. Wallander M, Bartnik M, Efendic S, Hamsten A, Malmberg K, Ohrvik J, et al. Beta cell dysfunction in patients with acute myocardial infarction but without previously known type 2 diabetes: a report from the GAMI study. Diabetologia. 2005; 48: 2229-35.
- 5. Feinberg MS, Schwartz R, Tanne D, Fisman EZ, Hod H, Zahger D, et al. Impact of the metabolic syndrome on the clinical outcomes of non-clinically diagnosed diabetic patients with acute coronary syndrome. Am J Cardiol. 2007; 99: 667-72.
- 6. Zeller M, Steg PG, Ravisy J, Laurent Y, Janin-Manificat L, L'Huillier I, et al. Prevalence and impact of MS on hospital outcomes in acute myocardial infarction. Arch Intern Med. 2005; 165: 1192-8.
- 7. Levantesi G, Macchia A, Marfisi RM, Franzoni MG, Maggioni AP, Nicolosi L, et al. Metabolic syndrome and risk of cardiovascular events after myocardial infarction. J Am Coll Cardiol. 2005; 46: 277-83.
- 8. Shaw JE, Hodge AM, de Courten M, Chitson P, Zimmet PZ. Isolated post-challenge hyperglycaemia confirmed as risk factor for mortality. Diabetologia. 1999; 42: 1050-4.
- 9. Shepherd J, Barter P, Carmena R, Deedwania P, Fruchart JC, Hoffner S, et al. Effect of lowering LDL cholesterol substantially below currently recommended levels in patients with coronary heart disease and diabetes: the Treating to New Targets (TNT) study. Diabetes Care. 2006; 29: 1220-6.
- 10. Julius S, Kjeldsen SE, Weber M, Brunner HR, Ekman S, Hanson L, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004; 363: 2022-31.
- 11. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005; 112: 2735-52.
- 12. Matthews DR, Hosker JP, Rudenski AS, Burnett MA, Darling P, Bown EG, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28: 412-9.
- 13. Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983; 51: 606.
- 14. Clavijo LC, Pinto TL, Kuchulakanti PK, Tenenbaum H, Fisman EZ, Tanne D, et al. Metabolic syndrome in patients with acute myocardial infarction is associated with increased infarct size and in-hospital complications. Cardiovasc Revasc Med. 2006; 7: 7-11.
- 15. Tenenbaum A, Adler Y, Boyko V, Sakai M, Nagata I, Doi K, et al. Insulin resistance is associated with increased risk of major cardiovascular events in patients with preexisting coronary artery disease. Am Heart J. 2007; 153: 559-65.
- 16. Taniguchi A, Nagasaka S, Fukushima M, Sakai M, Nagata I, Doi K, et al. Assessment of insulin sensitivity and insulin secretion from the oral glucose tolerance test in nonobese Japanese type 2 diabetic patients: comparison with minimal-model approach. Diabetes Care. 2000; 23: 1439-40.
- 17. Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006; 113: 1888-904.
- 18. Gokce N, Keaney JF Jr, Hunter LM, Watkins MT, Nedeljkovic ZC, Menzoiam JO, et al. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003; 41: 1769-75.
- 19. Huang PH, Chen JW, Lu TM, Yu-An Ding P, Lin SJ. Combined use of endothelial function assessed by brachial ultrasound and high-sensitive C-reactive protein in predicting cardiovascular events. Clin Cardiol. 2007; 30: 135-40.
- 20. Boulon C, Lafitte M, Richeboeuf V, Paviot B, Pradeou V, Coste P, et al. Prevalence of metabolic syndrome after acute coronary syndrome and its prognostic significance. Am J Cardiol. 2006; 98: 1429-34.
- 21. Marroquin OC, Kip KE, Kelley DE, Johnson BD, Shaw LJ, Bairey Merz CN, et al. Metabolic syndrome modifies the cardiovascular risk associated with angiographic coronary artery disease in women. A report from the Women's Ischemia Syndrome Evaluation. Circulation. 2004; 109: 714-21.
- 22. Bartnik M, Malmberg K, Norhammar A, Tenerz A, Ohrvik J, Rydén L. Newly detected abnormal glucose tolerance: an important predictor of long-term outcome after myocardial infarction. Eur Heart J. 2004; 25: 1990-7.
- 23. Tamita K, Katayama M, Takagi T, Akasaka I, Yamamura A, Kajis S, et al. Impact of newly diagnosed abnormal glucose tolerance on long-term prognosis in patients with acute myocardial infarction. Circ J. 2007; 71: 834-41.
- 24. Barr ELM, Zimmet PZ, Welborn TA, Jolley D, Magliano DJ, Dunstan DW, et al. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance. The Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation. 2007; 116: 151-7.
- 25. Bartinik M, Rydén L, Maimberg K, Ohrvik J, Pyöralä K, Standl E, et al. Oral glucose tolerance test is needed for appropriate classification of glucose regulation in patients with coronary artery disease: a report from the Euro Heart Survey on Diabetes and the Heart. Heart. 2007; 93: 72-7.
- 26. Schnell O. The links between diabetes and cardiovascular disease. J Intervent Cardiol. 2005; 18: 413-6.
- 27. Nesto RW, Lago RM. Glucose: a biomarker in acute myocardial infarction ready for prime time? Circulation. 2008; 117: 990-2.
Early glucometabolic profile in patients with acute coronary syndromes and metabolic syndrome
Publication Dates
-
Publication in this collection
06 Apr 2009 -
Date of issue
Feb 2009
History
-
Accepted
17 Mar 2008 -
Reviewed
10 Mar 2008 -
Received
20 Feb 2008