Abstracts
OBJECTIVE: To evaluate the effects of carbon dioxide infusion to abdominal wall adipocytes. METHODS: Fifteen volunteers were subjected to sessions of CO2 infusion for three consecutive weeks (two sessions per week with intervals of two to three days between each). The volume of carbon dioxide infused per session, at points previously marked, was always calculated on the basis of surface area to be treated, with a fixed infused volume of 250 ml/100cm² of treated surface. The infiltration points were marked respecting the limit of 2cm equidistant between them. At each point 10 ml was injected per session, with a flow of 80ml/min. Fragments were collected from subcutaneous tissue of the anterior abdominal wall before and after treatment. The number and histomorphological changes of adipocytes (mean diameter, perimeter, length, width and number of adipocytes per field of observation) were measured by computerized cytometry. The results were analyzed with paired Student t test, adopting a significance level of 5% (p <0.05). RESULTS: There was a significant reduction in the number of adipocytes in the abdominal wall, as well as the area, diameter, perimeter, length and width of the adipocytes, after the infusion of CO2 (p = 0.0001). CONCLUSION: The percutaneous infiltration of CO2 reduces the population of adipocytes of the anterior abdominal wall and modifies their morphology.
Adipocytes; Subcutaneous tissue; Image processing Computer-Assisted; Carbon dioxide
OBJETIVO: Avaliar os efeitos da infiltração de dióxido de carbono em adipócitos presentes na parede abdominal. MÉTODOS: Quinze voluntárias foram submetidas a sessões de infusão de CO2 durante três semanas consecutivas (duas sessões por semana com intervalos de dois a três dias entre cada sessão). O volume de gás carbônico infundido por sessão, em pontos previamente demarcados, foi sempre calculado com base na superfície da área a ser tratada, com volume infundido fixo de 250 mL/100cm² de superfície tratada. Os pontos de infiltração foram demarcados respeitando-se o limite eqüidistante 2cm entre eles. Em cada ponto se injetou 10mL, por sessão, com fluxo de 80mL/min. Foram colhidos fragmentos de tecido celular subcutâneo da parede abdominal anterior antes e após o tratamento. O número e as alterações histomorfológicas dos adipócitos (diâmetro médio, perímetro, comprimento, largura e número de adipócitos por campos de observação) foram mensurados por citometria computadorizada. Os resultados foram analisados com o teste t de Student pareado, adotando-se nível de significância de 5% (p<0,05). RESULTADOS: Encontrou-se redução significativa no número de adipócitos da parede abdominal e na área, diâmetro, perímetro, comprimento e largura após o uso da hipercapnia (p=0,0001). CONCLUSÃO: A infiltração percutânea de CO2 reduz a população e modifica a morfologia dos adipócitos presentes na parede abdominal anterior.
Adipócitos; Tela subcutânea; Processamento de imagem assistida por computador; Dióxido de carbono
ORIGINAL ARTICLE
IPhD, Department of Surgery, Faculty of Medicine, University of São Paulo - São Paulo, Brazil
IIAssistant Professor, Department of Surgery Faculty of Medicine, University of São Paulo - SãoPaulo, Brazil
IIIAssistant Professor, Department of Cell Biology, Institute of Biosciences, University of São Paulo - São Paulo, Brazil
IVTrainee. Laboratory of Lipids, Institute of Biosciences, University of São Paulo - São Paulo, Brazil
VAssociate Professor, Post-Graduate Program in Health Sciences, San Francisco University, Bragança Paulista - São Paulo, Brazil
VIProfessor, Human Structural Topography, Department of Surgery, Faculty of Medicine, University of São Paulo - São Paulo, Brazil
Correspondence to
ABSTRACT
OBJECTIVE: To evaluate the effects of carbon dioxide infusion to abdominal wall adipocytes.
METHODS: Fifteen volunteers were subjected to sessions of CO2 infusion for three consecutive weeks (two sessions per week with intervals of two to three days between each). The volume of carbon dioxide infused per session, at points previously marked, was always calculated on the basis of surface area to be treated, with a fixed infused volume of 250 ml/100cm2 of treated surface. The infiltration points were marked respecting the limit of 2cm equidistant between them. At each point 10 ml was injected per session, with a flow of 80ml/ min. Fragments were collected from subcutaneous tissue of the anterior abdominal wall before and after treatment. The number and histomorphological changes of adipocytes (mean diameter, perimeter, length, width and number of adipocytes per field of observation) were measured by computerized cytometry. The results were analyzed with paired Student t test, adopting a significance level of 5% (p <0.05).
RESULTS: There was a significant reduction in the number of adipocytes in the abdominal wall, as well as the area, diameter, perimeter, length and width of the adipocytes, after the infusion of CO2 (p = 0.0001).
CONCLUSION: The percutaneous infiltration of CO2 reduces the population of adipocytes of the anterior abdominal wall and modifies their morphology.
Key words: Adipocytes. Subcutaneous tissue. Image processing. Computer-Assisted;.Carbon dioxide/therapeutic use.
INTRODUCTION
In the early thirties, observations in France found that a simple water bath saturated with carbon dioxide (CO2) improved symptoms in patients with ischemic and inflammatory diseases, probably by increasing local circulation1. Later it was found that the percutaneous infiltration of CO2 in the subcutaneous tissue through needles not only improved blood flow in ischemic tissues, but also increased local oxygen concentration2,3. The therapeutic effects of subcutaneous infiltration of CO2 were attributed to local vasodilation, which caused the fall in peripheral vascular resistance, improving blood supply. This vasodilation, both arterial and venous, which increased local blood flow, was confirmed by studies that measured tissue blood perfusion by flowmetry 4,5. Another effect of CO2infiltration into the subcutaneous tissue is to increase local temperature, determining a lipolytic effect, which is not found when using other gas mixtures6. Still based on clinical observations, it was demonstrated in the early 21st century that the infiltration of CO2 in the subcutaneous tissue was able to reduce localized fat deposits7. However, despite these findings suggest that the reduction in fat deposits occurred by changes in the number and shape of adipocytes, there have been no histological studies that could confirm these suspicions.
The accumulation of fat located in the anterior abdomen is a cause of constant clinic and aesthetic concern. With the advent of liposuction in the abdominal wall, the formation of small deposits of subcutaneous fat during the postoperative follow-up has become a major cause of dissatisfaction with this technique8. Although the pathophysiological bases of these complications are not yet fully understood, different methods have been proposed in order to meet the best therapeutic strategy for its correction1. Thus, application of chemical or physical agents has been used indiscriminately, but the results are still controversial9,10. Unsatisfied with the outcome, many patients resort to alternative procedures (most often performed by non authorized individuals), which may worsen cosmetic results and lead to the onset of serious, at times fatal, complications11.
In 2004, it was demonstrated for the first time that the infiltration of CO2 in the subcutaneous tissue, when associated with liposuction procedures, was effective in the treatment of localized accumulations of fat or skin irregularities resulting from the operations1. Histological evidence of the beneficial effects of this treatment option in reducing the thickness of abdominal subcutaneous tissue was demonstrated, suggesting that the effects of the method could be related to increased local circulation, as well as from the direct action of CO2 on breaking the adipocyte cytoplasmic membrane. Improvement of skin elasticity was shown, with decrease in the accumulation of fat when the percutaneous infiltration of CO2 was carried out after liposuction3,12,13. Since then the method has been used with increasing frequency for treatment of different forms of lipodystrophy and in aesthetic medicine14-16.
Although applied for more than a decade, few studies have evaluated the histological changes in the fat cells located in the anterior abdominal wall after percutaneous infiltration of CO213. The literature shows an absolute lack of scientifically conducted studies evaluating different effects of the method, especially in relation to histological and cytometric alterations of the subcutaneous adipocyte layer17.
The aim of this study was to evaluate whether percutaneous infiltration of medical CO2 in areas of localized fat in the anterior abdominal wall is related to changes in cytometry and in the number of adipocytes analyzed.
METHODS
This study was approved by the Ethics Committee for Analysis of Research Projects (CAPPesq) of the University Hospital, University of São Paulo (number HU/USP 857/08) and was initiated after approval by the National Commission for Studies and Research (CONEP).
We prospectively evaluated 15 women from the University Hospital of the USP, with a Body Mass Index (BMI) between 20 and 25 (healthy) in the period between January 2007 and January 2009, who met the inclusion criteria (female, BMI between 20 and 25, aged 24 to 50 years, with an area of localized fat accumulation in the anterior abdominal wall, without signs of lipodystrophy). We excluded pregnant women, nursing mothers, postmenopausal women, those who had metabolic disorders or autoimmune diseases, those who underwent hormone replacement therapy, the ones previously submitted to abdominal surgery (including liposuction), patients with skin lesions on the skin of the abdomen and the ones that had sustained changes in body weight greater than two pounds during the treatment (three weeks). All volunteers, after clarification of all stages of the procedure, signed a consent form agreeing to participate in the study, as well as allowing the carrying out of the abdominal incision required for specimen collection.
Standardization of the method
Anthropometric measures
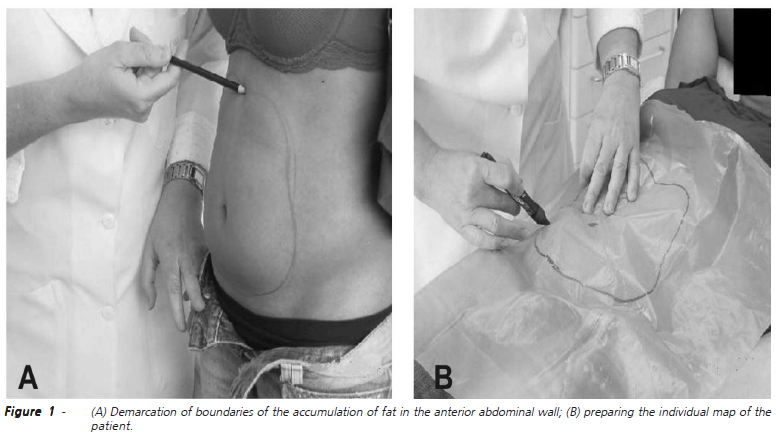
Once selected, all volunteers underwent the following anthropometric measures: body weight (kg), height (m) and BMI (Kg/m2). Demarcation and mapping of the perimeter of the area with accumulation of fat in the anterior abdominal wall was carried out (Figures 1A and 1B). Measures of waist circumference were always evaluated based on a plane parallel to the ground with the volunteers in standing position. Two circumferences were measured, the first passing by the umbilicus and the second at the highest point of the anterior superior right and left iliac crests (Figure 2). The same anthropometric measures were taken again seven days after the last session of CO2 infiltration.
Photographic documentation
Photographic documentation of the abdominal region was always performed with the same focal length in the planes (frontal, right oblique, right profile, left oblique, left profile). The photos were always performed in a standardized way, with the volunteers in standing position in a previously marked area on the ground, indicating the direction of the plans set out for photography. New photographs were taken of the same previously established plans seven days after the last infiltration of CO2.
Calculation of total body surface and area treated with CO2
The calculation of total body surface area (BSA), in square meters, was obtained according to the formula BSA=K.p2/3 (k = constant equal to 0.09; p = body weight in Kilograms)18. The calculation of the area of localized fat accumulation in the anterior abdominal wall to be treated by CO2 infiltration in absolute numbers (cm2) and percentage (%) in relation to the BSA was obtained for each volunteer by the method of photographic matching. The maps of individual areas of fat accumulation in the anterior abdomen of each volunteer were documented using a digital camera (DSC-S50 - Sony Brazil Ltda. SP, Brazil) next to a 10x10cm black square (100 cm2), whose image was used as a parameter for the subsequent scanning of images. The images taken by digital camera were transferred to a microcomputer, and with the help of an image analysis program (UTHSCSA Image Tool - University of Texas Health Science Center, SA, Texas, USA)19, different areas to be treated with CO2 were calculated. The black square was the basis for calculating the area in square centimeters and its percentage. Each area calculation was obtained by measuring the area to be subjected to infiltration of CO2 by the same researcher (Figures 3A and 3B).
Calculating the volume of infused CO2
The calculation of the volume of CO2 to be infused in the area of fat accumulation per session was established according to the methodology described above, which estimated the volume of CO2 to obtain therapeutic effect in 250ml20. The flow and time of application of CO2 was carried out by a specific equipment for this purpose, calibrated to always infiltrate CO2 at a flow rate of 80ml/ min, which gave a maximum time of application of eight seconds for each marked point (Advanced Carbatek; Estek, São Paulo, Brazil).
Demarcation and puncture of the infusion sites
With the volunteer in supine position and using the individual map that marked the perimeter of the area where there was fat accumulation, we marked the area again. With a proper pen, we drew 2cm equidistant points in this perimeter to establish the locations where the puncture for CO2 infiltration should be performed (Figures 4A and 4B).
The patients remained in supine position for the puncture and infusion of CO2 in the subcutaneous tissue. After antisepsis of the abdominal wall with an alcoholic solution of chlorhexidine puncture was performed using a 30-gauge needle, which penetrated the subcutaneous tissue at each previously marked point at a depth of 2cm. Ten milliliters of medicinal carbon gas were infused in all marked areas, thus completing the quantity for that area. The sessions happened twice a week for a total of six, with intervals of two to three days between them.
Biopsy and post-treatment
Biopsies of the subcutaneous tissue, before and after CO2 infiltration, were always performed with the same technique. The place of acquisition of the pre-treatment material was standardized for all volunteers before the first session. The fragment of skin and subcutaneous tissue was always harvested in a standard point located 3 cm to the right of the umbilicus, with the subject in supine position. Post-treatment biopsy was performed at a point 1cm below the one of the pre-treatment biopsy.
For the procedure we performed antisepsis of the site with a solution of chlorhexidine and alcohol, then we proceeded with the anesthetic infiltration of 2% lidocaine without vasoconstrictor. The abdominal wall was covered with a fenestrated sterile surgical cloth and an elliptical incision measuring 3 x 2 mm, comprehending the skin and subcutaneous tissue was made. Fragments of removed adipose tissue, measuring 1 cm length, were placed in 4% paraldehyde solution, where the specimen remained for 24 hours and then was transferred to another flask with 70% alcohol. We carried out the synthesis of subcutaneous tissue with one 5-0 polyglactin suture stitch and the skin with 6-0 intradermal nylon. After the intervention, the skin was covered with sterile tape. The stitches were removed on the eighth postoperative day, and a new occlusive dressing was made with the aim of better coaptation of the edges of the wound and of improving the appearance of the scar.
Histological technique
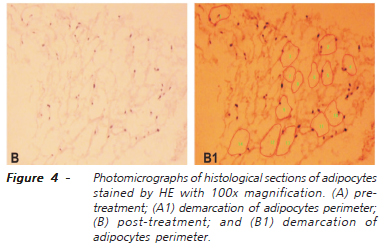
Surgical specimens (pre and post-treatment) were prepared in paraffin blocks, which subsequently were subjected to 4ì-thick histological sections to slide preparation, always stained by hematoxylin-eosin21. All slides were examined under an optical microscope by a pathologist who was unaware of the origin of the material, as well as of the objectives of the study. With the program of image analysis, computer-assisted Image-Pro Plus 6.0 (Media Cybernetics, Bethesda, MD, USA) we measured the area, mean diameter, perimeter, length and width of adipocytes present in the specimens (Figure 4). In each slide six random fields were analyzed, measuring each proposed variable22,23. We counted the number of adipocytes present in each field in the tissues obtained before and after treatment. We calculated the index of ellipticity of adipocytes through the ratio: L/W (L = length and W = width).
All individual anthropometric data, as well as the value of different histological variables studied, were recoded in specific spreadsheets for statistical analysis. We used the paired Student t test for comparison of the results obtained in the two study time points (pre and post-treatment). Variations of the results of anthropometric measurements were evaluated by Kruskal-Wallis test. For both tests we adopted a significance level of 5% (p<0.05). The calculations were performed using SPSS, version 13.0.
RESULTS
Table 1 shows the values of anthropometric measurements before and after infusion of CO2. No significant variation was found in anthropometric measurements of weight (p=0.64), umbilical circumference (p=0.31) and circumference at the iliac crest (p=0.32) before and after the sessions.
Table 2 shows the average, with respective standard deviation, of the values observed for the selected variables measured by computerized cytometry (area, diameter, perimeter, length, width, and number of adipocytes) by comparing the values before and after CO2 infusion. There was a significant reduction in the values of all variables after the use of percutaneous infiltration of CO2 (p <0.00001).
DISCUSSION
Brandi et al.7 first demonstrated, in 2001, that systematic, percutaneous infiltration of CO2 in regions of the abdominal wall with localized fat deposits could lead to adipocytes rupture, improving results after liposuction. The authors encouraged the use of the technique as an adjuvant therapy to obtain better cosmetic results. The formation of these unwanted fat deposits, in addition to compromising aesthetics, has not yet been subject to an alternative therapy consensus15,16. Lipolytic properties of percutaneous CO2 infiltration could be an effective alternative to handling unwanted fat deposits that often form after liposuction, providing better cosmetic results and patient satisfaction1,3,7,12.
A systematic literature review confirmed the clinical efficacy of this alternative approach12. The low cost, small number of formal contraindications (patients receiving anticoagulation, hemorrhagic diastases) and the low rate of fatal complications meant that the technique gained popularity by making it increasingly utilized24-26. Nevertheless, percutaneous CO2infiltration has been empirically applied, no studies existing to standardize the various technical aspects involved, such as: appropriate amount of CO2 infiltration, number and frequency of sessions, infusion speed . Few studies have evaluated the effects of the technique using modern methods to determine the true effects of CO2 on subcutaneous adipocytes13. Most publications evaluated the effects of the method, as well as the histological changes found in the subcutaneous tissue, in an empirical and subjective manner, not using any method that permits a more accurate and objective assessment. With the advent of procedures for image analysis (computer-assisted morphometry scan), it became possible to reliably measure histological changes occurring in different cells and tissues subjected to various experimental situations27,28. Computerized morphometry is being increasingly used in histopathological studies, proving to be valid for providing greater accuracy in histology measurements, easy to use and low cost29,30.
Aiming to standardize the sample of this study, we selected only voluntary patients who met the inclusion criteria (mean age 34 years, body mass index (BMI) between 20 and 25). This level of BMI was chosen to characterize a healthy condition. Exclusion criteria were postmenopausal women, pregnant women, nursing mothers and women in hormone replacement therapy, in order to prevent any hormonal interference that could alter the distribution of adipose tissue. The age range chosen includes adult women in reproductive age. The option to study the fat located in the anterior abdominal wall guaranteed to always research the same kind of fat, in an area with easy definition, measurement, documentation and control. Therefore, this study examined only the accumulation of fat in the anterior abdominal wall in healthy women. Volunteers subjected to any type of abdominal surgery (bariatric or plastic surgery) were also excluded from the study, as well as volunteers who accumulated or lost weight greater than two pounds, in order to avoid that variables related to scarring or changes in fat metabolism that could affect results.
To ensure uniformity in all anthropometric measures, both before and after treatment, we always measured at the umbilicus and on the higher ridges of the anterior-superior crests, always with the volunteers in standing position. These circles accompanied planes parallel to the ground. Similarly, tissue samples were always removed in the same abdominal wall place before and after treatment, avoiding comparison of samples originated from different regions. The results showed no significant variation in the measurements of weight, umbilical circumference and at the level of the iliac crests at the beginning and at the end of treatment. It is possible that more sessions could cause variations in these measures, as noted by others who adopted longer treatment periods7.
From these data, it was possible to correlate the area of localized fat accumulation in the anterior abdominal wall with other anthropometric parameters, in addition to BMI, aiming to correlate the extent of surface area to be treated with the method and the total body surface based on the previously proposed formula, which allows to estimate the area in square meters. With the demarcation of boundaries of the area of fat accumulation in the anterior abdominal wall, we could create maps of individual demarcation, and the strategy to photograph this area with the same focal length and using a juxtaposed 10x10cm square made it possible to calculate with accuracy the area of fat accumulation through the computational image analysis software. From the knowledge of the area being treated, it was possible to calculate the volume of CO2 to be applied in each session for each volunteer. This same strategy had been previously used31. Knowing the total body surface areas and the surface to be treated with CO2, the percentage was calculated in relation to total body surface. With this information, and the proposal to infuse always 250 ml of CO2 for each area of 100cm2, we obtained a reference to the precise amount of CO2 to be infused in each subject per session. The demarcation of equidistant points every 2 cm assured infiltration of equal volumes in each puncture site. The application of CO2 was performed with the aid of the equipment described, which allowed us to set the speed of application at 80ml/min. With the standardization of the infiltration time in eight seconds, it was possible to ensure that the same amount of gas (10 ml) was applied at each punctured point.
During each session we monitored the macroscopic skin condition at the point of infiltration. At some points, we noted a mild, regional, short-lived skin rash, as well as a small emphysema that persisted for a maximum of five minutes, remitting completely after this period. The volunteers reported the painful discomfort as of low intensity and bearable during the application. None of the volunteers throughout the study showed allergic reactions or post-application infections. These findings are also described in individuals who developed extensive subcutaneous emphysema after laparoscopic procedures12,26. The only noteworthy complication was the formation of small bruises in a few scattered puncture sites.
In this study the volume of CO2 used per session was established with the greatest care possible, based on the extent of the treated surface. We tried to strictly control the volume to be applied, reaching an estimated 10 ml for each puncture site. Despite the previous demonstration of therapeutic effects the studies stipulated the daily volume of CO2 applied in an arbitrary manner, ranging between 20 and 800 ml20,25,32. The standardization adopted in this study showed that for every 100 cm2 of to-be-treated area the volume of 250 ml, or 10 cm3 for each puncture site, showed therapeutic efficacy in reducing adipocytes' number and cytometry.
Another aspect that deserves consideration is the frequency and duration of treatment to achieve effective results. Previous studies have suggested series of applications on alternate days for a period of two to three weeks, with the possibility of extending the applications according to clinical evolution5,15,25. Therefore, the duration and the interval between sessions did not follow any pattern, causing the number of sessions to range between 5 and 20, without any scientific justification for this large variation. In this study, we chose the number of six sessions, with intervals of at least two days, to compose the treatment over the seven days of the week (21 days or three weeks). This standard was adopted in all the volunteers, allowing the analysis of results not to suffer the interference of irregular CO2 infiltrations.
Histological analysis was always performed by experienced personnel and had adopted a similar methodology in prior studies22,23. When considering that the sample consisted of healthy volunteers, pretreatment histological studies confirmed data normality, which gave security to the statement that we used a sample comprised of healthy volunteers. Similar statistical results in anthropometric data obtained by analysis of variance in the pre and post-treatment periods demonstrated that the target regions of the measurements were the same. Histological examination after treatment showed changes in adipocytes' cytometry, uniformly with respect to area, diameter, perimeter, length, width and length/width ratio, which renders the of ellipticity index. The image analysis software used, by confering the precise numerical quantification of all selected variables at two different times, allowed the use of parametric tests for paired samples that showed significant reduction in all variables after the percutaneous CO2. Similarly, the counting of adipocytes present in six fields of vision showed a reduction of one third (33.18%). Hence, the results suggest that the infiltration of CO2 in the volume, flow, time, number of sessions and the intervals applied was able to reduce the number and decrease the values of the cytometric variables of adipocytes located in the anterior abdominal wall of healthy women. It is prudent to imagine that the observed effects may have finite duration, i.e., they are temporary.
The fatty tissue is essentially a fat depot, with different characteristics according to its body location, however, in actuality, there is experimental evidence indicating that adipocytes produce and secrete highly active hormones and cytokines, with specific receptors and physiological importance, acting as a real endocrine organ. In future, properly conducted scientific research will need to address whether the infusion of medicinal CO2 influences these physiological functions recently attributed to fat tissue. The clarification of the role of percutaneous infiltration of CO2 in reduction of fat deposition for cosmetic purposes and in the importance of the technique in the functional aspects of the whole organism is thus an open field, with broad and promising perspectives,.
Under the conditions of this study, we conclude that the standardized infusion of CO2 into the subcutaneous tissue of the anterior abdominal wall of healthy women significantly reduces the number and size of adipocytes present at the site.
REFERENCES
- 1. Brandi C, D'Aniello C, Grimaldi L, Caiazzo E, Stanghellini E. Carbon dioxide therapy: effects on skin irregularity and its use as a complement to liposuction. Aesthetic Plast Surg. 2004;28(4):222-5.
- 2. Di Ció AV, Klein L. Action of subcutaneous injection of carbon on arterial tension. Prensa Med Argent. 1950;37(30):1707-9.
- 3. Ito T, Moore JI, Koss MC. Topical application of CO2 increases skin blood flow. J Invest Dermatol. 1989; 93(2):259-62.
- 4. Diji A. Local vasodilatador action of carbon dioxide on blood vessels of the hand. J Appl Physiol. 1959;14(3):414-6.
- 5. Volkmer E. Subcutaneous CO(2) insufflation therapy. Schmerz 1992;6(2)154-5.
- 6. Diji A, Greenfield AD. The local effect of carbon dioxide on uman blood vessels. Am Heart J. 1960;60:907-14.
- 7. Brandi C, D'Aniello C, Grimaldi L, Bosi B, Dei I, Lattarulo P, Alessandrini C. Carbon dioxide therapy in the treatment of localized adiposities: clinical study and histopathological correlations. Aesthetic Plast Surg. 2001;25(3):170-4.
- 8. Rosenbaum M, Prieto V, Hellmer J, Boschmann M, Krueger J, Leibel RL, Ship AG. An exploratory investigation of the morphology and biochemistry of cellulite. Plast Reconstr Surg. 1998;101(7):1934-9.
- 9. Sun Y, Wu SF, Yan S, Shi HY, Chen D, Chen Y. Laser lipolysis used to treat localized adiposis: a preliminary report on experience with Asian patients. Aesthetic Plast Surg. 2009;33(5):701-5.
- 10. Davis MD, Wright TI, Shehan JM. A complication of mesotherapy: noninfectious granulomatous panniculitis. Arch Dermatol. 2008;144(6):808-9.
- 11. Lehnhardt M, Homann HH, Daigeler A, Hauser J, Palka P, Steinau HU. Major and lethal complications of liposuction: a review of 72 cases in Germany between 1998 and 2002. Plast Reconstr Surg. 2008;121(6):396e-403e.
- 12. Brockow T, Hausner T, Dillner A, Resch KL. Clinical evidence of subcutaneous CO2 insufflations: a systematic review. J Altern Complement Med. 2000;6(5):391-403.
- 13. Ferreira JC, Haddad A, Tavares SA. Increase in collagen turnover induced by intradermal injection of carbon dioxide in rats. J Drugs Dermatol. 2008; 7(3):201-6.
- 14. Campos V, Bloch L, Cordeiro T. Carboxytherapy for gynoid lipodystrophy treatment: the Brazilian experience. J Am Acad Dermatol. 2007;56(2-Suppl.2):AB196.
- 15. Koutná N. Carboxytheraphy: a new non-invasive method in aesthetic medicine. Cas Lek Cesk. 2006;145(11):841-3.
- 16. Lee GSK. Carbon dioxide theraphy in the treatment of cellulite: an audit of clinical practice. Aesthetic Plast Surg. 2010;34(2):239-43.
- 17. Tavares-Ferreira JC. Carboxiterapia na cicatrização de feridas crônicas no HGeF. Rev Med HGF. 2007;8(2):14-5.
- 18. Guyton AC, Jones CE, Coleman TG. Hormonal cardiac output and its variation. In: Guyton AC. Cardiac output and its regulation. 2nd ed. Philadelphia: WB Sounders, 1973. p.3-29.
- 19. Wilcox CD, Dove SB, McDavid D, Greer DB. UTHSCSA Image Tool. Disponível em: http://ddsdx.uthscsa.edu/dig/itdesc.html Acesso em 7 nov 2008.
- 20. Di Ció AV. 2400 cases of intermittent claudication and gangrene of the extremities treated by subcutaneous injection of oxygencarbon dioxide mixture. Prensa Med Argent. 1956;43(1):1-23.
- 21. Gamble M. The hematoxylins and eosin. In: Bancroft JD, Gambe M, editors. Theory and pratice of histological techniques. New York: Churchill Livingstone. 2008; 2008. p.121-35.
- 22. Bing C, Russell S, Becket E, Pope M, Tisdale MJ, Trayhurn P, Jenkins JR. Adipose atrophy in cancer cachexia: morphologic and molecular analysis of adipose tissue in tumour-bearing mice. Brit J Cancer. 2006;95(8):1028-37.
- 23. Bing C, Trayhurn P. Regulation of adipose tissue metabolism in cancer cachexia. Curr Opin Clin Nutr Metab Care. 2008;11(3):201-7.
- 24. Heinicke HJ. Einführende mitteilung über die therapeutische subkutane Quellgasinsufflation mit kohlendioxid. Phys Rehab Kur Med 1985;37(3):171-6.
- 25. Taubert K. Carbon dioxide insufflation in headache and migraine. Z Arztl Fortbild (jena). 1991:85(1-2):23-30.
- 26. Wolf JS Jr, Carrier S, Stoller ML. Gas embolism: helium is more lethal than carbon dioxide. J Laparoendosc Surg. 1994;4(3):173-7.
- 27. Martinez CAR, Waisberg J, Palma RT, Silva FZ, Cimerman G, Goffi FS. Morphometric study of gastric mucosa in dogs submitted to proximal gastric vagotomy, splenectomy or proximal gastric vagotomy associated with splenectomy. Acta Cir Bras [serial on the internet]. 2002;17(5): 289-98. Disponível em http://www.scielo.br/acb
- 28. Sousa MV, Priolli DG, Portes AV, Cardinalli IA, Pereira JA, Martinez CAR. Evaluation by computerized morphometry of histopathological alterations of the colon wall in segments with and without intestinal transit in rats. Acta Cir Bras [serial on the internet]. 2008 23(5):417-24. Disponível em http://www.scielo.br/acb
- 29. Martinez CAR, Nonose R, Spadari APP, Máximo FR, Priolli DG, Pereira JA, Margarido NF. Quantification by computerized morphometry of tissue levels of sulfomucins and sialomucins in diversion colitis in rats. Acta Cir Bras [serial on the internet]. 2010;25(3):231-40. Disponível em http://www.scielo.br/acb
- 30. Nonose R, Spadari APP, Priolli DG, Máximo FR, Pereira JA, Martinez CAR. Tissue quantification of neutral and acid mucins in the mucosa of the colon with and without fecal stream in rats. Acta Cir Bras [serial on the internet]. 2009;24(4):267-75. Disponível em http://www.scielo.br/acb
- 31. Mauad RJ Jr, Shimizu MH, Mauad T, de Tolosa EM. Buflomedil and pentoxifylline in the viability of dorsal cutaneous flaps of rats treated with nicotine. J Plast Reconstr Aesthet Surg. 2006;59(4):387-92.
- 32. Joderko GZ, Nowicki L, Galaszek E. Subkutane CO2 gasinsufflation bei der arterieller ver-schlusskrankheit. Z Phis Med Balm med Klin (Sonder-helf 1). 1990:19-88.
Cytometric evaluation of abdominal subcutaneous adipocytes after percutaneous CO2 infiltration
Publication Dates
-
Publication in this collection
20 Apr 2011 -
Date of issue
Feb 2011
History
-
Accepted
12 Mar 2010 -
Received
10 Jan 2010