ABSTRACT
Peltogyne chrysopis is an arboreal legume endemic to the Atlantic Forest and known only from the state of Bahia, Brazil. Focal observations were made of anthesis, pollen availability, stigma receptivity, nectar production, and the presence of osmophores and UV-reflective pigments for the species. Floral visitors were also observed and classified based on the timing and frequency of their visits and their foraging behavior. The breeding system was inferred from the pollen-ovule ratio and pollen tube growth after pollination treatments. Peltogyne chrysopis was found to be melittophilous, with anthesis occurring from 02h00min to 05h00min, and protogynous and xenogamous, with flower scent emission and pollen release before sunrise. Xenochlora nigrofemorata was the main pollinator, as it effectively collected and transferred pollen grains. Nectar production appears to be a secondary resource to ensure the attraction of a diversity of floral visitors and potential pollinators in the absence of effective pollinators. The results of the present study contribute to understanding the pollination mechanisms of Peltogyne, a genus that has been neglected with regard to its reproductive mechanism, and documents, for the first time, the role of the bee genus Xenochlora in plant pollination.
Keywords:
anthesis; compatibility system; floral rewards; Leguminosae; melittophily; pollinator efficiency; Xenochlora nigrofemorata
Introduction
Studies on reproductive biology (e.g., floral biology, breeding system, pollination) have been widely recognized as important tools for identifying the factors associated with the reproductive success of plants and the subsequent maintenance of populations, especially when dealing with endemic, rare or endangered species (Schemske et al. 1994Schemske DW, Husband BC, Ruckelshaus MH, Goodwillie C, Parker IM, Bishop JG. 1994. Evaluating approaches to the conservation of rare and endangered plants. Ecology 75: 584-606.; Rodríguez-Pérez 2005Rodríguez-Pérez J. 2005. Breeding System, Flower Visitors and Seedling Survival of Two Endangered Species of Helianthemum (Cistaceae). Annals of Botany 95: 1229-1236.; Castro et al. 2008Castro S, Silveira P, Navarro L. 2008. How flower biology and breeding system affect the reproductive success of the narrow endemic Polygala vayredae Costa (Polygalaceae). Botanical Journal of the Linnean Society 157: 67-81.). However, many aspects of reproductive biology that influence the distribution and maintenance of tree species in fragmented tropical Atlantic forests remain poorly investigated. According to Girão et al. (2007Girão LC, Lopes AV, Tabarelli M, Bruna EM. 2007. Changes in tree reproductive traits reduce functional diversity in a fragmented Atlantic forest landscape. PLOS ONE 2: e908. doi: 10.1371/journal.pone.0000908.
https://doi.org/10.1371/journal.pone.000...
), forest fragmentation may be promoting shifts in the relative abundances of the reproductive traits of trees (e.g., hermaphroditic and xenogamic species predominate in forest fragments and in forest interiors over self-compatible species).
The Atlantic Forest phytogeographical domain has long been recognized as a biodiversity hotspot, although its forests have suffered massive fragmentation and serious environmental disturbances and currently occupy less than 8% of their original area of distribution (Galindo-Leal & Câmara 2005Galindo-Leal C, Câmara IG. 2005. Status do hotspot Mata Atlântica: uma síntese. In: Galindo-Leal C, Câmara IG. (eds.) Mata Atlântica: biodiversidade, ameaças e perspectivas. São Paulo, Fundação SOS Mata Atlântica. p. 3-12.). Habitat destruction and fragmentation have been the main factors reducing the viabilities of plant populations and driving biodiversity losses and extinctions (e.g., Rathcke & Jules 1993Rathcke BJ, Jules ES. 1993. Habitat fragmentation and plant-pollinator interactions. Current Science 65: 273-277.; Schemske et al. 1994Schemske DW, Husband BC, Ruckelshaus MH, Goodwillie C, Parker IM, Bishop JG. 1994. Evaluating approaches to the conservation of rare and endangered plants. Ecology 75: 584-606.; Silva et al. 2017Silva TR, Medeiros MB, Noronha SE, Pinto JRR. 2017. Species distribution models of rare tree species as an evaluation tool for synergistic human impacts in the Amazon rainforest. Brazilian Journal of Botany 40: 963-971.), thus understanding general eco-evolutionary patterns may help maintain biodiversity levels (Fontúrbel & Murúa 2014Fontúrbel FE, Murúa MM. 2014. Microevolutionary Effects of Habitat Fragmentation on Plant-Animal Interactions. Advances in Ecology 2014: 379267. doi: 10.1155/2014/379267
https://doi.org/10.1155/2014/379267...
). Numerous studies have found that habitat fragmentation may affect plant reproductive success by disrupting plant-pollinator interactions (see Aguilar et al. 2006Aguilar R, Ashworth L, Galetto L, Aizen MA. 2006. Plant reproductive susceptibility to habitat fragmentation: review and synthesis through meta-analysis. Ecology Letters 9: 968-980. to review; Fontúrbel & Murúa 2014Fontúrbel FE, Murúa MM. 2014. Microevolutionary Effects of Habitat Fragmentation on Plant-Animal Interactions. Advances in Ecology 2014: 379267. doi: 10.1155/2014/379267
https://doi.org/10.1155/2014/379267...
; Franceschinelli et al. 2015Franceschinelli EV, Carmo RM, Silva Neto CM, Gonçalves BB, Bergamin LL. 2015. Reproductive success ofCabralea canjerana(Meliaceae) in Atlantic forest fragments, Brazil. Revista de Biología Tropical 63: 515-524.). Additionally, forest fragmentation and increased selfing rates (e.g., small and isolated populations) can reduce gene flow resulting in inbreeding depression in plant populations (O’Brien 1994O’Brien SJ. 1994. A role for molecular genetics in biological conservation. Proceedings of the National Academy of Sciences of the USA 91: 5748-5755. ; Buza et al. 2000Buza L, Young A, Thrall P. 2000. Genetic erosion, inbreeding and reduced fitness in fragmented populations of the endangered tetraploid pea Swainsona recta. Biological Conservation 93: 177-186.; Kang et al. 2005Kang M, Jiang M, Huang H. 2005. Genetic Diversity in Fragmented Populations of Berchemiella wilsonii var. pubipetiolata (Rhamnaceae). Annals of Botany 95: 1145-1151.).
Pollination is an essential ecosystem service and is particularly important in natural populations for guaranteeing genetic diversity and thus contributing to biodiversity maintenance (Schemske et al. 1994Schemske DW, Husband BC, Ruckelshaus MH, Goodwillie C, Parker IM, Bishop JG. 1994. Evaluating approaches to the conservation of rare and endangered plants. Ecology 75: 584-606.; Kevan 1999Kevan PG. 1999. Pollinators as bioindicators of the state of the environment: species, activity and diversity. Agriculture, Ecosystems & Environment 74: 373-393.). Despite the high number of plant species that exhibit diurnal floral activity, many of them rely on nocturnal or dim-light foragers for pollination, among which insects are the most common (Wolda & Roubik 1986Wolda H, Roubik DW. 1986. Nocturnal bee abundance and seasonal bee activity in a Panamanian forest. Ecology 67: 426-433.; Sourakov 2004Sourakov A. 2004. Night blooming plants and their insect pollinators. In: Capinera JL. (eds.) Encyclopedia of entomology. Dordrecht, Springer. p. 1556-1558.; Wcislo et al. 2004Wcislo WT, Arneson L, Roesch K, Gonzalez V, Smith A, Fernández H. 2004. The evolution of nocturnal behaviour in sweat bees. Xenochlora nigrofemorata genalis and M. ecuadoria (Hymenoptera: Halictidae): an escape from competitors and enemies? Biological Journal of the Linnean Society 83: 377-387.). Among bees, one of the greatest pollinator groups, diurnal and nocturnal foragers often exhibit morpho-physiological constraints. For example, enlarged ocelli and compound eyes are common characters for nocturnal or dim-light bees and have been associated with neurophysiological traits that enhance light sensitivity (Tierney et al. 2012Tierney SM, Sanjur O, Grajales GG, Santos LM, Bermingham E, Wcislo WT. 2012. Photic niche invasions: phylogenetic history of the dim-light foraging augochlorine bees (Halictidae). Proceedings of the Royal Society of London Series, Biological Sciences 279: 794-803.). Few studies have focused on the role of nocturnal or dim-light pollinators (e.g., Hopkins et al. 2000Hopkins MJG, Hopkins HCF, Sothers CA. 2000. Nocturnal pollination of Parkia velutina by Xenochlora nigrofemorata bees in Amazonia and its possible significance in the evolution of chiropterophily. Journal of Tropical Ecology 16: 733-746.; Franco & Gimenes 2011Franco EL, Gimenes M. 2011. Pollination of Cambessedesia wurdackii in Brazilian campo rupestre vegetation, with special reference to crepuscular bees. Journal of Insect Science 11: 1-13.; Braun et al. 2012Braun M, Dötterl S, Schlindwein C, Gottsberger G. 2012. Can nectar be a disadvantage? Contrasting pollination natural histories of two woody Violaceae from the Neotropics. International Journal of Plant Sciences 173: 161-171.; Krug et al. 2015Krug C, Garcia MVB, Gomes FB. 2015. A scientific note on new insights in the pollination of guarana (Paullinia cupana var. sorbilis). Apidologie 46: 164-166. ; Cordeiro et al. 2016Cordeiro GD, Pinheiro M, Dötterl S, Alves-dos-Santos I. 2016. Pollination of Campomanesia phaea (Myrtaceae) by night-active bees: a new nocturnal pollination system mediated by floral scent. Plant Biology 19: 132-139.; Oliveira et al. 2016Oliveira FDS, Ribeiro MHM, Nunez CV, Albuquerque PMC. 2016. Flowering phenology of Mouriri guianensis (Melastomataceae) and its interaction with the crepuscular bee Xenochlora nigrofemorata amoena (Halictidae) in the restinga of Lençóis Maranhenses National Park, Brazil. Acta Amazonica 46: 281-290.), while temporal niche partitioning between them and diurnal pollinators has been even less explored (Smith et al. 2017Smith AR, Kitchen SM, Toney RM, Ziegler C. 2017. Is nocturnal foraging in a tropical bee an escape from interference competition? Journal of Insect Science 17: 1-7. ).
The present work focused on Peltogyne chrysopis Barneby, a legume species endemic to the Atlantic Forest, and for which there are currently only records from the coastal ecosystems of Bahia State in northeastern Brazil. The species appears to be restricted to rainforest forest fragments (Lima & Cordula 2015Lima HC, Cordula E. 2015. Peltogyne. In: Lista de espécies da flora do Brasil. Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/jabot/floradobrasil/FB78741. 30 Sep. 2017.
http://floradobrasil.jbrj.gov.br/jabot/f...
), where it occurs primarily in riparian habitats. According to the IUCN Red List Categories and Criteria, P. chrysopis is currently considered "vulnerable" based on its limited extent of occurrence and few collection records.
There have been no published studies concerning the breeding systems or pollinators of species of Peltogyne, even though they are widely distributed in the Neotropics (Silva 1976Silva MF. 1976. Revisão taxonômica do gênero Peltogyne Vog. (Leguminosae-Caesalpinioideae). Acta Amazonica 6: 1-61.), and especially in tropical moist forest phytogeographical domains of Brazil (Amazonian = 16 species; Atlantic Forest = 7 species out of 24 for Brazil) (Lima & Cordula 2015Lima HC, Cordula E. 2015. Peltogyne. In: Lista de espécies da flora do Brasil. Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/jabot/floradobrasil/FB78741. 30 Sep. 2017.
http://floradobrasil.jbrj.gov.br/jabot/f...
). The genus includes several taxa of ecological, phytochemical, and/or economic importance (Silva 1976Silva MF. 1976. Revisão taxonômica do gênero Peltogyne Vog. (Leguminosae-Caesalpinioideae). Acta Amazonica 6: 1-61.).
We describe the floral biology of P. chrysopis and some aspects of its breeding system, with emphasis on the temporal availability of floral resources, plant-animal interactions during the flowering cycle, and pollination efficiency. To better understand the floral mechanisms underlying pollinator interactions, the following questions were addressed: (1) What is the pollination syndrome exhibited by P. chrysopis? (2) What are the floral resources offered and do they vary throughout the flowering cycle? (3) What are the floral visitors and potential pollinators? (4) Is P. chrysopis exclusively dependent on pollen vectors given its compatibility system? The present study was designed to contribute knowledge on the reproductive mechanisms of P. chrysopis, a legume species endemic to the Atlantic Forest, and generate useful information about its current state of conservation.
Materials and methods
Study species and site
Peltogyne chrysopis is a tree species of the subfamily Detarioideae (Leguminosae) that grows 20 to 25 m tall and has bi-foliolate leaves and small white flowers gathered in terminal panicles (Barneby 1994Barneby RC. 1994. A new Purpleheart (Peltogyne, Caesalpiniaceae) from South Bahian Atlantic Forest (Brazil). Brittonia 46: 270-272.). It is endemic to the Atlantic Forest (Lima & Cordula 2015Lima HC, Cordula E. 2015. Peltogyne. In: Lista de espécies da flora do Brasil. Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/jabot/floradobrasil/FB78741. 30 Sep. 2017.
http://floradobrasil.jbrj.gov.br/jabot/f...
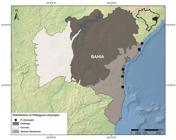
) where it occurs in low population densities (Thomas et al. 2009Thomas WW, Jardim JJG, Fiaschi P, Neto EM, Amorim AA. 2009. Composição florística e estrutura do componente arbóreo de uma área transicional de Floresta Atlântica no sul da Bahia, Brasil. Revista Brasileira de Botânica 32: 65-78.), with the few records available for it in herbaria collections being from Bahia State in northeastern Brazil (Fig. 1; data obtained from CRIA - Centro de Referência em Informação Ambiental, http://splink.cria.org.br/).
Geographic distribution of Peltogyne chrysopis Barneby based on herbaria collection records from the CRIA database (accessed: Aug. 8, 2017).
The present study was carried out from June to August 2016 (comprising four field expeditions for 10 days, totaling 105 hours of focal observation), with a population of P. chrysopis distributed along the anthropogenically impacted margins of the Jatimane River in the municipality of Nilo Peçanha, Bahia State. The vegetation, classified as dense ombrophilous forest or dense alluvial forest, is characterized by the presence of phanerophytes and large numbers of palm trees, lianas, and epiphytes (Veloso et al 1991Veloso HP, Rangel Filho ALR, Lima JCA. 1991. Classificação da vegetação brasileira adaptada a um sistema universal. Rio de Janeiro, IBGE.). The site has an elevation of 111 m and an Af-type climate, with intense rainfall throughout the year and no dry season. Mean monthly precipitation varies between 126 and 189 mm, while mean monthly temperature varies from 22.7 °C (July) to 26.7 °C (February) (Alvares et al. 2013Alvares CA, Stape JL, Sentelhas PC, Gonçalves JL, Sparovek G. 2013. Köppen’s climate classification map for Brazil. Meteorologische Zeitschrift 22: 711-728. ). A voucher specimen (I.M. Souza 322) was deposited in the herbarium at the Universidade Estadual de Feira de Santana.
Floral biology
The timing, sequence, and duration of anthesis were observed constantly in 35 pre-anthesis flower buds from 00:00 to 17h00min (on one individual) for three days during the course of a two-week period. The restricted number of focal individuals reflects the difficulty of accompanying flowering in such an arboreal species due to its crown heights and the absence of blooming in by other individuals in the small focal population (n = 3). Stigma receptivity was evaluated by exposing the stigmas of 30 flowers to 3 % of hydrogen peroxide (at one-hour intervals from 03h00min to 08h00min) to detect peroxidase activity (Dafni et al. 2005Dafni A, Kevan PG, Husband BC. 2005. Practical pollination biology. Ontario, Environquest Ltd.; Dey et al. 2016Dey K, Mondal S, Mandal S. 2016. Studies on stigma receptivity of Grewia asiatica L. with reference to esterase and peroxidase activity. International Journal of Engineering Research & Science 2: 120-122.).
Pollen availability (i.e., the number of anthers open with pollen) was assessed by observing 30 non-bagged floral buds during their entire floral cycle. Bagged flowers and pre-anthesis buds (n = 30) were fixed in FAA (50 %) at different time intervals throughout anthesis (03h00min, 04h00min, 05h00min, 06h00min, 12h00min, 15h00min) to estimate pollen viability with 2 % acetic carmine solution (Nadia et al. 2013Nadia TL, Menezes NL, Machado IC. 2013. Floral traits and reproduction of Avicennia schaueriana Moldenke (Acanthaceae): a generalist pollination system in the Lamiales. Plant Species Biology 28: 70-80. ; Costa & Machado 2017Costa ACG, Machado IC. 2017. Pin-monomorphism in Palicourea crocea (SW.) Roem. & Schult. (Rubiaceae): reproductive traits and role of floral visitors. Brazilian Journal of Botany 40: 1063-1070. ). Nectar production was assessed by monitoring: (i) the total volume of nectar accumulated in the flowers (n = 14) for 10 hours; and (ii) the volume of nectar secreted by a second group of flowers (n = 4) at pre-defined time intervals (06h00min, 08h00min, 10h00min, 12h00min, 14h00min, and 15h00min). These flowers were all bagged at pre-anthesis to avoid floral visitor intervention. Nectar volumes were measured using a graduated micro-syringe (0-25 µL, Hamilton, Reno, Nevada, USA). Non-parametric Wilcoxon test was used to determine if the nectar volumes secreted by the flowers varied during the day.
Breeding system inference using in vivo pollen germination techniques
We inferred the breeding system of P. chrysopis by estimating the pollen/ovule ratio (P/O), as proposed by Cruden (1977Cruden RW. 1977. Pollen-ovule ratios: A conservative indicator of breeding systems in flowering plants. Evolution 31: 32-46.). This ratio was obtained by counting all pollen grains from closed anthers of five flowers (under a microscope) and the number of ovules on the same flowers (under a stereomicroscope).
Pollination experiments were performed to test the hypothesis that P. chrysopis is self-incompatible and dependent on biotic vectors for pollen transfer. We analyzed the flowers of an individual tree (the only one in bloom) by performing the following treatments: (1) autonomous self-pollination (n = 10), which tested spontaneous pollen transference in isolated flowers; (2) manual self-pollination (n = 12), in which pollen grains from the anthers of one flower were manually transferred to the stigmas of the same flower; (3) manual cross-pollination, or geitonogamy (n = 12), in which pollen grains from one flower were transferred to the stigma of a second emasculated flower; and (4) natural pollination (control group) (n = 12), in which flowers were marked and left under natural conditions to evaluate natural pollen transfer. All of the pollination treatments, except natural pollination, were performed using pre-anthesis flower buds that were previously isolated in voile bags. After treatments 2 and 3, the flowers were again bagged to avoid floral visitor intervention, and approximately 10 hours after all of the treatments the flowers were fixed in 50% FAA.
Pistils were rinsed in distilled water, transferred to 70 % ethanol, and rinsed in 10N NaOH at 60 °C for 10 minutes and subsequently transferred to distilled water overnight. The pistils were then cleared in 2 % sodium hypochlorite for 1 h, rinsed in distilled water, and subsequently stained with 0.2 % aniline blue (modified from Tangmitcharoen & Owens 1997Tangmitcharoen S, Owens JN. 1997. Floral biology, pollination, pistil receptivity, and pollen tube growth of teak (Tectona grandis Linn f.). Annals of Botany 79: 227-241.). Each pistil was sliced in half and then placed on a microscope slide to be squashed under a coverslip. The presence of pollen tubes in the stigmatic tissue and/or in the style was then evaluated using epi-fluorescence microscopy.
Floral visitors and pollinators
Focal observations were made constantly from 05h00min to 17h00min for five days, for a total of 60 h of observations (distributed over two weeks), with the time and frequency of visits, and foraging behavior being recorded. The insects were captured with an entomological net, mounted, dried, deposited in the Museu de Zoologia da Universidade Estadual de Feira de Santana - MZUEFS, and identified by experts on their respective groups. The activities of the floral visitors were filmed and photographed to aid in describing their behaviors.
The floral visitors were classified as potential pollinators, occasional pollinators, or thieves based on their body size, visitation time, frequency and duration of visits, and foraging behavior on the flowers. Potential pollinators combined: (i) visitation during periods of stigmatic receptivity and pollen availability; (ii) high frequency of visits; and (iii) body size large enough to carry pollen grains and to come into contact with the stigmas of the flowers during visits. Occasional pollinators were those visitors with body sizes and behaviors suitable for pollen transfer, but with low visitation frequencies and durations. Thieves were floral visitors that collected pollen and/or nectar without contacting the anthers and/or stigma (Inouye 1980Inouye DW. 1980. The terminology of floral larceny. Ecology 61: 1251-1253.).
The efficiency of potential pollinators was tested by bagging the flowers soon after their visits. The pistils were fixed in 50 % FAA 10 hours after bagging the flowers, and the presence of pollen tubes in the stigmatic tissues and/or style were evaluated by preparing pistils for epi-fluorescence microscopy analysis, as previously described.
Results
Floral biology and pollination syndrome
The flowers of P. chrysopis are arranged in terminal panicles, subtended by a leaf, with a mean length of 15.6 ± 3.3 cm, ca. 123 ± 37.65 flowers per inflorescence, and ca. 500 flowers per branch. The flowers are hermaphroditic, slightly zygomorphic, and with four greenish sepals (6-8 × 5-7 mm) and five white petals (6.5-9.5 × 2-4.1 mm). The androecium is composed of 10 stamens with rimose yellow anthers (2-2.5 × 1-1.5 mm); the ovary is pubescent, superous and stipitate (4 × 3 mm) with seven ovules; the style is elongated (ca. 6 mm long) with a capitate stigma and exhibits slight herkogamy (in which the most internal whorl of five stamens is shorter than the pistil, while the anthers in the most external whorl are the same length as the pistil) (Fig. 2). Nectariferous tissue is found at the base of the ovary surrounding the stipe (Fig. 2G).
Floral biology of Peltogyne chrysopis Barneby. A-C. Anthesis development, with white arrows indicating stigmatic receptivity (A. 03:00 h; B. 04:00 h; C. 05:00 h); and red arrows indicating anther dehiscence (B. Closed anthers; C. Opened anthers; Scale bar: 2 mm); D. Xenochlora nigrofemorata collecting pollen on a flower (white arrow indicates the position of the stigma; red arrow indicates empty anthers); E. Pollen tubes growing in the style after visitation by Xenochlora nigrofemorata; F. Pollen tubes growing on the stigmatic surface after manual cross-pollination treatments (geitonogamy); G. Nectar droplets at the base of the ovary, indicated by a green arrow; H. Distribution of UV pigments in the flower, indicated by the yellow color; I. Distribution of osmophores in the flower, indicated by reddish dots.
The species has melittophilous flowers; anthesis begins near 02h00min with the slow distension of the sepals and petals; by approximately 05h00min the flowers are completely open with a receptive stigma and dehiscent anthers. The pistil becomes projected out of the floral bud in the first hours of anthesis (ca. 03h00min), with a receptive stigma that suggests protogyny (Fig. 2A). The flowers have osmophores on the margins of sepals and petals that release a slight sweet scent at the end of anthesis (Fig. 2I). UV pigments are present in the sepals, petals, and at the base of the filaments (Fig. 2H). The number of flowers opening per day varied from 5 to 43 during the study period; they lasted ca. 24 h, with the calyx and corolla persisting as they withered.
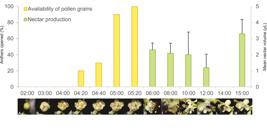
Anther dehiscence begins at approximately 04h20min and lasts 40-60 minutes (Figs. 2C, 3) with pollen viability varying from 99.75 ± 0.5 % (03h00min) to 97.75 ± 1.89 % (15h00min). Nectar is secreted at the base of ovary (as droplets) beginning at the end of anthesis and is produced throughout the day in volumes varying from 1.2 to 3.3 µL (Fig. 3); the secreted volumes did not vary significantly among different times of the day (Tab. 1). The total volume of nectar accumulated for a single day was ca. 1.6 ± 0.41 µL per flower. Nectar concentration was not precisely assessed due to methodological problems.
Temporal availability of floral resources of Peltogyne chrysopis Barneby and the records of floral visitors throughout the day: yellow bars = pollen grain availability; green bars = nectar production (black bars above each green bar represent standard deviations). A-G. Anthesis development and anther dehiscence (D-G); H. Xenochlora nigrofemorata collecting pollen; I. Dipteran searching for pollen; J-M. Trigona hyalinata feeding on nectar.
Comparison of nectar volume (µL) secreted by flowers of Peltogyne chrysopis Barneby at different times during the flowering cycle. Wilcoxon test results are indicated below the diagonal while P-values are above.
Breeding system
The pollen/ovule ratio for P. chrysopis was 5191.43 ± 1389.91, indicating it is a xenogamous species. The results of the breeding system experiments indicated that the species is self-compatible, as high percentages of stigmas were found with germinating pollen grains after geitonogamy and manual self-pollination treatments (Tab. 2). The species did not exhibit evidences of sporophytic or gametophytic self-incompatibility mechanisms (Fig. 2E-F) and depends on biotic vectors for pollen transfer.
Results of the treatments used to investigate the reproductive system of Peltogyne chrysopis in rainforest vegetation along the margins of the Jetimane River, Nilo Peçanha, Bahia State, Brazil.
Flower visitors and pollinators
We recorded visits by one beetle (Coleoptera), two dipterans (Diptera), and four different bee species (Hymenoptera) to P. chrysopis flowers. The beetle had a small body and simply walked on the petals and sepals while feeding on nectar (Fig. 2G); the dipterans landed on the anthers and fed on residual pollen grains (Fig. 3I). These floral visitors did not touch the stigma nor damaged the flowers and were therefore classified as thieves.
Among the bee species, Xenochlora nigrofemorata (Halictidae) was considered a potential pollinator. These bees visited all of the flowers as soon as anthesis ended (between 05h20min and 06h20min) with higher frequencies (one to four visits per flower); they visited the flowers searching for pollen and almost depleting the anthers. The relatively large body size of this species (in relation to the flower) and its foraging behavior (embracing a set of anthers, buzzing them, and then collecting pollen) enabled pollen transfer since the bees touched the stigma during their visits (Figs. 2D, 3H). The pollination efficiency of X. nigrofemorata was confirmed by epi-fluorescence microscopy analyses, with the presence of germinating pollen grains on the stigmatic surfaces and in the styles of all the pistils they visited (Fig. 2E, Tab. 2).
The bee Centris sp. (Apidae) was classified as an occasional pollinator since it was observed at the beginning of the morning (ca. 05h20min), but was not a frequent visitor to individual flowers (one visit per flower) and visited few flowers (n = 2). Its pollination efficiency could not be assed due to the few recorded visits. Trigonisca sp. was classified as pollen thieving. This small bee visited the flowers at the beginning of the morning (ca. 06h20min), landing on each anther and searching for residual pollen grains, but they did not carry great amounts of pollen nor touch the stigma. Trigona hyalinata visited the flowers during the entire day (first records ca. 08h00min), landing on the petals or embracing the pistil and positioning its head toward the base of the ovary to feed on nectar (Fig. 3J-M). During visits by this species, the anthers were almost completely empty, and thus this species was classified as nectar thief.
Discussion
Peltogyne is a poorly-studied legume genus with respect to its mating system, with information available for basically only its fruiting phenology (Rocha et al. 2006Rocha OJ, Vílchez B, Araya AL. 2006. A mast fruiting episode of Peltogyne
purpurea (Caesalpinaceae) in the Osa Peninsula, Costa Rica. Revista de Biología Tropical 52: 1151-1155.). There is also information in the literature concerning its taxonomy (e.g., Silva et al. 1976Silva MF. 1976. Revisão taxonômica do gênero Peltogyne Vog. (Leguminosae-Caesalpinioideae). Acta Amazonica 6: 1-61.), phytochemistry (e.g., Almeida et al. 1974Almeida MEde, Gottlieb OR, Sousa JR, Teixeira MA. 1974. New peltogynoids from three Peltogyne species. Phytochemistry 13: 1225-1228.; Gutiérrez-Macías et al. 2016Gutiérrez-Macías P, Peralta-Cruz J, Borja-de-la-Rosa A, Barragán-Huerta BE. 2016. Peltomexicanin, a peltogynoid quinone methide from Peltogyne Mexicana Martínez purple heartwood. Molecules 21: 186. doi:10.3390/molecules21020186.
https://doi.org/10.3390/molecules2102018...
), herbivory ecology (e.g., Nascimento & Proctor 1996Nascimento ΜΤ, Proctor J. 1996. Seed attacks by beetles and leaf-cutter ants on Peltogyne gracilipes Ducke (Caesalpiniaceae) on Maracá Island, Brazilian Amazonia. Journal of Tropical Ecology 2: 723-727.; 1997Nascimento MT, Proctor J. 1997. Population dynamics of five tree species in a monodominant Peltogyne forest and two other forest types on Maracá Island, Roraima, Brazil. Forest Ecology and Management 94: 115-128.), and population ecology (e.g., Nascimento & Proctor 2001Nascimento MT, Proctor J. 2001. Leaf herbivory on three tree species in a monodominant and two other Terra Firme forests on Maracá Island, Brazil. Acta Amazonica 31: 27-38.). Thus, the present study is the first to investigate some of the aspects of the breeding system of a species of Peltogyne. Our results indicate that P. chrysopis, an endemic species of the Atlantic Forest, possesses melittophilous and protogynic flowers that are mainly xenogamous and self-compatible. In addition, the present study is the first to document in detail pollination mediated by the poorly known bees of the genus Xenochlora.
Individuals of Peltogyne chrysopis initiate anthesis during predawn, with the flowers becoming completely opened at the beginning of the morning (ca. 05h00min), yet during dim-light. The flowers release a slight sweet scent that attracts Xenochlora nigrofemorata, the first floral visitors and a poorly documented bee species belonging to a small, poorly known and rarely collected bee genus (Engel et al. 1997Engel MS, Brooks RW, Yanega D. 1997. New genera and subgenera of Augochlorine bees (Hymenoptera: Halictidae). Scientific Papers, Natural History Museum, The University of Kansas 5: 1-21.; Engel 2000Engel MS. 2000. Classification of the bee tribe Augochlorini (Hymenoptera: Halictidae). Bulletin of the American Museum of Natural History 250: 1-90.; Santos & Melo 2013Santos LM, Melo GAR. 2013. Taxonomic notes and description of the male of Xenochlora nigrofemorata (Smith, 1879) (Hymenoptera: Apidae: Halictinae). Zootaxa 3670: 371-377.). Despite anthesis occurring during in the dark, the set of floral traits of P chrysopis (e.g., small and slightly zygomorphic flowers, presence of osmophores and UV-reflective pigments, and pollen as the main floral resource) fit traditional melittophily (Faegri & Pijl 1979Faegri K, Pijl L. 1979. The principles of pollination ecology. 3rd. edn. Oxford, Pergamon Press.).
Pollination is a key process for sexual reproduction of most flowering plants (Aguilar et al. 2006Aguilar R, Ashworth L, Galetto L, Aizen MA. 2006. Plant reproductive susceptibility to habitat fragmentation: review and synthesis through meta-analysis. Ecology Letters 9: 968-980. ), and bee-pollination has been shown to be prevalent in many different ecosystems. Although mechanisms related to pollination and the reproductive success of tree species have been poorly investigated (especially in the Brazilian Atlantic Forest), empirical evidence indicates that bee-pollination is prevalent, even in the canopies of trees growing in humid habitats (Dulmen 2001Dulmen A. 2001. Pollination and phenology of flowers in the canopy of two contrasting rain forest types in Amazonia, Colombia. Plant Ecology 153: 73-85.; Fidalgo & Kleinert 2009Fidalgo AO, Kleinert AMP. 2009. Reproductive biology of six Brazilian Myrtaceae: is there a syndrome associated with buzz-pollination? New Zealand Journal of Botany 47: 355-365. ; Vieira et al. 2010Vieira FA, Appolinário V, Fajardo CG, Carvalho D. 2010. Reproductive biology of Protium spruceanum (Burseraceae), a dominant dioecious tree in vegetation corridors in Southeastern Brazil. Revista Brasileira de Botânica 33: 711-715. ; Braun et al. 2012Braun M, Dötterl S, Schlindwein C, Gottsberger G. 2012. Can nectar be a disadvantage? Contrasting pollination natural histories of two woody Violaceae from the Neotropics. International Journal of Plant Sciences 173: 161-171.; Franceschinelli et al. 2015Franceschinelli EV, Carmo RM, Silva Neto CM, Gonçalves BB, Bergamin LL. 2015. Reproductive success ofCabralea canjerana(Meliaceae) in Atlantic forest fragments, Brazil. Revista de Biología Tropical 63: 515-524.), as confirmed by the present study with legitimate visits of X. nigrofemorata.
Xenochlora is a Neotropical bee genus of the tribe Augochlorini with only four species recorded from tropical moist forests (Michener 2007Michener CD. 2007 The bees of the world. 2nd. edn. Baltimore, Johns Hopkins.). The genus is closely related to Megalopta (Tierney et al. 2012Tierney SM, Sanjur O, Grajales GG, Santos LM, Bermingham E, Wcislo WT. 2012. Photic niche invasions: phylogenetic history of the dim-light foraging augochlorine bees (Halictidae). Proceedings of the Royal Society of London Series, Biological Sciences 279: 794-803.; Gonçalves 2016Gonçalves RB. 2016. A molecular and morphological phylogeny of the extant Augochlorini (Hymenoptera, Apoidea) with comments on implications for biogeography. Systematic Entomology 41: 430-440. ), which is widely recognized for the nocturnal or crepuscular activity (Wolda & Roubik 1986Wolda H, Roubik DW. 1986. Nocturnal bee abundance and seasonal bee activity in a Panamanian forest. Ecology 67: 426-433.; Hopkins et al 2000Hopkins MJG, Hopkins HCF, Sothers CA. 2000. Nocturnal pollination of Parkia velutina by Xenochlora nigrofemorata bees in Amazonia and its possible significance in the evolution of chiropterophily. Journal of Tropical Ecology 16: 733-746.; Franco & Gimenes 2011Franco EL, Gimenes M. 2011. Pollination of Cambessedesia wurdackii in Brazilian campo rupestre vegetation, with special reference to crepuscular bees. Journal of Insect Science 11: 1-13.; Carvalho et al. 2012Carvalho AT, Maia ACD, Ojima PY, Santos AA, Schlindwein C. 2012. Nocturnal bees are attracted by widespread floral scents. Journal of Chemical Ecology 3: 315-318.), but Xenochlora differs basically by having smaller ocelli and stiff, black setae on the hindlegs (Engel 2000Engel MS. 2000. Classification of the bee tribe Augochlorini (Hymenoptera: Halictidae). Bulletin of the American Museum of Natural History 250: 1-90.).
Xenochlora has been recognized as a diurnal genus (Engel et al. 1997Engel MS, Brooks RW, Yanega D. 1997. New genera and subgenera of Augochlorine bees (Hymenoptera: Halictidae). Scientific Papers, Natural History Museum, The University of Kansas 5: 1-21.; Tierney et al. 2012Tierney SM, Sanjur O, Grajales GG, Santos LM, Bermingham E, Wcislo WT. 2012. Photic niche invasions: phylogenetic history of the dim-light foraging augochlorine bees (Halictidae). Proceedings of the Royal Society of London Series, Biological Sciences 279: 794-803.), in contrast with its closest relative Megalopta. However, this recognition is based only on D. Roubik's collection (1991), who mentions that the holotype of the genus was collected using "flowers and baits" (Engel et al. 1997Engel MS, Brooks RW, Yanega D. 1997. New genera and subgenera of Augochlorine bees (Hymenoptera: Halictidae). Scientific Papers, Natural History Museum, The University of Kansas 5: 1-21.). Morphological attributes (e.g., smaller ocelli) have also been used to support the hypothesis of diurnal behavior for the genus (Michener 2007Michener CD. 2007 The bees of the world. 2nd. edn. Baltimore, Johns Hopkins.; Wcislo & Tierney 2009Wcislo WT, Tierney SM. 2009. Behavioural environments and niche construction: the evolution of dim-light foraging in bees. Biological Reviews 84: 19-37. ). Nevertheless, not all dim-light foraging bees have enlarged ocelli and compound eyes, especially facultative dim-light foragers (Wcislo & Tierney 2009Wcislo WT, Tierney SM. 2009. Behavioural environments and niche construction: the evolution of dim-light foraging in bees. Biological Reviews 84: 19-37. ). Despite the lack of confirmed ethological data in the literature, it is not possible to rule out facultative dim-light activity for species of Xenochlora (Tierney et al. 2012Tierney SM, Sanjur O, Grajales GG, Santos LM, Bermingham E, Wcislo WT. 2012. Photic niche invasions: phylogenetic history of the dim-light foraging augochlorine bees (Halictidae). Proceedings of the Royal Society of London Series, Biological Sciences 279: 794-803.). In the present study we recorded visits of X. nigrofemorata only at the beginning of the morning (i.e., 05h20min), during the transition from dim-light to sunrise. In addition, visits recorded some time later were associated with cloudy days.
Xenochlora nigrofemorata is considered the main pollinator of P. chrysopis due to: (i) bees visiting flowers when the stigma was receptive and pollen grains viable; (ii) bee body size and foraging behavior favoring contact with the stigmatic surface during visits; and, (iii) bee pollination efficiency (i.e., pollen transference from the anthers to the stigma), as confirmed by the presence of pollen tubes on the stigmatic surface and in the styles of visited flowers. All the information available in the literature thus far for the genus consists of taxonomic treatments (Engel et al. 1997Engel MS, Brooks RW, Yanega D. 1997. New genera and subgenera of Augochlorine bees (Hymenoptera: Halictidae). Scientific Papers, Natural History Museum, The University of Kansas 5: 1-21.; Engel 2000Engel MS. 2000. Classification of the bee tribe Augochlorini (Hymenoptera: Halictidae). Bulletin of the American Museum of Natural History 250: 1-90.; Santos & Melo 2013Santos LM, Melo GAR. 2013. Taxonomic notes and description of the male of Xenochlora nigrofemorata (Smith, 1879) (Hymenoptera: Apidae: Halictinae). Zootaxa 3670: 371-377.) and phylogenetic analyses of the tribe Augochloroni (Tierney et al. 2012Tierney SM, Sanjur O, Grajales GG, Santos LM, Bermingham E, Wcislo WT. 2012. Photic niche invasions: phylogenetic history of the dim-light foraging augochlorine bees (Halictidae). Proceedings of the Royal Society of London Series, Biological Sciences 279: 794-803.; Gonçalves 2016Gonçalves RB. 2016. A molecular and morphological phylogeny of the extant Augochlorini (Hymenoptera, Apoidea) with comments on implications for biogeography. Systematic Entomology 41: 430-440. ), and reports on nesting biology and social behavior for two species (Tierney et al. 2008Tierney SM, Gonzales-Ojeda T, Wcislo W. 2008. Nesting biology and social behavior of Xenochlora bees (Hymenoptera: Halictidae: Augochlorini) from Perú. Journal of the Kansas Entomological Society 81: 61-72.). Thus, the present study is the first to report on the association between flowers and a species of the genus Xenochlora, with detailed information on pollination mediated by X. nigrofemorata - pollen transference via floral sonication behavior (i.e., buzzing the anthers) as indicated by epi-fluorescence microscopy, which contradicts that assumed by Cardinal et al. (2018Cardinal S, Buchmann SL, Russell AL. 2018. The evolution of floral sonication, a pollen foraging behavior used by bees (Anthophila). Evolution 72: 590-600.) for the genus.
In addition to X. nigrofemorata, another bee species also buzzed the anthers of P. chrysopis to collect pollen from their flowers: individuals of Centris sp. made fast and punctual visits soon after anthesis ended. Their body size, foraging behavior, and timing and numbers of visits (few visits when the stigma was receptive and pollen was viable) classifies them as occasional pollinators. The behavior of buzzing anthers to collect pollen has been widely associated with pollination (i.e., buzz-pollination with explosive pollen release) of several flowering plants (e.g., Harder & Barclay 1994Harder LD, Barclay RMR. 1994. The functional significance of poricidal anthers and buzz pollination: controlled pollen. Functional Ecology 8: 509-517.; Dupont & Olesen 2006Dupont YL, Olesen JM. 2006. Andromonoecy and buzz pollination in Solanum species (Solanaceae) endemic to the Canary Islands. Annales del Jardín Botánico de Madri 63: 63-66.; Fidalgo & Kleinert 2009Fidalgo AO, Kleinert AMP. 2009. Reproductive biology of six Brazilian Myrtaceae: is there a syndrome associated with buzz-pollination? New Zealand Journal of Botany 47: 355-365. ; Franco et al. 2011Franco AM, Goldenberg R, Varassin IG. 2011. Pollinator guild organization and its consequences for reproduction in three synchronopatric species of Tibouchina (Melastomataceae). Revista Brasileira de Entomologia 55: 381-388.; Souza et al 2012Souza IM, Coutinho K, Funch LS. 2012. Estratégias fenológicas de Senna cana (Nees & Mart.) H. S. Irwin & Barneby (Fabaceae: Caesalpinioideae) como mecanismo eficiente para atração de polinizadores. Acta Botanica Brasilica 26: 435-443. ).
Buzz-pollination may have evolved as a feature that favored successful fertilization and the control of pollen removal by pollen thieves or less efficient pollinators (Luca & Vallejo-Marín 2013Luca PA, Vallejo-Marín M. 2013. What's the ‘buzz’ about? The ecology and evolutionary significance of buzz-pollination. Current Opinion in Plant Biology 16: 429-435.). Empirical evidence indicates that the bee behavior of embracing anthers and then contracting flight muscles at high frequencies (King 1993King MJ. 1993. Buzz foraging mechanism of bumble bees. Journal of Apicultural Research 32: 41-49. ) is not performed only on flowers with poricidal anthers, but is more closely associated with the actual abilities of the bees distributed among seven hymenopteran families to perform buzzing (Proença 1992Proença CEB. 1992. Buzz pollination - older and more widespread than we think? Journal of Tropical Ecology 8: 115-120. ; Freitas & Sazima 2003Freitas L, Sazima M. 2003. Floral biology and pollination mechanisms in two Viola species - from nectar to pollen flowers? Annals of Botany 91: 311-317.; Fidalgo & Kleinert 2009Fidalgo AO, Kleinert AMP. 2009. Reproductive biology of six Brazilian Myrtaceae: is there a syndrome associated with buzz-pollination? New Zealand Journal of Botany 47: 355-365. ; Luca & Vallejo-Marín 2013Luca PA, Vallejo-Marín M. 2013. What's the ‘buzz’ about? The ecology and evolutionary significance of buzz-pollination. Current Opinion in Plant Biology 16: 429-435.; Cardinal et al 2018Cardinal S, Buchmann SL, Russell AL. 2018. The evolution of floral sonication, a pollen foraging behavior used by bees (Anthophila). Evolution 72: 590-600.), as seen here with X. nigrofemorata and Centris sp. during their visits to P. chrysopis flowers.
The other two bee species that visited flowers of P. chrysopis did not collect pollen by buzzing the anthers. The small species Trigonisca sp. visited flowers soon after anthesis ended, but did not touch the stigmatic surface during their visits. Trigona hyalinata visited the flowers throughout the day while feeding on nectar, but as they appeared after visits by efficient pollinators (i.e., X. nigrofemorata) and the anthers were virtually empty, they did not carry pollen grains on their bodies. Both of these bee species (Trigonisca sp. and T. hyalinata) were therefore classified as nectar thieves. Nevertheless, it is important to highlight the potential for pollen transfer by T. hyalinata (in the absence of main pollinators) in light of appropriate aspects of its morphology and behavior. Besides, a species of Trigona do mediate effective pollination in a population of a congeneric legume species (P. pauciflora) in northern Bahia State (IM Souza unpubl. res.).
Our observations suggest temporal segregation of floral visitors according to floral resource availability, with anther dehiscence before sunrise attracting the pollinators X. nigrofemorata and Centris sp., followed by the secretion of highly viscous nectar droplets soon after the end of anthesis, and persisting throughout the day, attracting T. hyalinata. This temporal segregation is an important feature commonly associated with reduced overlap among the temporal niches of bee species (Willmer & Corbet 1981Willmer PG, Corbet SA. 1981. Temporal and microclimatie partitioning of the floral resources of Justicia aurea amongst a concourse of pollen vectors and nectar robbers. Oecologia 51: 67-8.; Biesmeijer & Richter 1999Biesmeijer JC, Richter JAP. 1999. Niche differentiation in nectar-collecting stingless bees: the influence of morphology, oral choice and interference competition. Ecological Entomology 24 380-388.; Barônio & Torezan-Silingardi 2017Barônio GJ, Torezan-Silingardi HM. 2017. Temporal niche overlap and distinct bee ability to collect floral resources on three species of Brazilian Malpighiaceae. Apidologie 48: 168-180. ). This partitioning of floral visitation reduces competition for floral resources and might optimize pollination and, consequently, reproductive success of P. chrysopis, since the species is exclusively dependent on biotic vectors for pollen transference.
Assuming that xenogamy (which improves gene flow and genetic variation among populations) is the main reproductive strategy of P. chrysopis, self-compatibility (e.g., geitonogamy) may still provide advantages of reproductive assurance (O’Brien 1994O’Brien SJ. 1994. A role for molecular genetics in biological conservation. Proceedings of the National Academy of Sciences of the USA 91: 5748-5755. ; Buza et al. 2000Buza L, Young A, Thrall P. 2000. Genetic erosion, inbreeding and reduced fitness in fragmented populations of the endangered tetraploid pea Swainsona recta. Biological Conservation 93: 177-186.; Navarro & Guitián 2002Navarro L, Gutián J. 2002. The role of floral biology and breeding system on the reproductive success of the narrow endemic Petrocoptis viscosa rothm. (Caryophyllaceae). Biological Conservation 103: 125-132.). Self-compatibility can also be associated with reproductive flexibility for colonizing harsh habitats (Navarro & Guitián 2002Navarro L, Gutián J. 2002. The role of floral biology and breeding system on the reproductive success of the narrow endemic Petrocoptis viscosa rothm. (Caryophyllaceae). Biological Conservation 103: 125-132.) or disturbed areas - such as the Atlantic Forest fragments where P. chrysopis is found.
The flowering of P. chrysopis appears to be asynchronous (only one individual bloomed during the study period) and occurs in the coldest months of the year, since the study region experiences relatively low temperatures in June, July, and August (23.3, 22.7, and 23.3 °C respectively) (Alvares et al. 2013Alvares CA, Stape JL, Sentelhas PC, Gonçalves JL, Sparovek G. 2013. Köppen’s climate classification map for Brazil. Meteorologische Zeitschrift 22: 711-728. ). The supposed asynchronous flowering of P. chrysopis, combined with its self-compatibility, may assure its reproductive success.
The information gathered here concerning the floral biology and pollination strategies of this endemic Atlantic Forest legume species (P. chrysopis) contributes to the knowledge of the reproductive biology of the genus Peltogyne as a whole and the role of X. nigrofemorata, which belongs to a poorly documented genus, in the pollination of plant species. Future challenges will involve broadening research efforts to cover other related species and synthesizing that information in a phylogenetic context to help elucidate evolution within the subfamily Detarioideae (LPWG 2017LPWG. 2017. A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny. Taxon 66: 44-77.).
Acknowledgements
The authors would like to thank the Coordenação de Aperfeiçoamento de Pessoal do Nível Superior (CAPES) for the grant awarded to the first author; the Universidade Estadual de Feira de Santana for providing the infrastructure necessary for the data processing; Richard Onoo for logistic support during the field expeditions; Karoline Coutinho de Santana for preparing figure 1; and the Dr. Favízia Freitas de Oliveira for identifications of bee species. LPQ is supported by research productivity grants (CNPq).
References
- Aguilar R, Ashworth L, Galetto L, Aizen MA. 2006. Plant reproductive susceptibility to habitat fragmentation: review and synthesis through meta-analysis. Ecology Letters 9: 968-980.
- Almeida MEde, Gottlieb OR, Sousa JR, Teixeira MA. 1974. New peltogynoids from three Peltogyne species. Phytochemistry 13: 1225-1228.
- Alvares CA, Stape JL, Sentelhas PC, Gonçalves JL, Sparovek G. 2013. Köppen’s climate classification map for Brazil. Meteorologische Zeitschrift 22: 711-728.
- Barneby RC. 1994. A new Purpleheart (Peltogyne, Caesalpiniaceae) from South Bahian Atlantic Forest (Brazil). Brittonia 46: 270-272.
- Barônio GJ, Torezan-Silingardi HM. 2017. Temporal niche overlap and distinct bee ability to collect floral resources on three species of Brazilian Malpighiaceae. Apidologie 48: 168-180.
- Biesmeijer JC, Richter JAP. 1999. Niche differentiation in nectar-collecting stingless bees: the influence of morphology, oral choice and interference competition. Ecological Entomology 24 380-388.
- Braun M, Dötterl S, Schlindwein C, Gottsberger G. 2012. Can nectar be a disadvantage? Contrasting pollination natural histories of two woody Violaceae from the Neotropics. International Journal of Plant Sciences 173: 161-171.
- Buza L, Young A, Thrall P. 2000. Genetic erosion, inbreeding and reduced fitness in fragmented populations of the endangered tetraploid pea Swainsona recta Biological Conservation 93: 177-186.
- Cardinal S, Buchmann SL, Russell AL. 2018. The evolution of floral sonication, a pollen foraging behavior used by bees (Anthophila). Evolution 72: 590-600.
- Carvalho AT, Maia ACD, Ojima PY, Santos AA, Schlindwein C. 2012. Nocturnal bees are attracted by widespread floral scents. Journal of Chemical Ecology 3: 315-318.
- Castro S, Silveira P, Navarro L. 2008. How flower biology and breeding system affect the reproductive success of the narrow endemic Polygala vayredae Costa (Polygalaceae). Botanical Journal of the Linnean Society 157: 67-81.
- Cordeiro GD, Pinheiro M, Dötterl S, Alves-dos-Santos I. 2016. Pollination of Campomanesia phaea (Myrtaceae) by night-active bees: a new nocturnal pollination system mediated by floral scent. Plant Biology 19: 132-139.
- Costa ACG, Machado IC. 2017. Pin-monomorphism in Palicourea crocea (SW.) Roem. & Schult. (Rubiaceae): reproductive traits and role of floral visitors. Brazilian Journal of Botany 40: 1063-1070.
- Cruden RW. 1977. Pollen-ovule ratios: A conservative indicator of breeding systems in flowering plants. Evolution 31: 32-46.
- Dafni A, Kevan PG, Husband BC. 2005. Practical pollination biology. Ontario, Environquest Ltd.
- Dey K, Mondal S, Mandal S. 2016. Studies on stigma receptivity of Grewia asiatica L. with reference to esterase and peroxidase activity. International Journal of Engineering Research & Science 2: 120-122.
- Dulmen A. 2001. Pollination and phenology of flowers in the canopy of two contrasting rain forest types in Amazonia, Colombia. Plant Ecology 153: 73-85.
- Dupont YL, Olesen JM. 2006. Andromonoecy and buzz pollination in Solanum species (Solanaceae) endemic to the Canary Islands. Annales del Jardín Botánico de Madri 63: 63-66.
- Engel MS. 2000. Classification of the bee tribe Augochlorini (Hymenoptera: Halictidae). Bulletin of the American Museum of Natural History 250: 1-90.
- Engel MS, Brooks RW, Yanega D. 1997. New genera and subgenera of Augochlorine bees (Hymenoptera: Halictidae). Scientific Papers, Natural History Museum, The University of Kansas 5: 1-21.
- Faegri K, Pijl L. 1979. The principles of pollination ecology. 3rd. edn. Oxford, Pergamon Press.
- Fidalgo AO, Kleinert AMP. 2009. Reproductive biology of six Brazilian Myrtaceae: is there a syndrome associated with buzz-pollination? New Zealand Journal of Botany 47: 355-365.
- Fontúrbel FE, Murúa MM. 2014. Microevolutionary Effects of Habitat Fragmentation on Plant-Animal Interactions. Advances in Ecology 2014: 379267. doi: 10.1155/2014/379267
» https://doi.org/10.1155/2014/379267 - Franceschinelli EV, Carmo RM, Silva Neto CM, Gonçalves BB, Bergamin LL. 2015. Reproductive success ofCabralea canjerana(Meliaceae) in Atlantic forest fragments, Brazil. Revista de Biología Tropical 63: 515-524.
- Franco AM, Goldenberg R, Varassin IG. 2011. Pollinator guild organization and its consequences for reproduction in three synchronopatric species of Tibouchina (Melastomataceae). Revista Brasileira de Entomologia 55: 381-388.
- Franco EL, Gimenes M. 2011. Pollination of Cambessedesia wurdackii in Brazilian campo rupestre vegetation, with special reference to crepuscular bees. Journal of Insect Science 11: 1-13.
- Freitas L, Sazima M. 2003. Floral biology and pollination mechanisms in two Viola species - from nectar to pollen flowers? Annals of Botany 91: 311-317.
- Galindo-Leal C, Câmara IG. 2005. Status do hotspot Mata Atlântica: uma síntese. In: Galindo-Leal C, Câmara IG. (eds.) Mata Atlântica: biodiversidade, ameaças e perspectivas. São Paulo, Fundação SOS Mata Atlântica. p. 3-12.
- Girão LC, Lopes AV, Tabarelli M, Bruna EM. 2007. Changes in tree reproductive traits reduce functional diversity in a fragmented Atlantic forest landscape. PLOS ONE 2: e908. doi: 10.1371/journal.pone.0000908.
» https://doi.org/10.1371/journal.pone.0000908 - Gonçalves RB. 2016. A molecular and morphological phylogeny of the extant Augochlorini (Hymenoptera, Apoidea) with comments on implications for biogeography. Systematic Entomology 41: 430-440.
- Gutiérrez-Macías P, Peralta-Cruz J, Borja-de-la-Rosa A, Barragán-Huerta BE. 2016. Peltomexicanin, a peltogynoid quinone methide from Peltogyne Mexicana Martínez purple heartwood. Molecules 21: 186. doi:10.3390/molecules21020186.
» https://doi.org/10.3390/molecules21020186 - Harder LD, Barclay RMR. 1994. The functional significance of poricidal anthers and buzz pollination: controlled pollen. Functional Ecology 8: 509-517.
- Hopkins MJG, Hopkins HCF, Sothers CA. 2000. Nocturnal pollination of Parkia velutina by Xenochlora nigrofemorata bees in Amazonia and its possible significance in the evolution of chiropterophily. Journal of Tropical Ecology 16: 733-746.
- Inouye DW. 1980. The terminology of floral larceny. Ecology 61: 1251-1253.
- Kang M, Jiang M, Huang H. 2005. Genetic Diversity in Fragmented Populations of Berchemiella wilsonii var. pubipetiolata (Rhamnaceae). Annals of Botany 95: 1145-1151.
- Kevan PG. 1999. Pollinators as bioindicators of the state of the environment: species, activity and diversity. Agriculture, Ecosystems & Environment 74: 373-393.
- King MJ. 1993. Buzz foraging mechanism of bumble bees. Journal of Apicultural Research 32: 41-49.
- Krug C, Garcia MVB, Gomes FB. 2015. A scientific note on new insights in the pollination of guarana (Paullinia cupana var. sorbilis). Apidologie 46: 164-166.
- Lima HC, Cordula E. 2015. Peltogyne In: Lista de espécies da flora do Brasil. Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/jabot/floradobrasil/FB78741 30 Sep. 2017.
» http://floradobrasil.jbrj.gov.br/jabot/floradobrasil/FB78741 - LPWG. 2017. A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny. Taxon 66: 44-77.
- Luca PA, Vallejo-Marín M. 2013. What's the ‘buzz’ about? The ecology and evolutionary significance of buzz-pollination. Current Opinion in Plant Biology 16: 429-435.
- Michener CD. 2007 The bees of the world. 2nd. edn. Baltimore, Johns Hopkins.
- Nadia TL, Menezes NL, Machado IC. 2013. Floral traits and reproduction of Avicennia schaueriana Moldenke (Acanthaceae): a generalist pollination system in the Lamiales. Plant Species Biology 28: 70-80.
- Nascimento ΜΤ, Proctor J. 1996. Seed attacks by beetles and leaf-cutter ants on Peltogyne gracilipes Ducke (Caesalpiniaceae) on Maracá Island, Brazilian Amazonia. Journal of Tropical Ecology 2: 723-727.
- Nascimento MT, Proctor J. 1997. Population dynamics of five tree species in a monodominant Peltogyne forest and two other forest types on Maracá Island, Roraima, Brazil. Forest Ecology and Management 94: 115-128.
- Nascimento MT, Proctor J. 2001. Leaf herbivory on three tree species in a monodominant and two other Terra Firme forests on Maracá Island, Brazil. Acta Amazonica 31: 27-38.
- Navarro L, Gutián J. 2002. The role of floral biology and breeding system on the reproductive success of the narrow endemic Petrocoptis viscosa rothm. (Caryophyllaceae). Biological Conservation 103: 125-132.
- O’Brien SJ. 1994. A role for molecular genetics in biological conservation. Proceedings of the National Academy of Sciences of the USA 91: 5748-5755.
- Oliveira FDS, Ribeiro MHM, Nunez CV, Albuquerque PMC. 2016. Flowering phenology of Mouriri guianensis (Melastomataceae) and its interaction with the crepuscular bee Xenochlora nigrofemorata amoena (Halictidae) in the restinga of Lençóis Maranhenses National Park, Brazil. Acta Amazonica 46: 281-290.
- Proença CEB. 1992. Buzz pollination - older and more widespread than we think? Journal of Tropical Ecology 8: 115-120.
- Rathcke BJ, Jules ES. 1993. Habitat fragmentation and plant-pollinator interactions. Current Science 65: 273-277.
- Rocha OJ, Vílchez B, Araya AL. 2006. A mast fruiting episode of Peltogyne purpurea (Caesalpinaceae) in the Osa Peninsula, Costa Rica. Revista de Biología Tropical 52: 1151-1155.
- Rodríguez-Pérez J. 2005. Breeding System, Flower Visitors and Seedling Survival of Two Endangered Species of Helianthemum (Cistaceae). Annals of Botany 95: 1229-1236.
- Santos LM, Melo GAR. 2013. Taxonomic notes and description of the male of Xenochlora nigrofemorata (Smith, 1879) (Hymenoptera: Apidae: Halictinae). Zootaxa 3670: 371-377.
- Schemske DW, Husband BC, Ruckelshaus MH, Goodwillie C, Parker IM, Bishop JG. 1994. Evaluating approaches to the conservation of rare and endangered plants. Ecology 75: 584-606.
- Silva MF. 1976. Revisão taxonômica do gênero Peltogyne Vog. (Leguminosae-Caesalpinioideae). Acta Amazonica 6: 1-61.
- Silva TR, Medeiros MB, Noronha SE, Pinto JRR. 2017. Species distribution models of rare tree species as an evaluation tool for synergistic human impacts in the Amazon rainforest. Brazilian Journal of Botany 40: 963-971.
- Smith AR, Kitchen SM, Toney RM, Ziegler C. 2017. Is nocturnal foraging in a tropical bee an escape from interference competition? Journal of Insect Science 17: 1-7.
- Sourakov A. 2004. Night blooming plants and their insect pollinators. In: Capinera JL. (eds.) Encyclopedia of entomology. Dordrecht, Springer. p. 1556-1558.
- Souza IM, Coutinho K, Funch LS. 2012. Estratégias fenológicas de Senna cana (Nees & Mart.) H. S. Irwin & Barneby (Fabaceae: Caesalpinioideae) como mecanismo eficiente para atração de polinizadores. Acta Botanica Brasilica 26: 435-443.
- Tangmitcharoen S, Owens JN. 1997. Floral biology, pollination, pistil receptivity, and pollen tube growth of teak (Tectona grandis Linn f.). Annals of Botany 79: 227-241.
- Thomas WW, Jardim JJG, Fiaschi P, Neto EM, Amorim AA. 2009. Composição florística e estrutura do componente arbóreo de uma área transicional de Floresta Atlântica no sul da Bahia, Brasil. Revista Brasileira de Botânica 32: 65-78.
- Tierney SM, Gonzales-Ojeda T, Wcislo W. 2008. Nesting biology and social behavior of Xenochlora bees (Hymenoptera: Halictidae: Augochlorini) from Perú. Journal of the Kansas Entomological Society 81: 61-72.
- Tierney SM, Sanjur O, Grajales GG, Santos LM, Bermingham E, Wcislo WT. 2012. Photic niche invasions: phylogenetic history of the dim-light foraging augochlorine bees (Halictidae). Proceedings of the Royal Society of London Series, Biological Sciences 279: 794-803.
- Veloso HP, Rangel Filho ALR, Lima JCA. 1991. Classificação da vegetação brasileira adaptada a um sistema universal. Rio de Janeiro, IBGE.
- Vieira FA, Appolinário V, Fajardo CG, Carvalho D. 2010. Reproductive biology of Protium spruceanum (Burseraceae), a dominant dioecious tree in vegetation corridors in Southeastern Brazil. Revista Brasileira de Botânica 33: 711-715.
- Wcislo WT, Arneson L, Roesch K, Gonzalez V, Smith A, Fernández H. 2004. The evolution of nocturnal behaviour in sweat bees. Xenochlora nigrofemorata genalis and M. ecuadoria (Hymenoptera: Halictidae): an escape from competitors and enemies? Biological Journal of the Linnean Society 83: 377-387.
- Wcislo WT, Tierney SM. 2009. Behavioural environments and niche construction: the evolution of dim-light foraging in bees. Biological Reviews 84: 19-37.
- Willmer PG, Corbet SA. 1981. Temporal and microclimatie partitioning of the floral resources of Justicia aurea amongst a concourse of pollen vectors and nectar robbers. Oecologia 51: 67-8.
- Wolda H, Roubik DW. 1986. Nocturnal bee abundance and seasonal bee activity in a Panamanian forest. Ecology 67: 426-433.
Publication Dates
-
Publication in this collection
Jul-Sep 2018
History
-
Received
06 July 2018 -
Accepted
09 Aug 2018