Abstract
In this study, two species of Romaleidae grasshoppers, Radacridium mariajoseae and R.nordestinum, were analyzed after CMA3/DA/DAPI sequential staining and fluorescence in situ hybridization (FISH) to determine the location of the 18S and 5S rDNA and histone H4 genes. Both species presented karyotypes composed of 2n = 23, X0 with exclusively acrocentric chromosomes. CMA3+ blocks were detected after CMA3/DA/DAPI staining in only one medium size autosome bivalent and in the X chromosome in R. mariajoseae. On the other hand, all chromosomes, except the L1 bivalent, of R. nordestinum presented CMA3+ blocks. FISH analysis showed that the 18S genes are restricted to the X chromosome in R. mariajoseae, whereas these genes were located in the L2,S9 and S10 autosomes in R. nordestinum.In R. mariajoseae, the 5S rDNA sites were localized in the in L1 and L2 bivalents and in the X chromosome. In R. nordestinum, the 5S genes were located in the L2,L3,M4 and M5 pairs. In both species the histone H4 genes were present in a medium size bivalent. Together, these data evidence a great variability of chromosome markers and show that the 18S and 5S ribosomal genes are dispersed in the Radacridium genome without a significant correlation.
cytogenetics; FISH; fluorochromes; grasshoppers; rDNA
Chromosome mapping of ribosomal genes and histone H4 in the genus Radacridium (Romaleidae)
Allison Anjos; Vilma Loreto; Maria José de Souza
Departamento de Genética, Centro de Ciências Biológicas, Universidade Federal de Pernambuco, Recife, PE, Brazil
Send correspondence to Send correspondence to Vilma Loreto Departamento de Genética, Centro de Ciências Biológicas, Universidade Federal de Pernambuco s/n, Cidade Universitária 50740-600 Recife, PE, Brasil E-mail: vloreto@bol.com.br
ABSTRACT
In this study, two species of Romaleidae grasshoppers, Radacridium mariajoseae and R.nordestinum, were analyzed after CMA3/DA/DAPI sequential staining and fluorescence in situ hybridization (FISH) to determine the location of the 18S and 5S rDNA and histone H4 genes. Both species presented karyotypes composed of 2n = 23, X0 with exclusively acrocentric chromosomes. CMA3+ blocks were detected after CMA3/DA/DAPI staining in only one medium size autosome bivalent and in the X chromosome in R. mariajoseae. On the other hand, all chromosomes, except the L1 bivalent, of R. nordestinum presented CMA3+ blocks. FISH analysis showed that the 18S genes are restricted to the X chromosome in R. mariajoseae, whereas these genes were located in the L2,S9 and S10 autosomes in R. nordestinum.In R. mariajoseae, the 5S rDNA sites were localized in the in L1 and L2 bivalents and in the X chromosome. In R. nordestinum, the 5S genes were located in the L2,L3,M4 and M5 pairs. In both species the histone H4 genes were present in a medium size bivalent. Together, these data evidence a great variability of chromosome markers and show that the 18S and 5S ribosomal genes are dispersed in the Radacridium genome without a significant correlation.
Keywords: cytogenetics, FISH, fluorochromes, grasshoppers, rDNA.
Introduction
The Romaleidae family accounts for more than 200 species of grasshoppers comprising three subfamilies: Romaleinae, Aucacrinae and Trybliophorinae. It is the second most diverse family of the superfamily Acridoidea occurring from semiarid regions to tropical rainforests (Carbonell, 1977; Roberts and Carbonell, 1982; Carbonell, 1984, 1986, 2002). The genus Radacridium is dispersed across the Northeast region of Brazil, where it seems to be well-adapted to the severely arid conditions (Carbonell, 1984). Two species are found in the state of Pernambuco: Radacridium mariajoseae, which is typical of the Agreste region, and Radacridium nordestinum, typical of the Caatinga biome (Carbonell, 1984, 1996).
Cytogenetic studies involving representatives of Romaleidae have revealed a great karyotypic conservation (Mesa et al., 1982, 2004; Souza and Kido, 1995; Rocha et al., 1997; Pereira and Souza, 2000). Most of analyzed species, including R. mariajoseae and R. nordestinum, presented karyotypes with 2n = 23 male, 24 female, an X0/XX sex chromosome system and exclusively acrocentric chromosomes. The constitutive heterochromatin (CH) in Romaleidae is predominantly located in the pericentromeric regions of all chromosomes, as observed in Xyleus angulatus, Brasilacris gigas, Chromacris nuptialis, Radacridium nordestinum e Phaeoparia megacephala (Souza and Kido, 1995; Rocha et al., 1997; Pereira and Souza, 2000). Analyses with DAPI and CMA3 in romaleids showed a predominance of GC-rich (CMA3+) regions. In Xyleus angulatus and Xestotrachelus robustus, CMA3+ blocks were observed in all chromosomes (Souza et al., 1998; Souza et al., 2003). In contrast, few GC-rich CH regions were observed by Loreto et al. (2005) in two Chromacris species. C. nuptialis had only one bivalent (M6) with a pericentromeric CMA3+ block, whereas C. speciosa presented CMA3+ blocks in two autosomal bivalents, a proximal one in M6 and a telomeric one in L2.
A large part of the eukaryotic genome is formed by repetitive DNA, including tandem sequences mainly comprising satellite DNA and multigene families. Ribosomal DNA and histone gene families include a variable number of copies with various genome locations (Charlesworth et al., 1994; Nei and Rooney, 2005). Fluorescence in situ hybridization (FISH) with rDNA and histone genes probes has proven useful for mapping and their location and in clarifying the genome organization of several organisms. Among invertebrates, rDNA gene mapping has been used in several groups, including worms (Vitturi et al., 2002), mollusks (Colomba et al., 2002), insects (Cabrero and Camacho, 2008; Cabral-de-Mello et al., 2011a; Panzera et al., 2012), echinoderms (Caradonna et al., 2007) and others. The studies involving histone genes mapping in grasshoppers included some species of the Acrididae family (Cabrero et al., 2009; Oliveira et al., 2011) and four species of the Proscopiidae family (Cabral-de-Mello et al., 2011b) were mapped.
This study aimed at understanding the pattern of organization of multigene families in two species of Radacridium. Our results showed a great variability in the number and location of rDNA clusters in both species, whereas the histone H4 genes were highly conserved in number. These results are compared with data from other grasshopper species and are discussed based on the possible mechanisms involved in repetitive DNA diversification.
Material and Methods
We analyzed ten individuals of R. mariajoseae from Gravatá (08º12'04" S; 35º33'53" W) and Bezerros (08º14'00" S; 35º47'49" W) and ten adult males of Radacridium nordestinum from Surubim (07º49'59" S; 35º45'17" W), all located in the Agreste region of the state of Pernambuco, Brazilian Northeast. Specimens were processed and their testes were fixed in Carnoy solution (3:1 ethanol:acetic acid). Chromosome preparations were obtained by the testicular follicles squashing technique, in one drop of 45% acetic acid. The coverslips were removed after liquid nitrogen immersion.
CMA3/DA/DAPI sequential staining was performed according to Schweizer (1976). The slides were stained with CMA3 (0.5 mg/mL) for one hour, washed with distilled water and stained with DA (Distamicine A, 0.1 mg/mL) for 45 min. The slides were rewashed and stained with DAPI (2 µg/mL) for 20 min and mounted in glycerol/McIlvaine buffer/MgCl2.
Probes were obtained by PCR performed according to Ayres (2002), with modifications, using genomic DNA from both species and the primers: 18S DNAr -Sca18S1F (F 5' -CCC CGT AAT CGG AAT GAG TA -3'); Sca18S1R (R 5'-GAG GTT TCC CGT GTT GAG TC 3'), 5SrDNA F(5'-AAC GAC CAT ACC ACG CTG AA 3'); R (5'-AAG CGG TCC CCC ATC TAA GT -3'), Histone H4 F-1 (5' -TSC GIG AYA ACA TYC AGG GIA TCA C -3') and R-1 (5' -CKY TTI AGI GCR TAI ACC ACR TCC AT -3'). The PCR products analyzed after electrophoresis in a 1% agarose gel had the expected sizes. The 18S rDNA and histone H4 probes were labeled with bio-tin-16-dUTP and the 5S rDNA probe, with digoxigenin-11-dUTP.
FISH was performed according to Cabral-de-Mello et al. (2011c), with some modifications. The chromosome preparations were submitted to an alcohol series pretreatment and then treated with RNase and pepsin. The hybridization mix contained 1 µL of probe and the denaturation was performed in a humid chamber at 75 ºC, followed renaturation overnight at 37 ºC. Immunodetection was performed with mouse anti-biotin (M743, DAKO) and rabbit anti-mouse-TRITC (R270, DAKO) for biotin and sheep anti-digoxigenin (Roche 1 207 741) and sheep anti-rabbit-FITC (DAKO-F0135) for digoxigenin. Chromosomes were counterstained with 4,6 diamidine-2-phenyl indole (DAPI) and the slides were mounted with Vectashield (Vector). Images were obtained in a Leica epifluorescence microscope, captured with the CW4000 program (Leica) and adjusted in Adobe Photoshop CS5.
Results
Radacridium mariajoseae and R. nordestinum presented 2n = 23(male), a sex determination mechanism of the X0(male) type and exclusively acrocentric chromosomes. Chromosomes of both species were grouped in three large (L1-L3), five medium (M4-M8) and three small (S9-S11) pairs. The X chromosome was a medium size acrocentric in both species. CMA3/DA/DAPI sequential staining showed GC-rich (CMA3 positive) constitutive heterochromatin (CH) blocks in the interstitial region of only one medium-sized bivalent (M5) and in the pericentromeric region of the X chromosome in R. mariajoseae (Figure 1a). In R. nordestinum, CMA3+ blocks were present in the interstitial region of the L2 bivalent and in the pericentromeric region of all chromosomes, except the L1 bivalent (Figure 1c). DAPI staining was homogeneous in both species (Figure 1b and d).
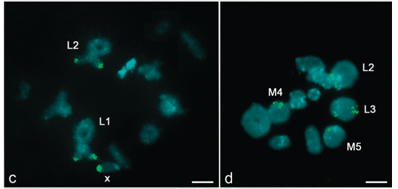
After FISH, the 18S rDNA probe labeled a single site in the pericentromeric region of the X chromosome in R. mariajoseae (Figure 2a) and three pericentromeric sites, in the bivalents L2,S9 and S10 of R. nordestinum (Figure 2b). The 5S rDNA genes were located in the pericentromeric region of the two largest bivalents (L1 and L2) and in the X chromosome of R. mariajoseae, (Figure 2c) and in the bivalents L2,L3 M4 and M5 of R. nordestinumm (Figure 2d). The histone H4 probe was mapped in a proximal location in a medium-sized autosome bivalent (M5) in both species (Figure 2e and f).
Discussion
The karyotypic similarities detected after conventional analysis between the two species of Radacridium did not extend to their CMA3 and DAPI staining patterns. Our results showed GC-richness CH heterogeneity. In R. mariajoseae only one autosome pair and the X chromosome presented CMA3+ blocks, which resembled the scarcity in GC-rich regions of other Romaleidae, as two species of Chromacris with a single CMA3+ block (Loreto et al., 2005). The presence of a large number of CMA3+ blocks is more frequent in Romaleidae, as observed in Xyleus angulatus, Phaeoparia megacephala and Xestotrachelus robustus, in which all CH was shown to be GC-rich (Souza et al., 1998; Pereira and Souza, 2000; Souza et al., 2003).
A great difference in the 18S and 5S rDNA distribution was observed between the species studied. R. mariajoseae presented a single 18S rDNA site in the X chromosome, confirming the finding of only one active nucleolus organizer region (NOR) after silver nitrate impregnation (AgNO3) by Rocha et al. (1997). Although three autosome bivalents showed 18S hybridization signals in R. nordestinum, only the L2 pair had a corresponding active NOR identified by Rocha et al. (1997). These data indicate a possible dispersion of the 18S rDNA sequences, which may have been caused by structural chromosome rearrangements, ectopic recombination and transpositions (Cabrero and Camacho, 2008). These kind of events have been observed in other insects (Nguyen et al., 2010; Cabral-de-Mello et al., 2011c). In the genus Radacridium, the ancestral rDNA site could have been present in the M9 bivalent in a common ancestor. Two points support this hypothesis: the presence of a 18S rDNA site in this pair in R. nordestinum, which was considered as a megameric chromosome by Rocha et al. (1997) and the preferential localization of NORs is in this type of chromosome in several species of grasshoppers (Rufas et al., 1985). The 18S rDNA probe location in the X chromosome of R. mariajoseae could have resulted from a chromosome rearrangement, such as a translocation or transposition, that moved it from M9 to the X, as a meiotic association between this chromosome and the megameric one has been described (Loreto et al., 2008a).
18S rDNA sites restricted to autosomes, as observed in R. nordestinum, were also detected in Xestotrachelus robustus, Chromacris nuptialis and C. speciosa (Souza et al., 2003; Loreto et al., 2005). On the other hand, 18S rDNA sites located both in autosomes and in the sex chromosome have been described, for example, in Xyleus discoideus angulatus (Souza et al., 1998; Loreto et al., 2008b). 18S rDNA sites have been frequently observed associated with GC-rich regions in Romaleidae (Pereira and Souza, 2000; Loreto et al., 2005), Acrididae (Loreto and Souza, 2000; Rocha et al., 2004), Proscopiidae (Souza and Moura, 2000) and Ommexechidae (Carvalho et al., 2011), a pattern also observed in the two species analyzed herein.
The histone H4 genes were located in a single chromosome pair (M5) in both species. A single chromosome pair bearing histone genes was also described in other species of grasshoppers. Cabrero et al. (2009) analyzed the location of the histone H3 and H4 genes in 35 species of grasshoppers from the Acrididae family. They observed that the great majority of species analyzed showed only one histone site, located in an autosome pair. In the same work, double FISH performed in 11 randomly chosen species revealed that, in all cases, both genes were present in the same chromosome site, indicating a great conservation of histone gene location in Acrididae. Cabral-de-Mello et al. (2011b), using a histone H3 probe in four species of Proscopiidae (Tetanorhynchus silvai, Scleratoscopia protopeirae, S. spinosa and Stiphra robusta), observed a single site in the M4 bivalent in all the species. Oliveira et al. (2011) observed multiples sites of histone H3 in Rhammatocerous brasiliensis (Acrididae) and concluded that this would be a derived condition and the presence of a single histone site, as observed in both species studied herein, would be the ancestral form.
The 5S and the 18S genes co-localized in the L2 chromosome of R. nordestinum and in the X chromosome of R. mariajoseae. Similarly to what we observed in both Radacridium species, an extensive variation in 5S rDNA distribution has been found in others grasshoppers, in which species presenting single sites and extending to all chromosome pairs have been described (Loreto et al., 2008b; Cabral-de-Mello et al., 2011a).
The results obtained in this study indicate a great level of karyotypic differentiation between R. mariajoseae and R. nordestinum. These data reinforce the fact that the high conservation observed at the chromosome level, including chromosome number and morphology, in Radacridium and other romaleids, is not reflected at the genomic level. Our results also contribute to the understanding of chromosome evolution patterns in the family Romaleidae.
Acknowledgments
We are grateful to Dr. Carlos Salvador Carbonell, Universidad de Montevidéo, Uruguai, for the taxonomic identification of the specimens used in this study. We are thankful to Dr. Diogo Cavalcanti Cabral-de-Mello for the final review of the manuscript. This work was supported by grants from the Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq) and Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE -APQ 1099-2.02/10).
Received: December 14, 2012; Accepted: March 12, 2013.
Associate Editor: Yatiyo Yonenaga-Yassuda
License information: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Ayres CFJ (2002) Genetic diversity in Brazilian populations of Aedes albopictus Mem Inst Oswaldo Cruz 97:871-875.
- Cabral-de-Mello DC, Cabrero J, Lopez-Leon MD and Camacho JPM (2011a) Evolutionary dynamics of 5S rDNA location in acridid grasshoppers and its relationship with H3 histone gene and 45S rDNA location. Genetica 139:921-931.
- Cabral-de-Mello DC, Martins C, Souza MJ and Moura RC (2011b) Cytogenetic mapping of 5S and 18S rRNAs and H3 histone genes in 4 ancient Proscopiidae grasshopper species: Contribution to understanding the evolutionary dynamics of multigene families. Cytogenet Genome Res 132:89-93.
- Cabral-de-Mello DC, Moura RC and Martins C (2011c) Cytogenetic mapping of rRNAs and histone H3 genes in 14 species of Dichotomius (Coleoptera, Scarabaeidae, Scarabaeinae) beetles. Cytogenet Genome Res 134:127-135.
- Cabrero J and Camacho JPM (2008) Location and expression of ribosomal RNA genes in grasshoppers: Abundance of silent and cryptic loci. Chromosome Res 16:595-607.
- Cabrero J, López-León MD, Teruel M and Camacho JPM (2009) Chromosome mapping of H3 and H4 histone gene clusters in 35 species of acridid grasshopper. Chromosome Res 17:397-404.
- Caradonna F, Bellavia D, Clemente AM, Sisino G and Barbieri R (2007) Chromosomal location and molecular characterization of three different 5S ribosomal DNA clusters in the sea urchin Paracentrotus lividus Genome 50:867-870.
- Carbonell CS (1977) Origin, evolution and distribution of the Neotropical acridomorph fauna (Orthoptera): A preliminary hypothesis. Rev Soc Entomoló Argentina 36:153-175.
- Carbonell CS (1984) Radacridium nordestinum: A new genus and species of Romaleid grasshopper from the Brazilian Caatinga (Orthoptera, Acridoidea). Acad Nat Sci Philadelphia 136:123-129.
- Carbonell CS (1986) Revision of the Neotropical genus Tropidacris (Orthoptera, Acridoidea, Romaleidae, Romaleinae). Acad Nat Sci Philadelphia 138:366-402.
- Carbonell CS (1996) New species of the genus Radacridium Carbonell 1984 (Acridoidea, Romaleidae, Romaleinae) from the Brazilian Northeast. J Orthoptera Res 5:37-41.
- Carbonell CS (2002) The Grasshopper Tribe Phaeopariini (Acridoidea, Romaleidae). Publications on Orthoptera Diversity. The Orthopterists' Society, Philadelphia, 148 pp.
- Carvalho DB, Rocha MF, Loreto V, Silva AEB and Souza MJ (2011) Ommexecha virens (Thunberg, 1824) and Descampsacris serrulatum (Serville, 1831) (Orthoptera, Ommexechidae): Karyotypes, constitutive heterochromatin and nucleolar organizing regions. Comp Cytogenet 5:123-132.
- Charlesworth B, Sniegowski P and Stephan W (1994) The evolutionary dynamics of repetitive DNA in eukaryotes. Nature 371:215-220.
- Colomba MS, Vitturi R, Castriota L, Bertoni R and Libertini A (2002) FISH mapping of 18S-28S and 5S ribosomal DNA, (GATA)n and (TTAGGG)n telomeric repeats in the periwinkle Melarhaphe neritoides (Prosobranchia, Gastropoda, Caenogastropoda). Heredity 88:381-384.
- Loreto V and Souza MJ (2000) Karyotype, constitutive heterochromatin and nucleolar organizer regions in Belosacris coccineipes (Acrididae-Leptysminae). Genet Mol Biol 23:1-5.
- Loreto V, Stadtler E, Melo NF and Souza MJ (2005) A comparative cytogenetic analysis between the grasshopper species Chromacris nuptialis and C. speciosa (Romaleidae): Constitutive heterochromatin variability and rDNA sites. Genetica 125:253-260.
- Loreto V, Cabrero J, López-León MD, Camacho JP and de Souza MJ (2008a) Comparative analysis of rDNA location in five neotropical gomphocerine grasshopper species. Genetica 132:95-101.
- Loreto V, Cabrero J, López-León MD, Camacho JP and Souza MJ (2008b) Possible autosomal origin of macro B chromosomes in two grasshopper species. Chromosome Res 16:233-241.
- Mesa A, Ferreira A and Carbonell GS (1982) Cariologia de los acridoideos neotropicales: Estado actual de su conocimiento y nuevas contribuciones. Ann Soc Entomol France 18:507-526.
- Mesa A, Garcia-Novo P, Portugal CB and Miyoshi AR (2004) Karyology of species belonging to the genera Agriacris Walker 1870 and Staleochlora with some considerations of romaleid phallic structures (Orthoptera, Acridoidea). J Orthoptera Res 13:15-18.
- Nei M and Rooney AP (2005) Concerted and birth-and-death evolution of multigene families. Annu Rev Genet 39:121-152.
- Nguyen P, Sahara K, Yoshido A and Marec F (2010) Evolutionary dynamics of rDNA clusters on chromosomes of moths and butterflies (Lepidoptera). Genetica 138:343-354.
- Oliveira NL, Cabral-de-Mello DC, Rocha MF, Loreto V, Martins C and Moura RC (2011) Chromosomal mapping of rDNAs and H3 histone sequences in the grasshopper Rhammatocerus brasiliensis (Acrididae, Gomphocerinae): Extensive chromosomal dispersion and co-localization of 5S rDNA/H3 histone clusters in the A complement and B chromosome. Mol Cytogenet 4:24-30.
- Panzera Y, Pita S, Ferreiro MJ, Ferrandis I, Lages C, Pérez R, Silva AE, Guerra M and Panzera F (2012) High dynamics of rDNA cluster location in kissing bug holocentric chromosomes (Triatominae, Heteroptera). Cytogenet Genome Res 138:56-67.
- Pereira LG and Souza MJ (2000) Nature and distribution of constitutive heterochromatin and NOR location in the grasshopper Phaeoparia megacephala (Romaleidae, Orthoptera). Cytobios 103:111-119.
- Roberts HR and Carbonell CS (1982) Revision of the grasshopper genera Chromacris and Xestotrachelus (Orthoptera, Romaleidae). Proc Calif Acad Sci 43:43-58.
- Rocha MF, Souza MJ and Tashiro T (1997) Karyotypic variability in the genus Radacridium (Orthoptera, Romaleidae, Romaleinae). Cytologia 62:53-60.
- Rocha MF, Souza MJ and Moura RC (2004) Karyotypic analysis, constitutive heterochromatin and NOR distribution in five grasshopper species of the subfamily Leptysminae (Acrididae). Caryologia 57:107-116.
- Rufas JS, Esponda P and Gosalvez J (1985) Complete dependence between Ag-NOR's and C-positive heterochromatin revealed by simultaneous Ag-NOR C-banding method. Cell Biol Int Rep 7:275-281.
- Schweizer D (1976) Reverse fluorescent chromosome banding with chromomycin and DAPI. Chromosoma 58:307-324.
- Souza MJ and Kido LMH (1995) Variability of constitutive heterochromatin in karyotypes of representatives of the family Romaleidae (Orthoptera). Braz J Genet 18:517-520.
- Souza MJ, Rufas JS and Orellana J (1998) Constitutive heterochromatin, NOR location and FISH in the grasshopper Xyleus angulatus (Romaleidae). Caryologia 51:73-80.
- Souza MJ and Moura RC (2000) Karyotypic characterization and constitutive heterochromatin in the grasshopper Stiphra robusta (Orthoptera, Proscopiidae). Cytobios 101:137-144.
- Souza MJ, Haver PRO and Melo F (2003) Karyotype, C-and fluorescence banding patterns, NOR location and FISH in the grasshopper Xestotrachelus robustus (Romaleidae). Caryologia 56:261-267.
- Vitturi R, Colomba MS, Pirrone AM and Mandrioli M (2002) rDNA (18S-28S and 5S) Colocalization and linkage between ribosomal genes and (TTAGGG)n telomeric sequence in the earthworm, Octodrilus complanatus (Annelida, Oligochaeta, Lumbricidae), revealed by single-and double-color FISH. J Hered 93:279-282.
Send correspondence to
Publication Dates
-
Publication in this collection
19 July 2013 -
Date of issue
2013
History
-
Received
14 Dec 2012 -
Accepted
12 Mar 2013