Abstract
Silicon carbide nanotube (SiCNTs) has been proven as a suitable material for wide applications in high power, elevated temperature and harsh environment. For the first time, we reported in this article an effective synthesis of SiCNTs by microwave heating of SiO2 and MWCNTs in molar ratio of 1:1, 1:3, 1:5 and 1:7. Blend of SiO2 and MWCNTs in the molar ratio of 1:3 was proven to be the most suitable for the high yield synthesis of β-SiCNTs as confirmed by X-ray diffraction pattern. Only SiCNTs were observed from the blend of MWCNTs and SiO2 in the molar ratio of 1:3 from field emission scanning electron microscopy imaging. High magnification transmission electron microscopy showed that tubular structure of MWCNT was preserved with the inter-planar spacing of 0.25 nm. Absorption bands of Si-C bond were detected at 803 cm-1 in Fourier transform infrared spectrum. Thermal gravimetric analysis revealed that SiCNTs from ratio of 1:3 showed the lowest weight loss. Thus, our synthetic process indicates high yield conversion of SiO2 and MWCNTs to SiCNTs was achieved for blend of SiO2 and MWCNTs in molar ratio of 1:3.
Keyword:
Silicon Carbide Nanotube; Multi-walled Carbon Nanotube; Microwave Processing; Synthesis; Vapor-Solid Reaction
1. Introduction
Silicon carbide (SiC) has attracted much attention and has being studied for the potential in many applications such as biosensors11 Oliveros A, Guiseppi-Elie A, Saddow SE. Silicon carbide: a versatile material for biosensor applications. Biomedical Microdevices. 2013;15(2):353-368., photo-catalysts22 Zhou W, Yan L, Wang Y, Zhang Y. SiC nanowires: A photocatalytic nanomaterial. Applied Physics Letters. 2006;89(1):013105. and hydrogen storage33 Mahdizadeh SJ, Goharshadi EK. Hydrogen storage on silicon, carbon, and silicon carbide nanotubes: A combined quantum mechanics and grand canonical Monte Carlo simulation study. International Journal of Hydrogen Energy. 2014;39(4):1719-1731. because it possesses several excellent properties and is also an important biocompatible material44 Ribeiro S, Ribeiro GC, Oliveira MR. Properties of SiC Ceramics Sintered via Liquid Phase Using Al2O3 + Y2O3, Al2O3 + Yb2O3 and Al2O3 + Dy2O3 as Additives: a Comparative Study. Materials Research. 2015;18(3):525-529.. Compared to bulk SiC, one-dimensional (1D) semiconductor nanostructured SiC such as SiC nano-rods, nanotubes and nanowires have been studied more extensively during the last decade owing to their versatile application in fabrication of optoelectronic, electronic and sensor devices on nanometer scale55 Wu R, Zhou K, Yue CY, Wei J, Pan Y. Recent progress in synthesis, properties and potential applications of SiC nanomaterials. Progress in Materials Science. 2015;72:1-60.. Silicon carbide nanotubes (SiCNTs) were known as one dimensional nanostructure material along with silicon carbide nanowires. SiCNTs have advantages over carbon nanotube (CNTs) for high temperature and harsh environment applications because they possess high reactivity on exterior surfaces, facilitating sidewall decoration and greater stability at high temperature and in highly oxidative environment66 Bosi M, Attolini G, Negri M, Frigeri C, Buffagni E, Ferrari C, et al. Optimization of a buffer layer for cubic silicon carbide growth on silicon substrates. Journal of Crystal Growth. 2013;383:84-94..
The main method for SiC production is the Acheson process, which is carbothermal reduction of SiO2 by coke at 2200-2500°C77 Károly Z, Mohai I, Klébert S, Keszler A, Sajó IE, Szépvölgyi J. Synthesis of SiC powder by RF plasma technique. Powder Technology. 2011;214(3):300-305.. Due to high reaction temperatures and long reaction time, the synthesized SiC has often large particle size and is contaminated with impurities such as oxygen and metals. SiCNTs are currently synthesized by using chemical vapor deposition (CVD)88 Xie Z, Tao D, Wang J. Synthesis of silicon carbide nanotubes by chemical vapor deposition. Journal of Nanoscience and Nanotechnology. 2007;7(2):647-652. and carbothermal reduction of the silica by using conventional heating99 Yang Z, Xia Y, Mokaya R. High Surface Area Silicon Carbide Whiskers and Nanotubes Nanocast Using Mesoporous Silica. Chemistry of Materials. 2004;16(20):3877-3884.. However, both of these methods have the downsides in the synthesis of the SiCNTs such as CVD method can only synthesize small amounts of SiCNTs and resulted in the presence of impurities in the SiCNTs due to extensive use of chemical during the synthesis of SiCNTs. Meanwhile, long heating duration, slow heating rate and requirement for further purification step to remove impurities was the problem always associated with the use of conventional heating method in carbothermal reduction of silica. Additional production cost was also needed since large consumption of energy was required to for the synthesis of SiCNTs. In addition, Latu-Romain et. al1010 Latu-Romain L, Ollivier M, Mantoux A, Auvert G, Chaix-Pluchery O, Sarigiannidou E, et al. From Si nanowire to SiC nanotube. Journal of Nanoparticle Research. 2011;13(10):5425.,1111 Latu-Romain L, Ollivier M, Thiney V, Chaix-Pluchery O, Martin M. Silicon carbide nanotubes growth: an original approach. Journal of Physics D: Applied Physics. 2013;46(9):092001. in their study of synthesis of the SiCNTs from silicon nanowire (Si NW) has successfully synthesized SiCNTs by hot filament CVD at temperature of 1100°C for 30 minutes using gold as catalyst. Continuous diffusion of Si gas into the layer of SiC has resulted in the formation of SiCNTs. However, the use of gold as catalyst incurred high cost and the need to synthesize Si NW required additional processing step which are time and energy consuming.
Recently, many researchers have ultilized microwave heating to synthesize SiC because of special characteristics of microwave heating in which microwave can volumetrically heat materials with favorable dielectric properties and can synthesize SiC with uniform grain size at higher synthesis rate and reduced reaction time1212 van Laar JH, Slabber JFM, Meyer JP, van Der Walt IJ, Puts GJ, Crouse PL. Microwave-plasma synthesis of nano-sized silicon carbide at atmospheric pressure. Ceramics International. 2015;41(3 Pt B):4326-4333.,1313 Kahar SM, Voon CH, Lee CC, Gopinath SCB, Arshad MK, Lim BY, et al. Synthesis of silicon carbide nanowhiskers by microwave heating: effect of heating duration. Materials Research Express. 2017;4(1):015005.. It is more economical to synthesize materials using microwave heating as compared to conventional methods such as mechanical milling, carbothermal reduction and laser synthesis since microwave heating is well known for low energy consumption, shorter reaction time and very low impurities such as oxygen and metals reside in the end products after synthesis1414 Hashimoto S, Ohashi S, Hirao K, Zhou Y, Hyuga H, Honda S, et al. Mechanism for the formation of SiC by carbothermal reduction reaction using a microwave heating technique. Journal of the Ceramic Society of Japan. 2011;119(10):740-744.
15 Dzido G, Markowski P, Malachowska-Jutsz A, Prusik K, Jarzebski AB. Rapid continuous microwave-assisted synthesis of silver nanoparticles to achieve very high productivity and full yield: from mechanistic study to optimal fabrication strategy. Journal of Nanoparticle Research. 2015;17:27.-1616 Galvão NKAM, Vasconcelos G, Santos MVR, Campos TMB, Pessoa RS, Guerino M, et al. Growth and Characterization of Graphene on Polycrystalline SiC Substrate Using Heating by CO2 Laser Beam. Materials Research. 2016;19(6):1329-1334.. The characteristic of microwave heating is attributed to the fact that microwave is a form of electromagnetic energy with a frequency range from 300 MHz to 300 GHz and microwave can couple with certain materials which have excellent dielectric properties such as carbon and it can be absorbed into these materials volumetrically which then transformed to heat from the inside of materials1717 Menéndez JA, Arenillas A, Fidalgo B, Fernández Y, Zubizarreta L, Calvo EG, et al. Microwave heating processes involving carbon materials. Fuel Processing Technology. 2010;91(1):1-8.,1818 Shi L, Hu X, Huang Y. Fast microwave-assisted synthesis of Nb-doped Li4Ti5O12 for high-rate lithium-ion batteries. Journal of Nanoparticle Research. 2014;16:2332.. Conventional heating method involves the transfer of heat between objects by the mechanisms of conduction, convection and radiation1919 Oghbaei M, Mirzaee O. Microwave versus conventional sintering: A review of fundamentals, advantages and applications. Journal of Alloys and Compounds. 2010;494(1-2):175-189.. Heat loss to surrounding and irregular heat transfer between the heat source and material often becomes the issue of conventional heating and causes low heating rate and high energy consumption. Li et. al2020 Li J, Shirai T, Fuji M. Rapid carbothermal synthesis of nanostructured silicon carbide particles and whiskers from rice husk by microwave heating method. Advanced Powder Technology. 2013;24(5):838-843. have reported that by using microwave heating, one can successfully synthesize nanostructured β-SiC in argon atmosphere. Compared to conventional heating, microwave heating was proven to be an efficient approach to synthesize SiC in terms of low energy consumption and time saving. Carbon materials such as MWCNTs are well known as good microwaves absorber2121 Kim T, Lee J, Lee KH. Microwave heating of carbon-based solid materials. Carbon Letters. 2014;15(1):15-24.. It is expected that by microwaves heating the blend of MWCNTs and SiO2, SiCNTs can be synthesized from the reaction of MWCNTs and SiO2. To date, no study was reported on the synthesis of SiCNTs by microwaves heating of blends of MWCNTs and SiO2.
In the previous study, several researchers have investigated the effect of ratio of raw material on the synthesis of SiC nano-material2222 Ding J, Deng C, Yuan W, Zhu H, Zhang X. Novel synthesis and characterization of silicon carbide nanowires on graphite flakes. Ceramics International. 2014;40(3):4001-4007.,2323 Zhang J, Li W, Jia Q, Lin L, Huang J, Zhang S. Molten salt assisted synthesis of 3C-SiC nanowire and its photoluminescence properties. Ceramics International. 2015;41(10 Pt B):12614-12620.. For example, Ding et al2424 Ding J, Zhu H, Li G, Deng C, Li J. Growth of SiC nanowires on wooden template surface using molten salt media. Applied Surface Science. 2014;320:620-626. in their study synthesis of SiC nanowires by using molten salt synthesis (MSS) method at temperature of 1400 °C indicates that ratio of raw materials is an important factor to synthesize SiC nanowires and they revealed that intensity of β-SiC peaks of ratio 1:3 was higher compared to Si and salts containing carbon of ratio 1:1, 1:2 and 1:4 which suggested that formation of SiC nanowires was highest when Si and salts containing carbon of ratio 1:3 was used. Besides that, Bi et al2525 Bi S, Ma L, Mei B, Tian Q, Liu C, Zhong C, et al. Silicon carbide/carbon nanotube heterostructures: Controllable synthesis, dielectric properties and microwave absorption. Advanced Powder Technology. 2014;25(4):1273-1279. reported the synthesis of the SiC/CNTs hetero-structures from the sol-gel mixture containing the mixture of MWCNTs and silica gel in various ratios by using tube furnace at temperature of 1400°C for 60 minutes. They found out that increase of molar ratio of MWCNTs in mixture caused high residual of MWCNTs and revealed that almost all reactant of silica gel and MWCNTs in the ratio of 1:3 was consumed and formed SiC/CNTs hetero-structures. Therefore, these studies indicate that ratio of raw materials has significant effect in the purity and quality of the synthesized SiCNTs.
Bi et al2525 Bi S, Ma L, Mei B, Tian Q, Liu C, Zhong C, et al. Silicon carbide/carbon nanotube heterostructures: Controllable synthesis, dielectric properties and microwave absorption. Advanced Powder Technology. 2014;25(4):1273-1279. and Quah et al2626 Quah HJ, Cheong KY, Lockman Z. Stimulation of silicon carbide nanotubes formation using different ratios of carbon nanotubes to silicon dioxide nanopowders. Journal of Alloys and Compounds. 2009;475(1-2):565-568. also studied and reported the effect of different ratios of the SiO2 particles and CNTs. However, in this article we studied the effect of different ratios of SiO2 particles and MWCNTs with a different synthesis approach which is by using microwave heating rather than conventional heating method used by Bi et al and Quah et al. Thus, in this paper, the effect of molar ratio of MWCNTs and SiO2 on the synthesis of SiCNTs was studied and reported, considering its importance on the properties of the prepared materials. We revealed the morphology, composition, optical and quality of SiCNTs that were synthesized by microwave heating of the blend of SiO2 and MWCNTs in different ratios. In order to identify the effect of molar ratio of SiO2 and MWCNT for the synthesis of SiCNTs, the as-synthesized SiCNTs were characterized using X-ray diffraction, field emission scanning electron microscopy, transmission electron microscopy, photoluminescence spectroscopy, fourier transform infrared spectroscopy, and thermal gravimetric analysis.
2. Experimental
In this study, the materials used were multi-walled carbon nanotubes (MWCNTs) and silicon dioxide (SiO2), which were purchased from Sigma Aldrich. SiO2 (particles size of 44 µm and purity 99%) was mixed properly with MWCNTs (diameter range from 10 to 70 nm) in the ratio of 1:1, 1:3, 1:5 and 1:7 using ethanol as a liquid medium in ultrasonic bath for 2 hours. After the mixing process, the mixtures were dried using hot plate until ethanol was completely evaporated and the mixtures were pressed into solid pellet form with a thickness of 3 mm to ease the sample handling during transferring to and out of microwave cavity and also to minimize the loss of SiO gas produced to surroundings.
2.45 GHz multi-mode cavity microwave furnace model HAmiLab-V3 from SYNOTHERM was used to conduct microwave heating. Figure 1 shows the arrangement of alumina crucible in microwave cavity which filled with silica sand, graphite and SiC susceptor and blends of different molar ratios (1:1, 1:3, 1:5 and 1:7) were placed at the center of alumina crucible. Silica sand is a good heat insulator and it can reduce the heat loss to surroundings while SiC susceptor has ability to absorb the microwave energy at low temperature and acts as the external heat source which helps to achieve high heating rate. Meanwhile, graphite can absorb the microwave energy and produced heat energy which then transferred to samples to increase heating rate. The cavity was vacuumed and pure argon gas atmosphere was introduced into microwave cavity during the synthesis. Heating rate of 30°C/min were used during microwave heating to reach the required temperature at 1400°C and maintained for 40 minutes. The power of microwave used in this study was within the range of 0.3 to 2.85 kW and the power was adjusted automatically by the microwave furnace since automatic mode was used. Therefore, the power of microwave energy fluctuated during the synthesis so that the preset temperature and heating rate can be obtained. The specimens were cooled in the microwave cavity to room temperature.
The specimens were characterized using X-ray diffraction (XRD), field emission scanning electron microscopy (FESEM), transmission electron microscopy (TEM), photoluminescence spectroscopy (PL), fourier transform infrared spectroscopy (FTIR) and thermal gravimetric analysis (TGA). XRD Siemens Diffractometer Model D-5000 using Cu Kα radiation source in θ/2θ mode was used to investigate the crystalline phase present in the specimens. Measurements were made with fast duration scan (1s) and small step size (0.02°). Meanwhile, the morphologies of specimens were observed by using FESEM model Nova Nano 450 at magnification 200K and accelerating voltage of 5 kV. Tubular structure of SiCNTs was confirmed using transmission electron microscopy model Philips Tecnai F20 TEM. Optical properties of SiCNTs were identified by using the photoluminescence spectroscopy (PL FL3-11 J81040) with xenon lamp of 400 watt and excitation wavelength at 265 nm and photoluminescence baseline subtraction was conducted by using OriginPro 8 by using method of auto-create a modifiable baseline with baseline algorithm of end weighted. Reference baseline then was subtracted and the graph was plotted. Fourier transform infrared (FTIR MAGNA550 kBr) spectroscopy was used to scan the samples from 500 - 4000 cm-1 with a spectrum resolution of 4 cm-1. Thermal stability of SiCNTs was evaluated using Perkin-Elmer Pyris 6 TGA analyzer. Samples about 10 mg weight were heated from 30 to 1300°C with a heating rate of 10 °C/min in atmospheric air in order to investigate the thermal stability of the synthesized SiCNTs. Raman spectroscopy was conducted by using Raman Spectrometer model Renishaw InVia Raman Microscope with excitation of 633 nm HeNe laser and spectral range was taken from 650 to 1800 cm-1.
3. Results and Discussion
3.1 X-Ray diffraction (XRD) patterns
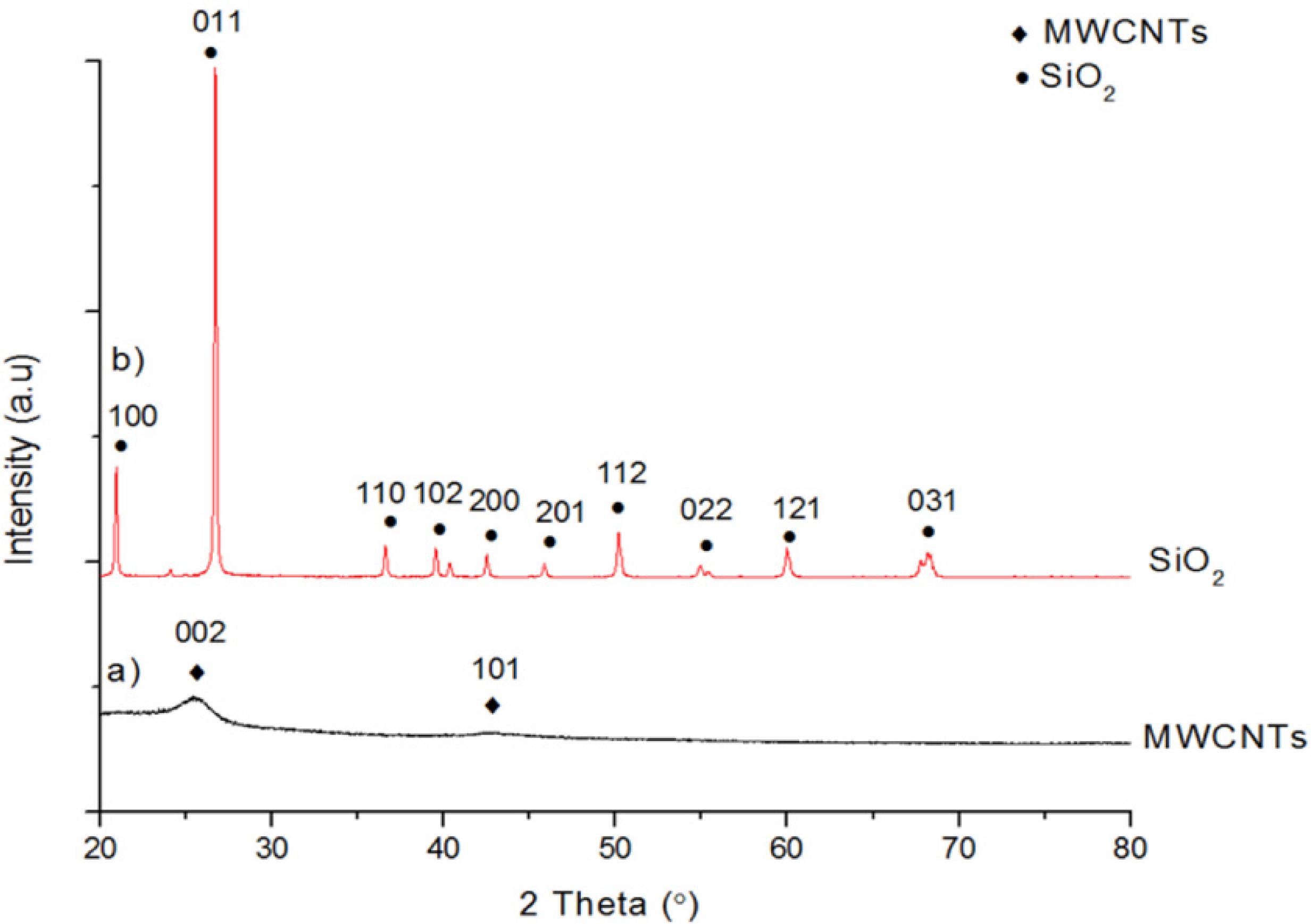
Figure 2 shows the XRD patterns of SiO2 particles and MWCNTs. Two broad peaks centered at 23° and 43° in Figure 2 a) shows that MWCNTs are amorphous while many sharp peaks in XRD pattern of SiO2 particles in Figure 2 b) revealed that SiO2 particles are crystalline. Meanwhile, Figure 3 shows XRD pattern of SiCNTs synthesized from blends of SiO2 and MWCNTs in the ratio of 1:1, 1:3, 1:5 and 1:7. It can be seen in Figure 3 (a)-(d) that the characteristic peaks of β-SiC centered at 2θ = 36.2°, 43°, 60° and 73.4°, which are associated with (111), (200), (220) and (311) planes of β-SiC (JCPDS Card No: 29-1129), respectively were observed in XRD patterns of all specimens synthesized from the blend of SiO2 and MWCNTs in the ratio of 1:1, 1:3, 1:5 and 1:7, respectively. This indicated that SiCNT was successfully synthesized from the blend of SiO2 and MWCNTs regardless of the ratio of MWCNTs and SiO2. It is worth mentioning that in XRD pattern of SiCNTs synthesized from the blend of SiO2 and MWCNTs in the ratio of 1:1 in Figure 3 (a), there is a peak corresponding to the residual of unreacted SiO2 at 2θ = 23° associated with (100) planes of SiO2 particles. Very small XRD peak corresponded to residual of unreacted MWCNTs were observed at 2θ = 27.3° associated with (002) planes of carbon. This shows that amount of MWCNTs in the blend to react with SiO2 was not sufficient when SiO2 and MWCNTs were in the ratio of 1:1. Thus, unreacted SiO2 was detected with very small residual MWCNTs observed and only small amounts of SiCNTs were produced.
XRD patterns of SiCNTs synthesized from blend of SiO2 and MWCNTs in the ratio of a) 1:1; b) 1:3; c) 1:5 and d) 1:7.
For SiCNTs synthesized from the blend of SiO2 and MWCNTs in the ratio of 1:3, XRD peaks corresponding to β-SiC can be observed from the XRD pattern as shown in Figure 3 (b). This indicated that the ratio 1:3 of SiO2 to MWCNTs is the suitable ratio to synthesize SiCNTs, because only very small residue of unreacted SiO2 and MWCNTs was observed in the XRD pattern. Meanwhile, XRD pattern for SiCNTs synthesized from the blend of SiO2 and MWCNTs in the ratio of 1:5 and 1:7 (Figure 3 (c) and (d)) show residual of unreacted MWCNTs at 2θ = 27° associated with (002) planes of carbon. These residual of MWCNTs was amorphous as indicated in XRD patterns in Figure 3 (c) and (d) which show broad peak of carbon peak. It is postulated that the reaction was not completed because there was no sufficient SiO2 to react with MWCNTs and thus significant amount of MWCNTs were not converted to SiCNTs. The result is consistent with the chemical reaction between the SiO2 and MWCNTs as shown by Equation (3.1) below which suggested that synthesized SiCNTs from ratio 1:3 gives highest yield conversion of SiO2 particles and MWCNTs to SiCNTs2525 Bi S, Ma L, Mei B, Tian Q, Liu C, Zhong C, et al. Silicon carbide/carbon nanotube heterostructures: Controllable synthesis, dielectric properties and microwave absorption. Advanced Powder Technology. 2014;25(4):1273-1279..
Similar observation was reported by Ding et al2222 Ding J, Deng C, Yuan W, Zhu H, Zhang X. Novel synthesis and characterization of silicon carbide nanowires on graphite flakes. Ceramics International. 2014;40(3):4001-4007. in his research to synthesize SiC nanowires from graphite and Si powder by molten salt synthesis method, in which they concluded that the ratio of Si powder to carbon (graphite) was crucial in order to synthesize SiC nanowires without any excess of unreacted graphite and Si powder and they revealed that the mixture of Si powder and graphite in the ratio of 1:3 was most favorable to synthesize SiC nanowires. Bi et al2525 Bi S, Ma L, Mei B, Tian Q, Liu C, Zhong C, et al. Silicon carbide/carbon nanotube heterostructures: Controllable synthesis, dielectric properties and microwave absorption. Advanced Powder Technology. 2014;25(4):1273-1279. also confirmed that the ratio 1:3 of SiO2 to MWCNTs can be used to synthesize SiCNTs by using the tube furnace with only small amounts of MWCNTs left as residue compared to other ratio as shown in XRD pattern.
3.2 Field emission scanning electron microscopy (FESEM) images
FESEM images of SiO2 particles, MWCNTs and SiCNTs synthesized from the blend of SiO2 and MWCNTs with a ratio ranged from 1:1, 1:3, 1:5 and 1:7 were shown in Figure 4. It can be observed that SiO2 particles were agglomerates of smaller particles which formed larger particles as shown in Figure 4 (a) while Figure 4 (b) shows the MWCNTs have smooth surface with diameter range from 11 to 70 nm. Meanwhile, it can be seen in Figure 4 (c) that for SiCNTs synthesized from the blend of SiO2 and MWCNTs of ratio 1:1, there is unreacted SiO2 (pointed out by red circle) due to the amount of MWCNTs available to react with SiO2 was insufficient when SiO2 and MWCNTs were in the ratio of 1:1. FESEM images in Figure 4 (d) show that SiCNTs synthesized from the blend of SiO2 and MWCNTs in the ratio of 1:3 is the suitable ratio for the synthesis of SiCNTs as high yield conversion of SiO2 particles and MWCNTs to SiCNTs was achieved as illustrated in FESEM images.
FESEM images of a) SiO2 particles, b) MWCNTs and SiCNTs synthesized from blend of SiO2 and MWCNTs in the ratio of c) 1:1; d) 1:3; e) 1:5 and f) 1:7. Red circle: Silicon dioxide particle (SiO2), Blue circle: Multi-walled carbon nanotubes (MWCNTs).
Meanwhile, FESEM images of SiCNTs synthesized from the blend of SiO2 and MWCNTs in the ratio of 1:5 and 1:7 as shown in Figure 4 (e) and (f) revealed that there were residuals of unreacted MWCNTs (pointed out by blue circle). This might be due to the fact that SiO2 was insufficient to react with MWCNTs and thus some MWCNTs were not converted to SiCNTs. These results from Figure 4 are in good agreement with the XRD pattern of the SiCNTs in Figure 3 in which high yield conversion of SiO2 and MWCNTs to β-SiCNTs was achieved, when the blend of SiO2 and MWCNTs was in the ratio of 1:3 with very small residual of SiO2 particles was detected. However, no SiO2 particle was observed in FESEM image of SiCNTs synthesized from the blend of SiO2 and MWCNTs in the ratio of 1:3 and this may due to the very small amount of residue of SiO2 particles which caused difficulty to detect SiO2 particles in FESEM image. It can be observed also from FESEM images of SiCNTs in Figure 3 (c) - (f) that diameter of the SiCNTs produced in this study was in the range of 11-71 nm while diameter of MWCNTs in Figure 3 (b) was in the range of 11-70 nm. This shows that there is no obvious change in the diameter of nanotube after MWCNTs were converted to SiCNTs.
3.3 Transmission electron microscopy (TEM) images
TEM images of MWCNTs and SiCNTs synthesized from blend of SiO2 and MWCNTs in the ratio of 1:3 were showed in Figure 5. Figure 5 (a) shows that the tubular structure of MWCNTs and it can be observed that MWCNTs has inter-planar spacing of 0.34 nm as reported by others2727 Kharissova OV, Kharisov BI. Variations of interlayer spacing in carbon nanotubes. RSC Advances. 2014;4(58):30807-30815.. TEM images in Figure 5 (b) showed that the SiCNTs has rough surface which may due to the reaction of SiO gas with carbon atoms at the surface of MWCNTs, resulted in the formation of defects as some of carbon atoms formed CO gas as explained later. Figure 5 (b) reveals that the SiCNTs has inter-planar spacing of 0.25 nm. This showed the SiCNTs was successfully formed after the synthesis and the tubular structure of MWCNTs was conserved. Similar result has been reported by Dai et al2828 Dai JX, Sha JJ, Zhang ZF, Wang YC, Krenkel W. Synthesis of high crystalline beta SiC nanowires on a large scale without catalyst. Ceramics International. 2015;41(8):9637-9641. in which they found that inter-planar spacing of SiC nanowire was observed to be 0.25 nm. It can be that diameter of SiCNTs in TEM image was in 13 nm which is in good consistence with the diameter of SiCNTs in the range of 11-71 nm as observed in FESEM image.
TEM images of a) SiCNTs synthesized from blend of SiO2 and MWCNTs in the ratio of 1:3 and b) HRTEM images of SiCNTs.
3.4 Photoluminescence spectroscopy (PL) spectra
PL spectra of SiCNTs synthesized from the blend of SiO2 and MWCNTs in the ratio of 1:1, 1:3, 1:5 and 1:7 are shown in Figure 6. Samples were excited by ultraviolet fluorescent light at 265 nm from a Xe lamp at room temperature. It can be observed that all the PL spectra of SiCNTs synthesized from the blend of SiO2 and MWCNTs in the ratio of 1:1, 1:3, 1:5 and 1:7 respectively have a strong PL peak of β-SiCNTs at wavelength of 465 nm corresponding to band gap of 2.67 eV. Compared to band gap of bulk 3C-SiC of 2.39 eV2929 Lee KM, Hwang JY, Urban B, Singh A, Neogi A, Lee SK, et al. Origin of broad band emissions of 3C-silicon carbide nanowire by temperature and time resolved photoluminescence study. Solid State Communications. 2015;204:16-18., the PL peaks of SiCNTs are considerably blue shifted. It is believed that this is due to quantum confinement effect. The results were in good agreement with the value reported by Chen et al3030 Chen J, Tang W, Xin L, Shi Q. Band gap characterization and photoluminescence properties of SiC nanowires. Applied Physics A. 2011;102(1):213-217., which reported that silicon carbide nanowire exhibits a strong and sharp emission at 470 nm and corresponded to band gaps of 2.64 eV. Nazarudin et al3131 Nazarudin NFFB, Noor NJBM, Rahman SA, Goh BT. Photoluminescence and structural properties of Si/SiC core-shell nanowires growth by HWCVD. Journal of Luminescence. 2015;157:149-157. also concluded that the PL of the SiC nano-crystalizes is strongly dependent on quantum confinement effects attributed to the size of the nano-crystallites embedded in the nanowires.
PL spectrum of SiCNTs synthesized from blend of SiO2 and MWCNTs in the ratio of a) 1:1; b) 1:3; c) 1:5 and d) 1:7.
In Figure 6 (a), PL spectrum of SiCNTs synthesized from the blend of SiO2 and MWCNTs in the ratio of 1:1 shows the presence of strong PL peak attributed to oxygen discrepancy in SiO2 at 387 nm and corresponded to band gaps of 3.2 eV. The presence of this peak is in good consistence with the XRD result of SiCNTs synthesized from the blend of SiO2 and MWCNTs in the ratio of 1:1 (Figure 3 (a)), which shows the presence of XRD peak corresponded to SiO2. This is due to the presence of SiO2 which was remained unreacted because of insufficient MWCNTs in the mixture. Similar result was also reported by Chiu and Li3232 Chiu SC, Li YY. SiC nanowires in large quantities: Synthesis, band gap characterization, and photoluminescence properties. Journal of Crystal Growth. 2009;311(4):1036-1041., they reported that there was an emission peak centered at 390 nm which was attributed to the oxygen discrepancy in SiO2 particles and thus indicated the presence of SiO2 particles after the synthesis of SiC.
PL spectrum of SiCNTs synthesized from the blend of SiO2 and MWCNTs in the ratio of 1:3 (Figure 6 (b)) consisted of single peak corresponding to β-SiCNTs at a wavelength of 465 nm with band gaps of 2.67 eV. This revealed that SiCNTs synthesized from the blend of SiO2 and MWCNTs in the ratio of 1:3 consisted only β-SiCNTs. However, XRD pattern in Figure 3 (b) shows the presence of very small peak of SiO2 particles but PL peak corresponded to SiO2 particles was not observed in Figure 6 (b). This may due to the amount of SiO2 particles was too small to be detected by PL. Meanwhile, for the PL spectra of SiCNTs synthesized from the blends of SiO2 and MWCNTs in the ratio of 1:5 and 1:7 in Figure 6 (c) and (d), it can be seen that only peak corresponding to β-SiCNTs were observed at wavelength of 465 nm although significant amount of MWCNTs remained unreacted and was detected in XRD patterns. No PL peak corresponded to MWCNTs was observed from the PL spectra of SiCNTs synthesized from the blend of SiO2 and MWCNTs in the ratio of 1:5 and 1:7. This was due to the quenching effect of the MWCNTs where the electrons are trapped in MWCNTs and thus decreases the PL intensity. MWCNTs are well known as good electron acceptors3333 Paul R, Kumbhakar P, Mitra AK. Synthesis and study of photoluminescence characteristics of carbon nanotube/ZnS hybrid nanostructures. Journal of Experimental Nanoscience. 2010;5(4):363-373. and thus can act as electron storage to trap electrons. Several researchers also have reported the similar result in which they reported no PL peak of MWCNTs was observed3434 Yu Y, Yu JC, Chan CY, Che YK, Zhao JC, Ding L, et al. Enhancement of adsorption and photocatalytic activity of TiO2 by using carbon nanotubes for the treatment of azo dye. Applied Catalysis B: Environmental. 2005;61(1-2):1-11.,3535 Lam SM, Sin JC, Abdullah AZ, Mohamed AR. Photocatalytic TiO2 /Carbon Nanotube Nanocomposites for Environmental Applications: An Overview and Recent Developments. Fullerenes, Nanotubes and Carbon Nanostructures. 2014;22(5):471-509.,3636 Abdullahi N, Saion E, Shaari AH, Al-Hada NM, Keiteb A. Optimisation of the Photonic Efficiency of TiO2 Decorated on MWCNTs for Methylene Blue Photodegradation. PLoS One. 2015;10(5):e0125511.. Besides that, it was also observed that the intensity of PL peak corresponded to β-SiC of SiCNTs in the ratio of 1:7 was lower than SiCNTs in the ratio of 1:5. This was due to the reduced electron-hole recombination rate for SiCNTs in the ratio of 1:7 comparing to the SiCNTs in the ratio of 1:5. Similar result was also reported by Gui et al3737 Gui MM, Chai SP, Xu BQ, Mohamed AR. Visible-light-driven MWCNT@TiO2 core-shell nanocomposites and the roles of MWCNTs on the surface chemistry, optical properties and reactivity in CO2 photoreduction. RSC Advances. 2014;4(46):24007-24013. in their study of TiO2/MWCNTs and roles of MWCNTs on surface chemistry, optical properties and reactivity in CO2 photo-reduction. They reported that increase of the MWCNTs loading in TiO2/MWCNTs caused great reduction of the PL intensity of TiO2 due to the inhibition of the electron-hole recombination in TiO2.
3.5 Fourier transform infrared (FTIR) spectra
To analyze the chemical bonding of the SiCNTs synthesized from the blend of SiO2 and MWCNTs in the ratio of 1:1, 1:3, 1:5 and 1:7, FTIR spectroscopy was used and the FTIR spectra of the SiCNTs are shown in Figure 7. FTIR peak corresponded to Si-C stretching bond was present at 1000-800 cm-1 in all FTIR spectra of SiCNTs synthesized from the blend of SiO2 and MWCNTs in the ratio of 1:1, 1:3, 1:5 and 1:7. From Figure 7 (a), it can be observed that SiCNTs synthesized from the blend of SiO2 and MWCNTs in the ratio of 1:1 has FTIR peak corresponded to stretching bond of Si-O bonding group at 1110 cm-13838 Raman V, Bhatia G, Mishra AK, Bhardwaj S, Sood KN. Synthesis of silicon carbide nanofibers from pitch blended with sol-gel derived silica. Materials Letters. 2006;60(29-30):3906-3911.. These indicated that some of SiO2 particles were not reacted with MWCNTs during the heating process.
FTIR spectrum of SiCNTs synthesized from blend of SiO2 and MWCNTs in the ratio of a) 1:1; b) 1:3; c) 1:5 and d) 1:7.
Meanwhile, SiCNTs synthesized from the blend of SiO2 and MWCNTs in the ratio of 1:3 in Figure 7 (b) shows FTIR absorption band centered at 806 cm-1 which corresponded to stretching vibration of Si-C bonds. This result is in good consistence with the XRD result of SiCNTs synthesized from the ratio of 1:3 (Figure 2 (b)) which shows high yield conversion of SiO2 particles and MWCNTs to β-SiCNTs when blend of ratio 1:3 of SiO2 particles and MWCNTs was used. Chen et al3939 Chen J, Shi Q, Xin L, Liu Y, Liu R, Zhu X. A simple catalyst-free route for large-scale synthesis of SiC nanowires. Journal of Alloys and Compounds. 2011;509(24):6844-6847. revealed similar result where strong FTIR peak centered at 820 cm-1 corresponded to transverse optical photon vibration mode of Si-C bond was observed from the FTIR spectra of synthesized 3C-SiC nanowires.
FTIR spectra of SiCNTs synthesized from the blend of SiO2 and MWCNTs in the ratio of 1:5 and 1:7 (Figure 7 (c) and (d)) revealed the presence of FTIR peaks corresponded to C=C stretching bonds centered at 1640 cm-14040 Yang X, Zhao-hui C, Feng C. High-temperature protective coatings for C/SiC composites. Journal of Asian Ceramic Societies. 2014;2(4):305-309.. The presence of these peaks indicated that some of the MWCNTs were not converted to SiCNTs and this may due to the insufficient amount of SiO2 particles to react with MWCNTs.
3.6 Thermal gravimetric analysis (TGA)
Thermal stability of SiCNTs synthesized from the blend of SiO2 and MWCNTs in the ratio of 1:1, 1:3, 1:5 and 1:7 was evaluated by using thermal gravimetric analysis. The TGA curves are shown in Figure 8. It can be observed from all TGA curves that no weight loss took place when SiC was heated up to 600 °C. The decomposition only started to occur when the temperature was higher than 600 °C which was attributed to the oxidation of MWCNTs. Giorcelli et al4141 Giorcelli M, Pavese M, Shahzad MI, Tagliaferro A. Silicon carbide hollow cylinders using carbon nanotubes structures as template. Materials Letters. 2015;151:12-15. reported similar observation in which SiC hollow cylinder synthesized by using MWCNTs has decomposed at 600 °C in their TGA study.
TGA analysis of SiCNTs synthesized from blend of SiO2 and MWCNTs in the ratio of 1:1; 1:3; 1:5 and 1:7.
It can be observed that SiCNTs synthesized from the blend of SiO2 and MWCNTs in the ratio of 1:1 has shown weight loss of 5% and started at 700 °C. It is believed that this weight loss of 5% was due to the decomposition of the residual MWCNTs and this result also shows good agreement with XRD pattern of SiCNTs in Figure 3 (a) in which very small XRD peak corresponded to carbon was observed and indicates the presence of small amount of residual MWCNTs. Li et al.4242 Zhou X, Li X, Gao Q, Yuan J, Wen J, Fang Y, et al. Metal-free carbon nanotube-SiC nanowires heterostructures with enhanced photocatalytic H2 evolution under visible light irradiation. Catalysis Science & Technology. 2015;5:2798-2806. also reported similar result in which they reported that decomposition of the MWCNTs occurred at temperature of 700 °C to 800 °C in SiC/MWCNTs and revealed the thermal stability of CNTs was enhanced in SiC/CNTs which led to higher resistance of MWCNTs toward oxidation.
Meanwhile, from Figure 8, TGA curved shows the SiCNTs synthesized from the blend of SiO2 and MWCNTs in the ratio of 1:3, 1:5 and 1:7 has weight loss of 4 %, 12 % and 35 %, respectively. This weight loss was attributed to the oxidation of residual MWCNTs. This result also shown a good agreement with XRD patterns of SiCNTs in Figure 3 which show there are very small amount of residual MWCNTs for ratio 1:3 while ratio 1:5 and 1:7 show significant amount of residual MWCNTs. Besides that, the amount of SiCNTs produced was reduced as the carbon in the blends increased from ratio of 1:3, 1:5 to 1:7. SiCNTs can help to protect MWCNTs from further oxidation by increasing the decomposition temperature of MWCNTs. As ratio 1:3 has produced high yield of SiCNTs as shown in XRD pattern in Figure 3, SiCNTs can effectively protect MWCNTs from decomposition. However, large weight loss of ratio 1:5 and 1:7 was due to the oxidation of MWCNTs since only small amount of SiCNTs was produced. Besides, the starting temperature for the decomposition was reduced to 650 °C and 600 °C for ratio 1:5 and 1:7, respectively due to the smaller amount of SiCNTs in the samples which cannot effectively improve the thermal stability of MWCNTs. Similar result was reported by Li et al.4242 Zhou X, Li X, Gao Q, Yuan J, Wen J, Fang Y, et al. Metal-free carbon nanotube-SiC nanowires heterostructures with enhanced photocatalytic H2 evolution under visible light irradiation. Catalysis Science & Technology. 2015;5:2798-2806. in which they reported in the thermal gravimetric analysis of pure MWCNTs, the decomposition started at 550ºC while for SiC/MWCNTs, decomposition of MWCNTs started at 700ºC, denoting the presence of SiCNTs can enhance the thermal stability of MWCNTs.
3.7 Raman spectroscopy
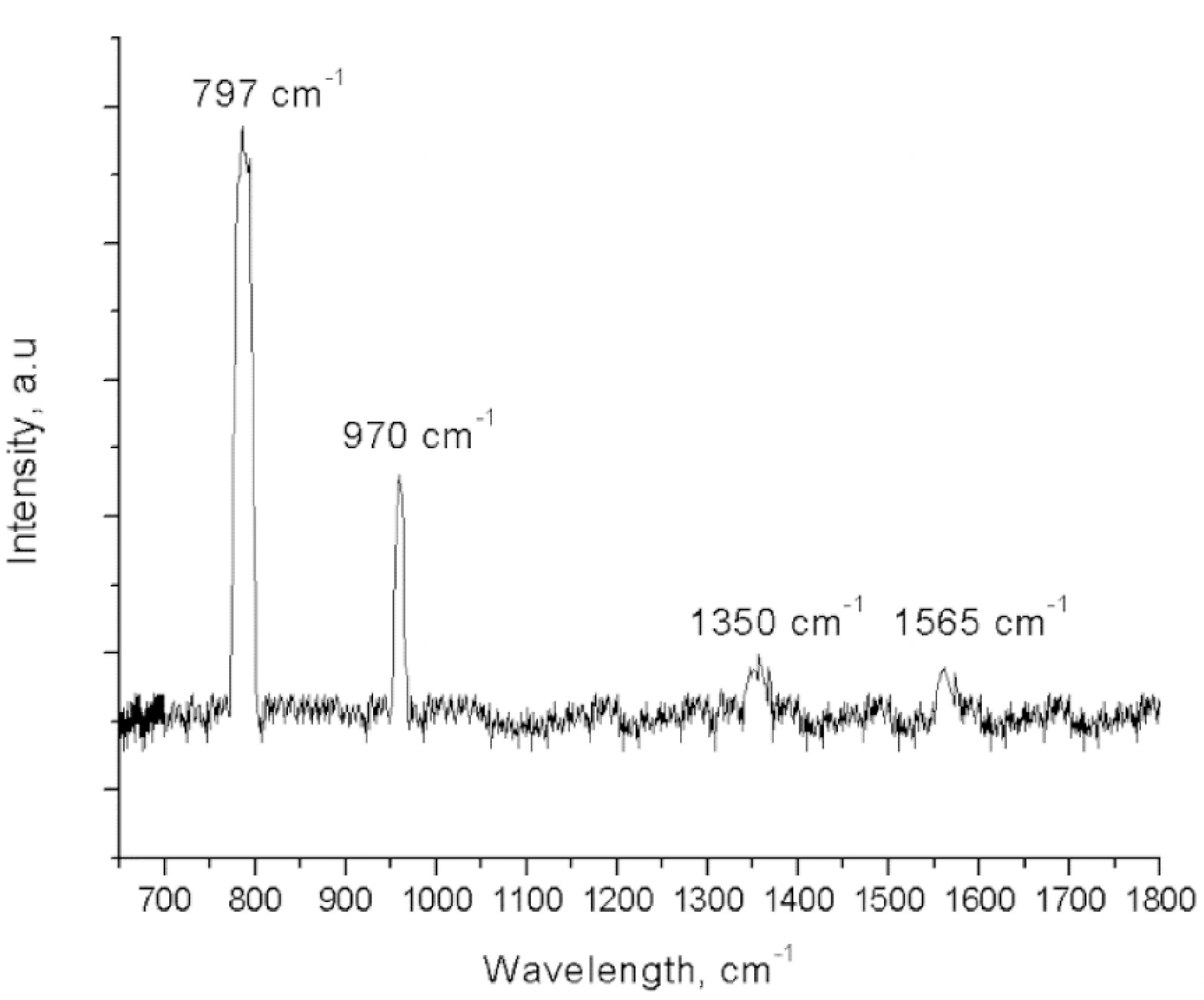
SiCNTs synthesized from blend of SiO2 particles and MWCNTs in the ratio of 1:3 shows peaks corresponded to β-SiC at 797 cm-1 and 970 cm-1, respectively in Raman spectrum in Figure 9. This peaks confirmed the presence of SiCNTs. Giorcelli et al4141 Giorcelli M, Pavese M, Shahzad MI, Tagliaferro A. Silicon carbide hollow cylinders using carbon nanotubes structures as template. Materials Letters. 2015;151:12-15. also showed similar result where peaks corresponded to transversal optical phonon (TO) and longitudinal optical phonon (LO) of 3C-SiC were observed at 797 cm-1 and 968 cm-1. Besides that, it can be observed that there are peaks corresponded to the CNTs at 1350 cm-1 and 1565 cm-1 which revealed the presence of small amount of residual of MWCNTs in SiCNTs. Pan et al4343 Pan Y, Zhu P, Wang X, Li S. Preparation and characterization of one-dimensional SiC-CNT composite nanotubes. Diamond and Related Materials. 2011;20(3):310-313. also reported similar Raman peaks which corresponded to the vibrations of carbon atoms with dangling bonds at 1346 cm-1 and 1568 cm-1 and thus revealed the presence of CNTs in SiC-CNTs composite. This result also show good agreement with XRD pattern of SiCNTs in ratio of 1:3 from Figure 3 (b) which shows the presence of very small amount of MWCNTs.
3.8 Growth mechanism of silicon carbide nanotubes (SiCNTs)
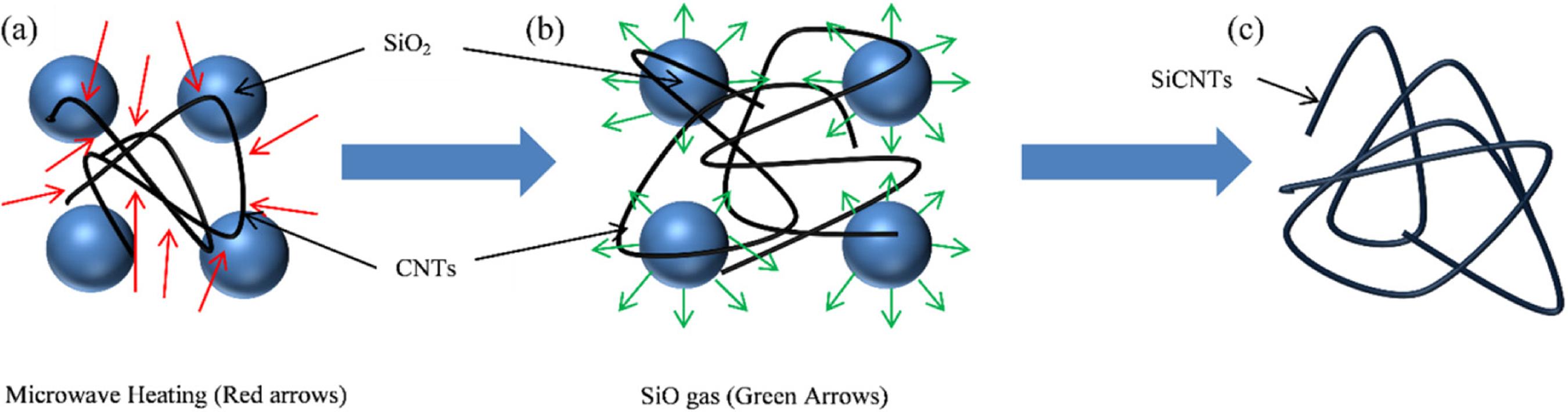
Several mechanisms for the growth of one dimensional nanostructure have been proposed such as vapor-liquid-solid (VLS)4444 Qi X, Liang J, Yu C, Ma S, Liu X, Xu B. Facile synthesis of interconnected SiC nanowire networks on silicon substrate. Materials Letters. 2014;116:68-70., vapor-solid (VS)4545 Wei J, Li K, Li H, Hou D, Zhang Y, Wang C. Large-scale synthesis and photoluminescence properties of hexagonal-shaped SiC nanowires. Journal of Alloys and Compounds. 2008;462(1-2):271-274. and solid-liquid-solid (SLS)4646 Czosnek C, Bućko MM, Janik JF, Olejniczak Z, Bystrzejewski M, Łabędź O, et al. Preparation of silicon carbide SiC-based nanopowders by the aerosol-assisted synthesis and the DC thermal plasma synthesis methods. Materials Research Bulletin. 2015;63:164-172. mechanisms. In this study, mechanism of vapor-solid (VS) was suggested to explain the synthesis of SiCNTs from the blend of SiO2 and MWCNTs at 1400 °C with heating rate of 30 °C/min and maintained for 40 minute. Synthesis by VS mechanism has advantages where it does not need catalyst for the formation of 1D morphology and thus no purification of the product is required. However, comparing to VLS mechanism, generally 1D material was not uniform and the diameter can vary over a wider range. The formation of SiCNTs involved three stages according to VS mechanism which are microwaves heating of blend of SiO2 and MWCNTs, vaporization of SiO2 to SiO gas and conversion of MWCNTs to SiCNTs.
In the first stage as shown in Figure 10 (a), the blend of SiO2 and MWCNTs was exposed to the microwave heating such that the temperature reached 1400 °C with heating rate of 30 °C/min and maintained for 40 minutes. When exposing to microwave irradiation, carbon materials such as MWCNTs were able to absorb the microwave irradiation as indicated by red arrows (Figure 10 (a)). Carbon materials can be heated by microwaves because of delocalized π electrons, which are free to move within the orbital structure of carbon. As the carbon continue to absorb the microwave energy, the kinetic energy of delocalized π electron increased and thus each carbon atom started to vibrate and move4747 Nwigboji IH, Ejembi JI, Wang Z, Bagayoko D, Zhao GL. Microwave absorption properties of multi-walled carbon nanotube (outer diameter 20-30nm)-epoxy composites from 1 to 26.5 GHz. Diamond and Related Materials. 2015;52:66-71.. These actions caused the heat to generate volumetrically after absorbing the microwave energy and thus temperature of carbon materials increased. Meanwhile, SiO2 is a microwave reflector, the microwave energy cannot penetrate the SiO2 due to characteristics of SiO2 being non-polar. Besides that, tetrahedral structure of SiO2 is bonded by strong covalent bonding between Si and O atom and its intermolecular force is very strong which caused the microwave energy cannot absorbed by the SiO24848 Peng Z, Hwang JY, Kim BG, Andriese M, Wang X. Microwave Dielectric Characterization of Silicon Dioxide. In: Hwang JY, Bai C, Carpenter J, Ikhmayies SJ, Li B, Monteiro SN, et al., eds. Characterization of Minerals, Metals, and Materials 2013. Hoboken: John Wiley & Sons; 2013. p. 389-395.. SiO2 particle was heated through the conduction of heat energy from carbon.
Schematic for the growth mechanism of SiCNTs from blend of SiO2 and MWCNTs in the ratio of 1:1, 1:3, 1:5 and 1:7 during exposing to microwave irradiation at 1400°C for 40 minute with heating rate of 30°C/min.
For the second stage, carbon reacted with the SiO2 particles at high temperature to form SiO gas (green arrows) and carbon monoxide (CO) gas (Figure 10 (b)) and the reaction is shown in Equation (3.2) below4949 Luo X, Ma W, Zhou Y, Liu D, Yang B, Dai Y. Synthesis and Photoluminescence Property of Silicon Carbide Nanowires Via Carbothermic Reduction of Silica. Nanoscale Research Letters. 2009;5(1):252-256.. This reaction is highly endothermic and required high temperature to occur.
SiO gas then further reacted with the remaining carbon to form SiCNTs with CO gas as the end of the reaction as shown in Figure 10 (c) in which only SiCNTs were synthesized. This reaction is shown by the Equation (3.3) as below and this was the last stage in the formation of SiCNTs in which each carbon atom in carbon molecule was bonded to 4 atoms of Si from the SiO gas and thus formed SiCNTs consisting of Si-C bond structure5050 Chiew YL, Cheong KY. A review on the synthesis of SiC from plant-based biomasses. Materials Science and Engineering: B. 2011;176(13):951-964..
This reaction of SiO2 particles and MWCNTs during the microwave heating of blend of SiO2 and MWCNTs can be concluded by overall chemical equation as shown in Equation (3.1) 5151 Cetinkaya S, Eroglu S. Chemical vapor deposition of C on SiO2 and subsequent carbothermal reduction for the synthesis of nanocrystalline SiC particles/whiskers. International Journal of Refractory Metals and Hard Materials. 2011;29(5):566-572.. The overall equation explained that the blend of SiO2 and MWCNTs in the ratio of 1:3 was the suitable ratio to synthesize SiCNTs.
4. Discussion
From the characterization and testing conducted by using XRD, FESEM, TEM, PL, FTIR, TGA and Raman spectroscopy, it can be observed that consistent results were obtained. Effective and rapid synthesis of SiCNTs can be obtained by using microwave heating assisted synthesis where special feature of the microwave heating which can rapidly produce heat through the interaction of the materials with microwave. By studying the blend ratio of the SiO2 particles and MWCNTs, high yield synthesis of SiCNTs can be achieved and thus produce high quality of SiCNTs. XRD pattern and FESEM images proved that ratio 1:3 of SiO2 particles and MWCNTs successfully synthesized high quality SiCNTs compared to others ratio in which high yield conversion of SiO2 particles and MWCNTs to SiCNTs was achieved. Similar with Bi et al2525 Bi S, Ma L, Mei B, Tian Q, Liu C, Zhong C, et al. Silicon carbide/carbon nanotube heterostructures: Controllable synthesis, dielectric properties and microwave absorption. Advanced Powder Technology. 2014;25(4):1273-1279. in their study synthesis of SiC/MWCNTs hetero-structures, they reported that the highest formation of SiCNTs was obtained from blend of SiO2 particles and MWCNTs in the ratio of 1:3. Furthermore, Zhang et al2323 Zhang J, Li W, Jia Q, Lin L, Huang J, Zhang S. Molten salt assisted synthesis of 3C-SiC nanowire and its photoluminescence properties. Ceramics International. 2015;41(10 Pt B):12614-12620. in their study of molten salt assisted synthesis of 3C-SiC nanowires also reported that blend of SiO2 particles and carbon in the ratio of 1:3 has showed the presence of XRD peak corresponded to β-SiC with highest intensity and indicated the high yield formation of SiC nanowires.
Besides that, it is worth mentioning that other advantages of synthesis of SiCNTs using microwave heating in this study are the minimal usage of chemical, simple procedure, environment friendly and economical method. Although, SiC has been successfully synthesized through carbothermal reduction using conventional heating5252 Keller N, Pham-Huu C, Ehret G, Keller V, Ledoux MJ. Synthesis and characterisation of medium surface area silicon carbide nanotubes. Carbon. 2003;41:2131-2139. and sol-gel5353 Najafi A, Fard FG, Rezaie HR, Ehsani N. Synthesis and characterization of SiC nano powder with low residual carbon processed by sol-gel method. Powder Technology. 2012;219:202-210. but, these processes are mainly energy consuming, require very high temperature, long heating duration and slow heating rate which in turn affect the synthesis of SiC. Obviously, the use of microwave heating for the synthesis of SiCNTs provides advantages such as rapid synthesis, simple procedure and no catalyst is required for the process. By study of the effect of ratio of raw materials, the high yield synthesis of the SiCNTs using microwave heating can be achieved and high quality SiCNTs could be obtained. This study also showed the novel way for the preparation and synthesis of SiCNTs using SiO2 particles and MWCNTs. Although, Bi et al and Quah et al also reported the similar study of the effect of different ratios of SiO2 particles and CNTs, in this study, we aimed to obtain high yield conversion of SiO2 particles and MWCNTs to SiCNTs by using microwave heating method which is more energy efficient and rapid comparing to conventional heating which is generally an energy and time consuming process.
5. Conclusions
For the first time, SiCNTs has been successfully synthesized from blend of SiO2 particles and MWCNTs in the ratios of 1:1, 1:3, 1:5 and 1:7 by using microwave heating as the new synthesis route which can synthesize high quality SiCNTs in a more time and energy efficient way. SiCNTs were characterized by using X-ray diffraction (XRD), field emission scanning electron microscopy (FESEM), transmission electron microscopy (TEM), photoluminescence spectroscopy (PL), fourier transform infrared (FTIR), thermal gravimetric analysis (TGA) and raman spectroscopy. The blend of SiO2 and MWCNTs in the ratio of 1:3 is the most suitable ratio for the synthesis of SiCNTs because of high yield conversion of SiO2 and MWCNTs was achieved and resulted in the formation of β-SiCNTs with only very small amount of residual unreacted SiO2 particles and MWCNTs. Besides that, the tubular structure of nanotube was completely retained and the band gap for SiCNTs was found to be 2.67 eV. These results evidenced the optimal condition for the generation of SiCNTs-based nanostructures. Through the implementation of the microwave heating in synthesis of SiC nano-materials, it has showed the novel way for the new processing method with simple procedure, high heating rate and low consumption of energy and thus improved the quality of synthesized SiC nano-materials compared to the currently used conventional heating method available for the synthesis of SiC nano-materials.
6. Acknowledgments
This work was supported by the Department of Higher Education, Ministry of Higher Education, and Malaysia [FRGS 9003-00441].
7. References
-
1Oliveros A, Guiseppi-Elie A, Saddow SE. Silicon carbide: a versatile material for biosensor applications. Biomedical Microdevices 2013;15(2):353-368.
-
2Zhou W, Yan L, Wang Y, Zhang Y. SiC nanowires: A photocatalytic nanomaterial. Applied Physics Letters 2006;89(1):013105.
-
3Mahdizadeh SJ, Goharshadi EK. Hydrogen storage on silicon, carbon, and silicon carbide nanotubes: A combined quantum mechanics and grand canonical Monte Carlo simulation study. International Journal of Hydrogen Energy 2014;39(4):1719-1731.
-
4Ribeiro S, Ribeiro GC, Oliveira MR. Properties of SiC Ceramics Sintered via Liquid Phase Using Al2O3 + Y2O3, Al2O3 + Yb2O3 and Al2O3 + Dy2O3 as Additives: a Comparative Study. Materials Research 2015;18(3):525-529.
-
5Wu R, Zhou K, Yue CY, Wei J, Pan Y. Recent progress in synthesis, properties and potential applications of SiC nanomaterials. Progress in Materials Science 2015;72:1-60.
-
6Bosi M, Attolini G, Negri M, Frigeri C, Buffagni E, Ferrari C, et al. Optimization of a buffer layer for cubic silicon carbide growth on silicon substrates. Journal of Crystal Growth 2013;383:84-94.
-
7Károly Z, Mohai I, Klébert S, Keszler A, Sajó IE, Szépvölgyi J. Synthesis of SiC powder by RF plasma technique. Powder Technology 2011;214(3):300-305.
-
8Xie Z, Tao D, Wang J. Synthesis of silicon carbide nanotubes by chemical vapor deposition. Journal of Nanoscience and Nanotechnology 2007;7(2):647-652.
-
9Yang Z, Xia Y, Mokaya R. High Surface Area Silicon Carbide Whiskers and Nanotubes Nanocast Using Mesoporous Silica. Chemistry of Materials 2004;16(20):3877-3884.
-
10Latu-Romain L, Ollivier M, Mantoux A, Auvert G, Chaix-Pluchery O, Sarigiannidou E, et al. From Si nanowire to SiC nanotube. Journal of Nanoparticle Research 2011;13(10):5425.
-
11Latu-Romain L, Ollivier M, Thiney V, Chaix-Pluchery O, Martin M. Silicon carbide nanotubes growth: an original approach. Journal of Physics D: Applied Physics 2013;46(9):092001.
-
12van Laar JH, Slabber JFM, Meyer JP, van Der Walt IJ, Puts GJ, Crouse PL. Microwave-plasma synthesis of nano-sized silicon carbide at atmospheric pressure. Ceramics International 2015;41(3 Pt B):4326-4333.
-
13Kahar SM, Voon CH, Lee CC, Gopinath SCB, Arshad MK, Lim BY, et al. Synthesis of silicon carbide nanowhiskers by microwave heating: effect of heating duration. Materials Research Express 2017;4(1):015005.
-
14Hashimoto S, Ohashi S, Hirao K, Zhou Y, Hyuga H, Honda S, et al. Mechanism for the formation of SiC by carbothermal reduction reaction using a microwave heating technique. Journal of the Ceramic Society of Japan 2011;119(10):740-744.
-
15Dzido G, Markowski P, Malachowska-Jutsz A, Prusik K, Jarzebski AB. Rapid continuous microwave-assisted synthesis of silver nanoparticles to achieve very high productivity and full yield: from mechanistic study to optimal fabrication strategy. Journal of Nanoparticle Research 2015;17:27.
-
16Galvão NKAM, Vasconcelos G, Santos MVR, Campos TMB, Pessoa RS, Guerino M, et al. Growth and Characterization of Graphene on Polycrystalline SiC Substrate Using Heating by CO2 Laser Beam. Materials Research 2016;19(6):1329-1334.
-
17Menéndez JA, Arenillas A, Fidalgo B, Fernández Y, Zubizarreta L, Calvo EG, et al. Microwave heating processes involving carbon materials. Fuel Processing Technology 2010;91(1):1-8.
-
18Shi L, Hu X, Huang Y. Fast microwave-assisted synthesis of Nb-doped Li4Ti5O12 for high-rate lithium-ion batteries. Journal of Nanoparticle Research 2014;16:2332.
-
19Oghbaei M, Mirzaee O. Microwave versus conventional sintering: A review of fundamentals, advantages and applications. Journal of Alloys and Compounds 2010;494(1-2):175-189.
-
20Li J, Shirai T, Fuji M. Rapid carbothermal synthesis of nanostructured silicon carbide particles and whiskers from rice husk by microwave heating method. Advanced Powder Technology 2013;24(5):838-843.
-
21Kim T, Lee J, Lee KH. Microwave heating of carbon-based solid materials. Carbon Letters 2014;15(1):15-24.
-
22Ding J, Deng C, Yuan W, Zhu H, Zhang X. Novel synthesis and characterization of silicon carbide nanowires on graphite flakes. Ceramics International 2014;40(3):4001-4007.
-
23Zhang J, Li W, Jia Q, Lin L, Huang J, Zhang S. Molten salt assisted synthesis of 3C-SiC nanowire and its photoluminescence properties. Ceramics International 2015;41(10 Pt B):12614-12620.
-
24Ding J, Zhu H, Li G, Deng C, Li J. Growth of SiC nanowires on wooden template surface using molten salt media. Applied Surface Science 2014;320:620-626.
-
25Bi S, Ma L, Mei B, Tian Q, Liu C, Zhong C, et al. Silicon carbide/carbon nanotube heterostructures: Controllable synthesis, dielectric properties and microwave absorption. Advanced Powder Technology 2014;25(4):1273-1279.
-
26Quah HJ, Cheong KY, Lockman Z. Stimulation of silicon carbide nanotubes formation using different ratios of carbon nanotubes to silicon dioxide nanopowders. Journal of Alloys and Compounds 2009;475(1-2):565-568.
-
27Kharissova OV, Kharisov BI. Variations of interlayer spacing in carbon nanotubes. RSC Advances 2014;4(58):30807-30815.
-
28Dai JX, Sha JJ, Zhang ZF, Wang YC, Krenkel W. Synthesis of high crystalline beta SiC nanowires on a large scale without catalyst. Ceramics International 2015;41(8):9637-9641.
-
29Lee KM, Hwang JY, Urban B, Singh A, Neogi A, Lee SK, et al. Origin of broad band emissions of 3C-silicon carbide nanowire by temperature and time resolved photoluminescence study. Solid State Communications 2015;204:16-18.
-
30Chen J, Tang W, Xin L, Shi Q. Band gap characterization and photoluminescence properties of SiC nanowires. Applied Physics A 2011;102(1):213-217.
-
31Nazarudin NFFB, Noor NJBM, Rahman SA, Goh BT. Photoluminescence and structural properties of Si/SiC core-shell nanowires growth by HWCVD. Journal of Luminescence 2015;157:149-157.
-
32Chiu SC, Li YY. SiC nanowires in large quantities: Synthesis, band gap characterization, and photoluminescence properties. Journal of Crystal Growth 2009;311(4):1036-1041.
-
33Paul R, Kumbhakar P, Mitra AK. Synthesis and study of photoluminescence characteristics of carbon nanotube/ZnS hybrid nanostructures. Journal of Experimental Nanoscience 2010;5(4):363-373.
-
34Yu Y, Yu JC, Chan CY, Che YK, Zhao JC, Ding L, et al. Enhancement of adsorption and photocatalytic activity of TiO2 by using carbon nanotubes for the treatment of azo dye. Applied Catalysis B: Environmental 2005;61(1-2):1-11.
-
35Lam SM, Sin JC, Abdullah AZ, Mohamed AR. Photocatalytic TiO2 /Carbon Nanotube Nanocomposites for Environmental Applications: An Overview and Recent Developments. Fullerenes, Nanotubes and Carbon Nanostructures 2014;22(5):471-509.
-
36Abdullahi N, Saion E, Shaari AH, Al-Hada NM, Keiteb A. Optimisation of the Photonic Efficiency of TiO2 Decorated on MWCNTs for Methylene Blue Photodegradation. PLoS One 2015;10(5):e0125511.
-
37Gui MM, Chai SP, Xu BQ, Mohamed AR. Visible-light-driven MWCNT@TiO2 core-shell nanocomposites and the roles of MWCNTs on the surface chemistry, optical properties and reactivity in CO2 photoreduction. RSC Advances 2014;4(46):24007-24013.
-
38Raman V, Bhatia G, Mishra AK, Bhardwaj S, Sood KN. Synthesis of silicon carbide nanofibers from pitch blended with sol-gel derived silica. Materials Letters 2006;60(29-30):3906-3911.
-
39Chen J, Shi Q, Xin L, Liu Y, Liu R, Zhu X. A simple catalyst-free route for large-scale synthesis of SiC nanowires. Journal of Alloys and Compounds 2011;509(24):6844-6847.
-
40Yang X, Zhao-hui C, Feng C. High-temperature protective coatings for C/SiC composites. Journal of Asian Ceramic Societies 2014;2(4):305-309.
-
41Giorcelli M, Pavese M, Shahzad MI, Tagliaferro A. Silicon carbide hollow cylinders using carbon nanotubes structures as template. Materials Letters 2015;151:12-15.
-
42Zhou X, Li X, Gao Q, Yuan J, Wen J, Fang Y, et al. Metal-free carbon nanotube-SiC nanowires heterostructures with enhanced photocatalytic H2 evolution under visible light irradiation. Catalysis Science & Technology 2015;5:2798-2806.
-
43Pan Y, Zhu P, Wang X, Li S. Preparation and characterization of one-dimensional SiC-CNT composite nanotubes. Diamond and Related Materials 2011;20(3):310-313.
-
44Qi X, Liang J, Yu C, Ma S, Liu X, Xu B. Facile synthesis of interconnected SiC nanowire networks on silicon substrate. Materials Letters 2014;116:68-70.
-
45Wei J, Li K, Li H, Hou D, Zhang Y, Wang C. Large-scale synthesis and photoluminescence properties of hexagonal-shaped SiC nanowires. Journal of Alloys and Compounds 2008;462(1-2):271-274.
-
46Czosnek C, Bućko MM, Janik JF, Olejniczak Z, Bystrzejewski M, Łabędź O, et al. Preparation of silicon carbide SiC-based nanopowders by the aerosol-assisted synthesis and the DC thermal plasma synthesis methods. Materials Research Bulletin 2015;63:164-172.
-
47Nwigboji IH, Ejembi JI, Wang Z, Bagayoko D, Zhao GL. Microwave absorption properties of multi-walled carbon nanotube (outer diameter 20-30nm)-epoxy composites from 1 to 26.5 GHz. Diamond and Related Materials 2015;52:66-71.
-
48Peng Z, Hwang JY, Kim BG, Andriese M, Wang X. Microwave Dielectric Characterization of Silicon Dioxide. In: Hwang JY, Bai C, Carpenter J, Ikhmayies SJ, Li B, Monteiro SN, et al., eds. Characterization of Minerals, Metals, and Materials 2013 Hoboken: John Wiley & Sons; 2013. p. 389-395.
-
49Luo X, Ma W, Zhou Y, Liu D, Yang B, Dai Y. Synthesis and Photoluminescence Property of Silicon Carbide Nanowires Via Carbothermic Reduction of Silica. Nanoscale Research Letters 2009;5(1):252-256.
-
50Chiew YL, Cheong KY. A review on the synthesis of SiC from plant-based biomasses. Materials Science and Engineering: B 2011;176(13):951-964.
-
51Cetinkaya S, Eroglu S. Chemical vapor deposition of C on SiO2 and subsequent carbothermal reduction for the synthesis of nanocrystalline SiC particles/whiskers. International Journal of Refractory Metals and Hard Materials 2011;29(5):566-572.
-
52Keller N, Pham-Huu C, Ehret G, Keller V, Ledoux MJ. Synthesis and characterisation of medium surface area silicon carbide nanotubes. Carbon 2003;41:2131-2139.
-
53Najafi A, Fard FG, Rezaie HR, Ehsani N. Synthesis and characterization of SiC nano powder with low residual carbon processed by sol-gel method. Powder Technology 2012;219:202-210.
Publication Dates
-
Publication in this collection
18 Sept 2017 -
Date of issue
Nov-Dec 2017
History
-
Received
12 Mar 2017 -
Reviewed
13 July 2017 -
Accepted
21 Aug 2017