Abstract
Methylmercury (MeHg) is present in the environment because of natural and anthropogenic causes. MeHg can reach the central nervous system (CNS) and cause neurological damage in humans and animals. Electric organ discharges (EODs) in the weak electric fish Gymnotus sylvius are produced by the electric organ and modulated by the CNS. These discharges are used for electrolocation and communication. The purpose of the present study was to investigate the effects of dietary MeHg exposure on EOD rate in G. sylvius. An oscilloscope was used to record the EOD rate. Two treatments were investigated: chronic MeHg administration (4 µg/kg MeHg every 2 days, with a total of nine dietary exposures to MeHg) and acute MeHg administration (a single dose of 20 µg/kg MeHg). The control data for both treatments were collected every 2 days for 18 days, with a total of nine sessions (day 1 until day 18). Data of fish exposed to MeHg were collected every 2 days, totaling nine sessions (day 19 until day 36). Chronic treatment significantly increased the EOD rate in G. sylvius (p < .05), especially with the final treatment (day 32 until day 36). Acute treatment resulted in an initial increase in the EOD rate, which was maintained midway through the experiment (day 26 until day 30; p < .05). The present study provides the first insights into the effects of MeHg on EODs in weak electric fish. The EOD rate is a novel response of the fish to MeHg administration.
electric organ discharge (EOD); methylmercury; electroreception; novel response
NEUROPSYCHOPHARMACOLOGY
Effects of methylmercury on electric organ discharges in the weak electric fish Gymnotus sylvius
Fernanda Dias de MoraesI; Caio MaximinoII; Fábio Alves de CarvalhoIII; Alceu Ferreira AlvesIV; Hugo Medeiros Garrido de PaulaIV,†; Amauri Gouveia Jr.II
IUniversidade Federal de São Carlos, São Carlos, SP, Brazil
IIUniversidade Federal do Pará, Belém, PA, Brazil
IIIUniversidade de São Paulo, Butantã, SP, Brazil
IVUniversidade Estadual Paulista, Bauru, SP, Brazil
†Deceased
Correspondence Correspondence: Fernanda Dias de Moraes Universidade Federal de São Carlos, Departamento de Genética e Evolução Rod. Washington Luiz Km 235 São Carlos, São Paulo CEP 13565-905, Brazil Phone: +55 016 33518376. Fax: +55 016 33518377 E-mail: fer.diasmoraes@gmail.com
ABSTRACT
Methylmercury (MeHg) is present in the environment because of natural and anthropogenic causes. MeHg can reach the central nervous system (CNS) and cause neurological damage in humans and animals. Electric organ discharges (EODs) in the weak electric fish Gymnotus sylvius are produced by the electric organ and modulated by the CNS. These discharges are used for electrolocation and communication. The purpose of the present study was to investigate the effects of dietary MeHg exposure on EOD rate in G. sylvius. An oscilloscope was used to record the EOD rate. Two treatments were investigated: chronic MeHg administration (4 µg/kg MeHg every 2 days, with a total of nine dietary exposures to MeHg) and acute MeHg administration (a single dose of 20 µg/kg MeHg). The control data for both treatments were collected every 2 days for 18 days, with a total of nine sessions (day 1 until day 18). Data of fish exposed to MeHg were collected every 2 days, totaling nine sessions (day 19 until day 36). Chronic treatment significantly increased the EOD rate in G. sylvius (p < .05), especially with the final treatment (day 32 until day 36). Acute treatment resulted in an initial increase in the EOD rate, which was maintained midway through the experiment (day 26 until day 30; p < .05). The present study provides the first insights into the effects of MeHg on EODs in weak electric fish. The EOD rate is a novel response of the fish to MeHg administration.
Keywords: electric organ discharge (EOD), methylmercury, electroreception, novel response.
Introduction
Mercury is present in the environment because of natural and anthropogenic events. Methylmercury (MeHg) is an organic form of mercury and the most toxic mercurial compound. The extensive use of MeHg in gold mining leads to aquatic pollution, which has been documented in Amazonian rivers (Akagi et al., 1995; Rabitto et al., 2011). MeHg is hazardous to wildlife and humans who consume contaminated fish. When MeHg reaches the aquatic environment, it can be incorporated into the food chain when absorbed by the gills or digestive tract of aquatic organisms (Pinheiro et al., 2000; Wang, Wong, & Wang, 2010; Dutton, & Fischer, 2011).
MeHg especially affects the nervous system, leading to recognized neurological diseases in humans and animals (Vilagi, Doczi, & Banczerowski-Pelyhe, 2000; Mieiro, Pereira, Duarte, & Pacheco, 2011). The mechanisms of MeHg neurotoxicity are still under investigation, but MeHg is known to disrupt protein synthesis by reacting with sulfhydryl (-SH) groups in enzymes and other small molecules such as glutathione (GSH; Bondy & Agrawal, 1980; Castoldi, Coccini, Ceccatelli, & Manzo, 2001; Farina, Rocha, Aschner, 2011). Alterations in GSH homeostasis can induce oxidative stress (Farina et al., 2011), leading to the oxidation of lipids, proteins, and DNA in mammals and fish (Grotto et al., 2011; Vicari, Ferraro, Ramsdorf, Mela, Ribeiro, & Cestari, 2012). Glutamate dyshomeostasis in the central nervous system (CNS) is another neurotoxic effect of MeHg (Aschner, Yao, Allen, & Tan, 2000; Farina et al., 2011). MeHg inhibits astrocytic glutamate uptake and increases glutamate release, leading to elevated extracellular glutamate levels and MeHg-induced excitotoxicity (Allen, Shanker, Tan, & Aschner, 2002; Aschner et al., 2000; Farina et al., 2011). Glutamate is a major excitatory neurotransmitter in the CNS, and extracellular glutamate accumulation can provoke overactivation of N-methyl-D-aspartate (NMDA)-type glutamate receptors, leading to an increase in Na2+ and Ca2+ influx into neurons and consequently the induction of cell death pathways (Farina et al., 2011).
Neurological effects associated with sensorial and behavioral disturbances in fish have been observed with MeHg accumulation in the nervous system (Baatrup, 1991; Hawryshyn & Mackay, 1979). The species Fundulus heteroclitus was exposed to MeHg and presented impairments in prey-capture behavior (Smith & Weis, 1997). Such an effect was attributed to reduced serotonin levels, which was also seen in Oreochromis niloticus when exposed to the mercurial compound (Tsai, Jang, & Wang, 1995). Schooling behavior and delayed spawning were observed in Notemigonus crysoteucas (Webber & Haines, 2003) and Pimephales promelas (Hammerschmidt, Sandheinrich, Wiener, & Rada, 2002), respectively, when they were contaminated with MeHg. MeHg also induces oxidative stress, alterations in serotonin levels, and anxiogenic-like behavior in Danio rerio (Maximino et al., 2011). The olfactory and visual systems in fish are also vulnerable to mercurial compounds (Baatrup, 1991; Tanan et al., 2006). Reports also indicate that MeHg intoxication affects neurophysiology and sensory-motor coordination in fish.
Gymnotus sylvius is a weak electric fish that emits low-voltage electric pulses at a discharge rate of 2070 Hz. The external morphology of this species is very similar to G. carapo, but it is biogeographically more restricted to central rivers in Brazil. In gymnotids, electric organ discharges (EODs) are used for electrolocation and communication (Hopkins, 1988; Stopa & Hoshino, 1999), which determines reproductive and non-reproductive interactions. The EOD is produced by electric organs situated along the lateral line, and the CNS is highly involved in the control of electric activity.
Despite the increased studies on the effects of MeHg in aquatic animals, no data are available about the effects of MeHg on electric activity in fish. The aim of the present study was to investigate the effects of dietary exposure to MeHg on EOD rate in G. sylvius, providing the first insights into the effects of MeHg on EODs in a weak electric fish.
Materials and Methods
Subjects
The experimental conditions and procedures were approved by the Research Ethics Committee of Universidade Estadual Paulista (UNESP) at the 12th Ordinary Meeting. Gymnotus fish, with an average weight of 29.7 ± 11.6 g and length of 15 ± 7 cm, were collected from the Tietê River, São Paulo state, Brazil. The fish were maintained under controlled laboratory conditions and kept individually in glass aquaria (.6 × .25 × .4 m) with hiding locations made of polyvinyl chloride tubes. The photoperiod (14 h light, 10 h dark), water temperature (23 ± 3ºC), pH (7.3 ± .5), and aeration (5 ± .5 mg dissolved O2) were controlled. Animals were fed daily with earthworms (Lumbricus sp.).
Cytogenetic analysis was conducted for species confirmation. The mitosis stimulation protocol was adapted to fish (Cole & Leavens, 1971; Oliveira, Almeida-Toledo, Foresti, & Toledo-Filho, 1988), and the mitotic chromosome preparation was described previously (Foresti, Oliveira, & Almeida-Toledo, 1993). The fish were anesthetized in an ice bath and sacrificed by transecting the spinal cord. All animals presented the 2n = 40 karyotype belonging to the species G. sylvius.
Apparatus
The EOD recordings were conducted in the Electric Engineering Laboratory (UNESP, Bauru). The animals were transferred to the laboratory on the days of data collection and individually kept in an aquarium (.3 × .14 × .2 m) with a polyvinyl chloride tube and placed inside a wire-mesh Faraday cage. The EODs were detected by two electrodes made of 50-Ώ impedance coaxial cables attached to the opposite ends of the hiding tube. A Tektronix digital oscilloscope (TDS2014) collected the signals from the electrodes and produced discharge rate curves that were stored on a computer. The EOD rates were obtained for each animal with minute-by-minute sampling for 30 continuous minutes per day. Therefore, 30 recordings were collected per fish per day.
Drug
MeHg chloride (CH3HgCl2) was purchased from Sigma-Aldrich (Saint-Quentin Fallavier, France). After the experiments, the contaminated material was acidified (pH < 2), neutralized in amide, and discarded for incineration.
Procedure
The chronic and acute effects of ingested MeHg chloride on EOD rate were investigated. The fish were exposed to MeHg through the ingestion of contaminated earthworms. The earthworms were injected with MeHg using insulin syringes at the time the fish were fed. Oral exposure to MeHg has important environmental significance because it is similar to the most common human exposure and avoids the discomfort of injections (Farina et al., 2011) and handling stress.
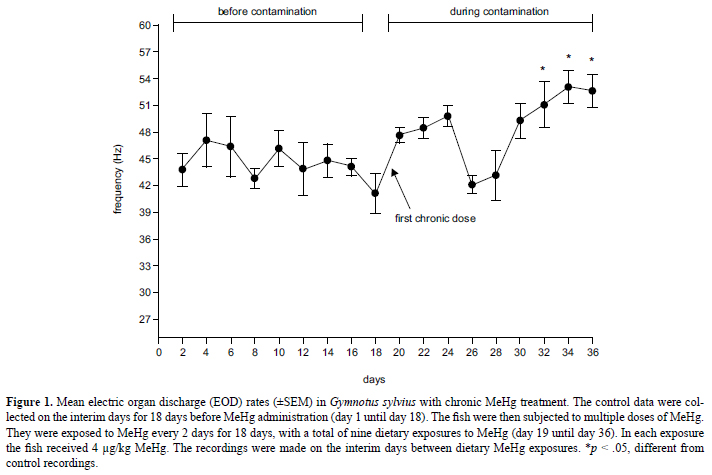
To evaluate the cumulative effects of MeHg administration over 18 days (day 19 until day 36), five fish (29.3 ± 9.0 g, 15.3 ± 2 cm) were subjected to multiple doses of MeHg (chronic treatment). They were exposed to MeHg every 2 days for 18 days, with a total of nine dietary exposures to MeHg. With each exposure, the fish were exposed to 4 µg/kg MeHg.
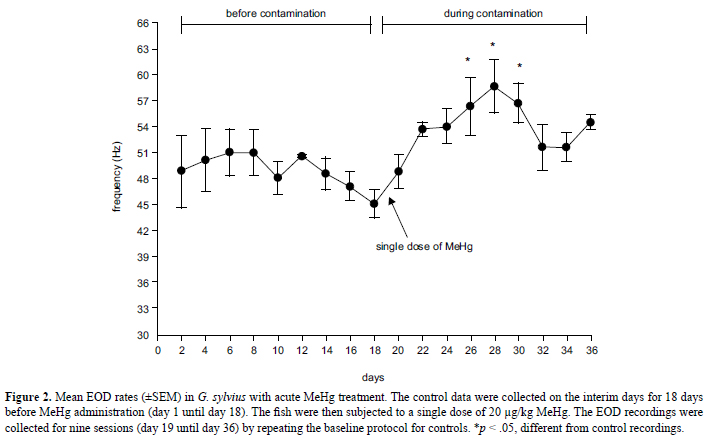
To evaluate the effect of methylmercury depuration over 18 days (day 19 until day 36), five fish (24.6 ± 13 g, 14.8 ± 2.5 cm) were subjected to a single dose of MeHg (acute treatment). They were exposed to 20 µg/kg MeHg.
Because all of the fish served as their own controls, the control data for both treatments were collected before dietary MeHg exposure. The control data for both treatments were collected every 2 days for 18 days (day 1 until day 18), with a total of nine sessions. In each session, the EOD recordings began immediately after a 5-min habituation period.
After the control EOD recordings, the fish were subjected to the acute and chronic experiments. The acutely treated animals were subjected to nine sessions of EOD recordings after MeHg administration, repeating the baseline protocol of the control sessions. The same occurred with the chronically treated animals, but the recordings were made on the interim days between dietary MeHg administration.
Statistical analysis
The 30 EOD rate measurements collected for each fish per day were averaged into one measure. The individual mean EOD rate was analyzed by comparing the data before and after MeHg administration for each treatment regimen. Each animal had its EOD rate recorded repeatedly after or during the treatments. Data were analyzed by one-way repeated-measures analysis of variance (ANOVA). The significance level was 95% (p < .05).
Results
Chronic MeHg treatment
The one-way repeated-measures ANOVA indicated that chronic MeHg treatment altered the EOD rate in G. sylvius (F = 5.01, df = 17, p < .001). The EOD rate exhibited oscillations during treatment (Figure 1). According to the Tukey post hoc test, the EOD rate significantly increased during the last three recordings (day 32 until day 36) when the estimated cumulative dose reached 36 µg/kg.
Acute MeHg treatment
One-way repeated-measures ANOVA indicated that acute MeHg treatment increased the EOD rate in G. sylvius (F = 3.63, df = 17, p < .001). From day 26 to day 30, the EOD rate significantly increased compared with the control recordings (Tukey test; p < .05; Figure 2). The prominent increase in EOD occurred on day 28 (Tukey test, p < .05).
Discussion
The effects of dietary MeHg administration were investigated on EOD rate in the weak electric fish G. sylvius using two treatment regimens. The chronic treatment was conducted to evaluate the consequences of small but constant exposure to MeHg, simulating trophic accumulation. The acute treatment was conducted to evaluate the effects of an elevated dose of MeHg and post-depuration events. In both treatments, MeHg altered the modulation of EOD, leading to an evident increase in the discharge rate of electric pulses.
Chronic MeHg treatment increased the EOD rate in G. sylvius, especially during the final stages of treatment. The progressively increasing pattern may be attributable to dose-dependent MeHg accumulation. The sequential doses of MeHg at short time intervals can accumulate in the organism (Oliveira-Ribeiro, Belger, Pelletier, & Rouleau, 2002) because of biomagnification caused by the high assimilation and low elimination rate of MeHg in fish (Wang et al., 2010; Dutton & Fischer, 2011). Horizontal cells in the retina in Hoplias malabaricus also presented increased responsiveness as a consequence of low-dose MeHg exposure (Tanan et al., 2006).
The acute treatment indicated that the MeHg depuration period affected the modulation of electric pulses in G. sylvius. Although the MeHg concentration was not quantified in G. sylvius tissues, it likely reached the brain of the fish, leading to alterations in the neural control of the EOD rate. MeHg is totally retained in several fish species, including 7 days after depuration in Oreochromis niloticus (Wang et al., 2010), 9 days after feeding on worms contaminated with MeHg in Fundulus heteroclitus (Dutton & Fischer, 2011), and 15 days after depuration in Salmo gairdneri (Hawryshyn & Mackay, 1979). MeHg can accumulate in the brain in S. gairdneri (Hawryshyn & Mackay, 1979) and gills, viscera, skin, liver, brain, and muscle in Fundulus heteroclitus, which presents the highest concentration of MeHg after 9 days of dietary exposure (Dutton & Fischer, 2011).
The increase in the EOD rate observed with both treatments in the present study can be related to MeHg neurotoxicity that occurs through an excitotoxic mechanism. Electric organ discharges are modulated by a series of neural nuclei including the electrosensorius nucleus, diencephalic pre-pacemaker nucleus, and bulbar pacemaker nucleus in the brain as described in G. carapo (Correa & Hoffman, 1999). This circuit possesses γ-aminobutyric acid (GABAergic) and glutamatergic projections in which the respective neurotransmitters interact with GABAA and NMDA receptors. Glutamate increases the EOD rate, and GABA reduces the EOD rate in the gymnotiform electric fish Hypopomus brevirostris (Kawasaki & Heiligenberg, 1990). MeHg neurotoxicity is related to glutamate dyshomeostasis in the CNS, which provokes extracellular glutamate accumulation in astrocytes. This phenomenon occurs by inhibiting astrocytic glutamate uptake and increasing glutamate release (Allen et al., 2002; Aschner et al., 2000; Farina et al., 2011). If MeHg mediates excitotoxicity via accumulation of extracellular glutamate levels, then the increase in EOD rate in G. sylvius may be a consequence of MeHg-induced excitotoxicity. The fine-tuning of the EOD rate is crucial for electrical communication among Gymnotus species, which determines reproductive and non-reproductive interactions and affects social and ecological aspects (Gouvêa Junior, Stopa, Paula, & Hoshino, 2002).
In summary, dietary MeHg administration affected the EOD in the weak electric fish G. sylvius, inducing a significant dose- and depuration-dependent increase in the EOD rate. Future investigations are needed to elucidate the effects of MeHg on glutamate homeostasis in the brain in G. sylvius and the direct effects of MeHg on the electric organ in the fish. The present study points to the EOD rate as novel response of fish to MeHg administration. Measuring EODs is inexpensive, useful, and non-invasive and may be used to monitor mercurial contamination in water and animals, especially in Amazonian rivers.
Acknowledgements
The authors are grateful to FAPESP (São Paulo Research Foundation, Process no. 04/14160-0) and CNPq (National Council for Scientific and Technological Development, Process no. 402817/2004-4). We also thank Helton Gonzales and Nelson Medeiros for technical assistance and Prof. Dr. Fábio Foresti and Diogo Teruo Hashimoto for assistance with the cytogenetic analysis.
Received 16 October 2012
Received in revised form 4 May 2013
Accepted 18 May 2013
Available online 27 June 2013
- Akagi, H., Malm, O., Kinjo, Y., Harada, M., Branches, F. J. P., Pfeiffer, W. C., & Kato, H. (1995). Methylmercury pollution in the Amazon, Brazil. Science of the Total Environment, 175, 85-95.
- Allen, J. W., Shanker, G., Tan, K. H., & Aschner, M. (2002). The consequences of methylmercury exposure on interactive functions between astrocytes and neurons. Neurotoxicology, 23, 755-759.
- Aschner, M., Yao, C. P., Allen, J. W., & Tan, K. H. (2000). Methylmercury alters glutamate transport in astrocytes. Neurochemistry International, 37, 199-206.
- Baatrup, E. (1991). Structural and functional effects of heavy metals on the nervous system, including sense organs, of fish. Comparative Biochemistry and Physiology Part C: Comparative Pharmacology, 100, 253-257.
- Bondy, S. C., & Agrawal, A. K. (1980). The inhibition of cerebral high affinity receptor sites by lead and mercury compounds. Archives of Toxicology, 46, 249-256.
- Castoldi, A. F., Coccini, T., Ceccatelli, S., & Manzo, L. (2001). Neurotoxicity and molecular effects of methylmercury. Brain Research Bulletin, 55, 197-203.
- Cole, C. J., & Leavens, C. R. (1971). Chromosome preparations of amphibians and reptiles: Improved technique. Herpetological Review, 3, 102.
- Correa, S. A. L., & Hoffmann, A. (1999). Effects of season and arousal state on the novelty response in Gymnotus carapo In A. L. Val, & V. M. F. Almeida e Val (Eds.), Biology of tropical fishes (pp. 149-160). Manaus: INPA.
- Dutton, J., & Fischer, N. S. (2011). Bioaccumulation of As, Cd, Cr, Hg (II), and MeHg in killfish (Fundulus heteroclitus) from amphipod and worm prey. Science of the Total Enviromment, 409, 3438-3447.
- Farina, M., Rocha, J. B. T., & Aschner, M. (2011). Mechanisms of methylmercury-induced neurotoxicity: Evidence from experimental studies. Life Sciences, 89, 555-563.
- Foresti, F., Oliveira, C., & Almeida-Toledo, L. F. (1993). A method for chromosome preparations from large fish specimens using in vitro short-term treatment with colchicine. Experientia, 49, 810-813.
- Gouvêa Junior, F., Stopa, R. M., Paula, H. M. G., & Hoshino, K. (2002). Suspensão das descargas de eletrolocação-comunicação e tamanho corporal no peixe-elétrico Gymnotus carapo Miller, 1966 (Osteichtyes, Gymnotidae). Revista Brasileira de Zoociências, 4, 203-214.
- Grotto, D., Valentini, J., Serpeloni, J. M., Monteiro, P. A. P., Latorraca, E. F., de Oliveira, R. S., ... Barbosa, F., Jr. (2011). Evaluation of toxic effects of a diet containing fish contaminated with methylmercury in rats mimicking the exposure in the Amazon riverside population. Environmental Research, 111, 1074-1082.
- Hammerschmidt, C. R., Sandheinrich, M. B., Wiener, J. G., & Rada, R. G. (2002). Effects of dietary methylmercury on reproduction of fathead minnows. Environmental Science and Technology, 36, 877-883.
- Hawryshyn, C. W., & Mackay, W. C. (1979). Toxicity and tissue uptake of methylmercury administered intraperitoneally to rainbow trout (Salmo gairdneri Richardson). Bulletin of Environmental Contamination and Toxicology, 23, 79-86.
- Hopkins, C. D. (1988). Neuroethology of electric communication. Annual Review of Neuroscience, 11, 497-535.
- Kawasaki M., & Heiligenberg W. (1990). Different classes of glutamate receptors and GABA mediate distinct modulations of a neuronal oscillator, the medullary pacemaker of a gymnotiform electric fish. Journal of Neuroscience, 10, 3896-3904.
- Mieiro, C. L., Pereira, M. E., Duarte, A. C., & Pacheco, M. (2011). Brain is a critical target in environmentally exposed fish (Dicentrarchus labrax): Bioaccumulation and oxidative stress profiles. Aquatic Toxicology, 103, 233-240.
- Oliveira, C., Almeida-Toledo, L. F., Foresti, F., & Toledo-Filho, S. A. (1988). Supernumerary chromosomes, Robertsonian rearrangements and multiple NORs in Corydoras aeneus (Pisces, Siluriformes, Callichthyidae). Caryologia, 41, 227-236.
- Oliveira-Ribeiro, C. A., Belger, L., Pelletier, E., & Rouleau, C. (2002). Histopathological evidence of inorganic mercury and methyl mercury toxicity in the arctic charr (Salvelinus alpinus). Environmental Research, 90, 217-225.
- Pinheiro, M. C. M., Guimarães, G. A., Nakanishi, J., Oikawa, T., Vieira, J. L., Quaresma, M., Cardoso, B., & Amoras, W. (2000). Total mercury in hair samples of inhabitants of Tapajós River, Pará State, Brazil. Revista da Sociedade Brasileira de Medicina Tropical, 33, 181-184.
- Rabitto, I. S., Bastos, W. R., Almeida, R., Anjos, A., Holanda, I. B. B., Galvão, R. C. F., ... Ribeiro, C. A. O. (2011). Mercury and DDT exposure risk to fish-eating human populations in Amazon. Environment International, 37, 56-65.
- Smith, G. M., & Weis, J. S. (1997). Predator-prey relationships in mummichogs (Fundulus heteroclitus (L.)): Effects of living in a polluted environment. Journal of Experimental Marine Biology and Ecology, 209, 75-87.
- Stopa, R. M., & Hoshino, K. (1999). Electrolocation-communication discharges of the fish Gymnotus carapo L. (Gymnotidae: Gymnotiformes) during behavioral sleep. Brazilian Journal of Medical and Biological Research, 32, 1223-1228.
- Tanan, C. L., Ventura, D. F., Souza, J. M., Grotzner, S. R., Mela, M., Gouveia, A., Jr., & Oliveira-Ribeiro, C. A. (2006). Effects of mercury intoxication on the response of horizontal cells of the retina of thraira fish (Hoplias malabaricus). Brazilian Journal of Medical and Biological Research, 39, 987-995.
- Tsai, C. L., Jang, T. H., & Wang, L. H. (1995). Effects of mercury on serotonin concentration in the brain of tilapia, Oreochromis mossambicus Neuroscience Letters, 184, 208-211.
- Vicari, T., Ferraro, M. V. M., Ramsdorf, W. A., Mela, M., Ribeiro, C. A. O., & Cestari, M. M. (2012). Genotoxic evaluation of different doses of methylmercury (CH3Hg+) in Hoplias malabaricus Ecotoxicology and Environmental Safety, 82, 47-55.
- Vilagi, I., Doczi, J., & Banczerowski-Pelyhe, I. (2000). Altered electrophysiological characteristics of developing rat cortical neurones after chronic methylmercury chloride treatment. International Journal of Developmental Neuroscience, 18, 493-499.
- Wang, R., Wong, M. H., & Wang, W. X. (2010). Mercury exposure in the freshwater tilapia Oreochromis niloticus Environmental Pollution, 158, 2694-2701.
- Webber, H. M., & Haines, T. A. (2003). Mercury effects on predator avoidance behavior of a forage fish, golden shiner (Notemigonus crysoteucas). Enviromental Toxicology and Chemistry, 22, 1556-1561.
Publication Dates
-
Publication in this collection
02 Oct 2013 -
Date of issue
June 2013
History
-
Received
16 Oct 2012 -
Accepted
18 May 2013 -
Reviewed
04 May 2013