Abstract
This work evaluated the effect of grape juice, red wine and resveratrol in liver parameters of rats submitted to high-fat diet. Experimental model was conducted with groups of adult females Rattus norvegicus: control (CG); high-fat (HG); grape juice (JG); red wine (RW) and resveratrol solution (RG). The high-fat diet significantly altered hepatocytes and Kupffer cells in all treated groups. HG group presented severe steatosis followed hepatocyte ballooning and tissue damages. JG group minimized hepatic histological lesion caused by high-fat diet and WG group also induced steatosis and inflammation in hepatocytes, similar to HG. Still, resveratrol protected the tissue against fatty liver disease by reducing fat infiltration and inflammation, indicating possible therapeutic effects on the liver. Cell cycle analysis showed that HG promoted damage to the tissue, reducing the viable cell content and increasing apoptosis, even when associated with wine consumption or isolated resveratrol. However, JG protected the liver against cell damage generated by the diet. Consumption of grape juice, even associated with a high-fat diet, represents a promising protection of the liver against cellular damage, but red wine further affects the tissue, and resveratrol alone was able to reduce damage but did not minimize cellular damage to the liver.
Key words
Grape juice; high-fat diet; liver; resveratrol; wine
INTRODUCTION
Diets play major role in both health care and disease progression (Pereira et al. 2014PEREIRA RA, DUFFEY KJ, SICHIERI R & POPKIN BM. 2014. Sources of excessive saturated fat, trans fat and sugar consumption in Brazil: an analysis of the first Brazilian nationwide individual dietary survey. Public Health Nutr 17: 113-121., Milić et al. 2014MILIĆ S, LULIĆ D & ŠTIMAC D. 2014. Non-alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations. WJG 20: 9330.). The Consumption of a high-fat diet is associated with increased body weight, metabolic changes, such as inflammation and oxidative stress, cell damage as well as chronic non-transmissible diseases involving various tissues such as the liver. (Milić et al. 2014MILIĆ S, LULIĆ D & ŠTIMAC D. 2014. Non-alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations. WJG 20: 9330., Oarada et al. 2012OARADA M, TSUZUKI T, NIKAWA T, KOHNO S, HIRASAKA K & GONOI T. 2012. Refeeding with a high-protein diet after a 48 h fast causes acute hepatocellular injury in mice. Br J Nutr 107: 1435-1444., Bedê et al. 2015BEDÊ TP, PASCOAL AC, FACÓ LH, DE SALVO CASTRO E, MATTOSO V, DIAS JF & DE AZEREDO VB. 2015. Effect of the intake of liquids rich in polyphenols on blood pressure and fat liver deposition in rats submitted to high-fat diet. Nutr Hosp 31: 2539-2545., Lozano et al. 2016LOZANO I ET AL. 2016. High-fructose and high-fat diet-induced disorders in rats: impact on diabetes risk, hepatic and vascular complications. Nutr Metab 13: 15.).
Excessive intake of lipids induces increased flow and oxidation of fatty acids in the liver with increased fat deposition in hepatocytes and promoted inflammation and lipotoxicity contributing to cell malfunction, cell death and cell lesions (Cohen et al. 2011COHEN JC, HORTON JD & HOBBS HH. 2011. Human fatty liver disease: old questions and new insights. Science 332: 1519-1523.). Associated with a high-fat diet, alcohol consumption is also reported as an etiological factor for hepatic steatosis, as well as other drugs or toxins (Noureddin & Rinella 2015NOUREDDIN M & RINELLA ME. 2015. Nonalcoholic fatty liver disease, diabetes, obesity, and hepatocellular carcinoma. Clin Liver Dis 19: 361-379.). Excessive fat deposition in the liver causes mitochondrial dysfunction and fatty acids oxidation produces high production of reactive oxygen species (ROS) as side product of that causes damage to cellular membranes, proteins, lipids and deoxyribonucleic acid (DNA) (Noureddin & Rinella 2015NOUREDDIN M & RINELLA ME. 2015. Nonalcoholic fatty liver disease, diabetes, obesity, and hepatocellular carcinoma. Clin Liver Dis 19: 361-379., Reynés 2015REYNÉS B, PALOU M & PALOU A. 2017. Gene expression modulation of lipid and central energetic metabolism related genes by high-fat diet intake in the main homeostatic tissues. Food Funct 8: 629-650.).
Chronic hepatic lipid accumulation results in non-alcoholic fatty liver disease (NAFLD) that may progress to non-alcoholic steatohepatitis (NASH), a condition characterized by chronic inflammation and fibrosis, associated with overweight. Hepatocytes respond to lesions by various mechanisms, such as apoptosis, non-apoptotic death and cellular autophagy (Widiker et al. 2010, Leung et al. 2016LEUNG A, TRAC C, DU J, NATARAJAN R & SCHONES D E. 2016. Persistent chromatin modifications induced by high fat diet. J Biol Chem 291: 10446-10455.). However, external environmental factors have been studied, such as nutrients that damage or stimulate hepatic cells for regeneration. (Dudley et al. 2011DUDLEY KJ, SLOBODA DM, CONNOR KL, BELTRAND J & VICKERS MH. 2011. Offspring of mothers fed a high fat diet display hepatic cell cycle inhibition and associated changes in gene expression and DNA methylation. PLoS ONE 6: 21662., Kim et al. 2011KIM S, JIN Y, CHOI Y & PARK T. 2011. Resveratrol exerts anti-obesity effects via mechanisms involving down-regulation of adipogenic and inflammatory processes in mice. Biochem Pharmacol 81: 1343-1351.). The high-fat diet leads to epigenetic changes in the hepatic tissue cell cycle (Czaja et al. 2013CZAJA MJ ET AL. 2013. Functions of autophagy in normal and diseased liver. Autophagy 9: 1131-1158.). Excess dietary fat leads to changes in chromatin and intracellular protein activation that influence the progression of different cycle phases. This can have a major impact on phenotypic results making cells vulnerable to apoptosis and favoring the inflammatory response in tissues (Mizushima et al. 2008MIZUSHIMA N, LEVINE B, CUERVO AM & KLIONSKY DJ. 2008. Autophagy fights disease through cellular self-digestion. Nature 451: 1069., Wellenk & Thompson 2010). Oxidative stress triggered by ROS overproduction also activates the lysosomal cell death pathway and results in cytotoxicity, leading to hepatic inflammation (Noureddin & Rinella 2015NOUREDDIN M & RINELLA ME. 2015. Nonalcoholic fatty liver disease, diabetes, obesity, and hepatocellular carcinoma. Clin Liver Dis 19: 361-379., Bantel 2012BANTEL H. 2012. Mechanisms of cell death in acute liver failure. Front Physiol 3: 79.).
Food consumption give several bioactive compounds that have health benefits (Laliena et al. 2012LALIENA A, MIGUEL B S, CRESPO I, ALVAREZ M, GONZÁLEZ-GALLEGO J & TUÑÓN M J. 2012. Melatonin attenuates inflammation and promotes regeneration in rabbits with fulminant hepatitis of viral origin. J Pineal Res 53: 270-278., Yuzefovych et al. 2013YUZEFOVYCH LV, MUSIYENKO SI, WILSON GL & RACHEK LI. 2013. Mitochondrial DNA damage and dysfunction, and oxidative stress are associated with endoplasmic reticulum stress, protein degradation and apoptosis in high fat diet-induced insulin resistance mice. PLoS ONE 8: e54059., Charradi et al. 2013CHARRADI K, ELKAHOUI S, LIMA M & AOUANI E. 2013. High-fat diet induced an oxidative stress in white adipose tissue and disturbed plasma transition metals in rat: prevention by grape seed and skin extract. J Physiol Sci 63: 445-455., Rahal et al. 2014RAHAL A, KUMAR A, SINGH V, YADAV B, TIWARI R, CHAKRABORTY S & DHAMA K. 2014. Oxidative stress, prooxidants, and antioxidants: the interplay. Biomed Res Int 2014: 19 p., Ghanim et al. 2011GHANIM H, SIA C L, KORZENIEWSKI K, LOHANO T, ABUAYSHEH S, MARUMGANTI A, CHAUDHRI T & DANDONA P. 2011. A resveratrol and polyphenol preparation suppresses oxidative and inflammatory stress response to a high-fat, high-carbohydrate meal. J Clin Endocrinol Metab 96: 1409-1414.). Grapes are rich in polyphenols, whose consumption presents health benefits, which makes the fruit known for its functional properties. Studies in the liver tissue show that grape compounds provide tissue with pro-ion in both disease prevention and disease development. (Poljsak 2011POLJSAK B. 2011. Strategies for reducing or preventing the generation of oxidative stress. Oxid Med Cell Longev 2011: 15 p., Cunha et al. 2016CUNHA AL, MOURA KS, BARBOSA JC & DOS SANTOS AF. 2016. Os metabólitos secundários e sua importância para o organismo. Diversitas Journal 1: 175-181.).
In this case, beverages derived from grapes, such as red wine and whole red grape juice, present a complex range of phenolic compounds, such as anthocyanins, resveratrol and quercetin, known not only for their strong antioxidant effect, but also for the prevention of oxidative reactions and formation of free radicals, and for their anti-proliferative and anti-inflammatory effects (Rindler et al. 2013RINDLER PM, PLAFKER SM, SZWEDA L & KINTER M. 2013. High dietary fat selectively increases catalase expression within cardiac mitochondria. J Biol Chem 288: 1979-1990., Giovinazzo & Grieco 2015GIOVINAZZO G & GRIECO F. 2015. Functional properties of grape and wine polyphenols. Plant Foods Hum Nutr 70: 454-462., Singh et al. 2016SINGH CK, SIDDIQUI IA, EL-ABD S, MUKHTAR H & AHMAD N. 2016. Combination chemoprevention with grape antioxidants. Mol Nutr Food Res 60: 1406-1415.).
Experimental models of diet-induced hepatotoxicity have contributed to elucidate the pathophysiology of various hepatic diseases and also aim to identify and evaluate possible hepatoprotective dietary agents (Milić et al. 2014MILIĆ S, LULIĆ D & ŠTIMAC D. 2014. Non-alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations. WJG 20: 9330., Oarada et al. 2012OARADA M, TSUZUKI T, NIKAWA T, KOHNO S, HIRASAKA K & GONOI T. 2012. Refeeding with a high-protein diet after a 48 h fast causes acute hepatocellular injury in mice. Br J Nutr 107: 1435-1444., Bedê et al. 2015BEDÊ TP, PASCOAL AC, FACÓ LH, DE SALVO CASTRO E, MATTOSO V, DIAS JF & DE AZEREDO VB. 2015. Effect of the intake of liquids rich in polyphenols on blood pressure and fat liver deposition in rats submitted to high-fat diet. Nutr Hosp 31: 2539-2545., Lozano et al. 2016LOZANO I ET AL. 2016. High-fructose and high-fat diet-induced disorders in rats: impact on diabetes risk, hepatic and vascular complications. Nutr Metab 13: 15.). It is therefore important to study the possible effect of consuming polyphenol-rich beverages under the liver that are attacked by a high-fat diet, evaluating their potential in liver cell regeneration.
Therefore, the aim of this study was to evaluate the effect of whole grape juice, red wine and resveratrol solution isolated in the hepatic tissue of rats submitted to the high fat diet, in relation to histological, cell cycle and apoptosis parameters.
MATERIALS AND METHODS
Experimental design and sampling
The study was conducted in the Laboratory of Experimental Nutrition at Department of Nutrition and Dietetics of Federal Fluminense University (LabNE-UFF). All animal procedures were approved by the Animal Ethics Committee from Federal Fluminense University (protocol under number 00216/10).
A total of 50 female Wistar rats, all adults (90 days), weighing 200±20g obtained at the LabNE-UFF were kept in cages in a controlled environment (24°±2°C, with a 12 h daylight cycle). The experiment lasted for 8 weeks.
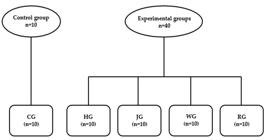
Animals were randomly divided into five groups (n=10/group): 1) Control Group (CG): received standard diet (4% fat) based on the AIN-93M (Reeves et al 1993); 2) High- fat group (HG): received high-fat diet (20% fat); 3) Grape juice group (JG): received high fat diet (20% fat) and whole grape juice (15 mL/day); 4) Red wine group (RW): received high fat diet (20% fat) and red wine (10 mL/day); 5) Resveratrol solution group (RG): received high fat diet (20% fat) and resveratrol solution (15 mL/day – 40 mg/L). All animals were given access to feed and water ad libitum. Juice, wine and resveratrol solution were offered in individual bottles. This dietary protocol is illustrated in Figure 1 and the Table I shows the composition of the diets of the different groups.
Dietary protocol. Experimental model: (CG) Control group supplemented with control feed and water; (HG) High-fat diet group received high-fat diet and water; (JG) Grape juice group represents animals submitted to high-fat diet treated with grape juice (15 mL/day) and water; (WG) Wine group represents animals submitted to high-fat diet treated with red wine (10 mL/day) and water; (RG) Resveratrol group represents animals submitted to high-fat diet treated with resveratrol solution isolated (15 mL/day - 40 mg/L) and water. Diets and solutions were administered during a period of 60 days.
Red wine (Cabernet Sauvignon) and grape juice were obtained from local market and were kept refrigerated (10 °C) during the study. Considering the concentration of total polyphenols of the red wine (1014-3718 mg gallic acid equivalent (GAE)/L, mean 2366 mg GAE/L) and whole grape juice (1617–2213, mean 1915 mg GAE/L ), the volume offered to the animals of grape juice (15mL/day) and wine (10mL/day) was determined to resemble the amount of polyphenols in the two drinks (Oliveira et al. 2009OLIVEIRA ACD, VALENTIM IB, GOULART MOF, SILVA CA, BECHARA EJH & TREVISAN MTS. 2009. Fontes vegetais naturais de antioxidantes. Quím Nova 32: 689-702., Roesler et al. 2007ROESLER R, MALTA LG, CARRASCO LC, HOLANDA RB, SOUSA CAS & PASTORE GM. 2007. Atividade antioxidante de frutas do cerrado. Food Sci Technol 27: 53-60., Sautter et al. 2005SAUTTER CK, DENARDIN S, ALVES AO, MALLMANN CA, PENNA NG & HECKTHEUER L H. 2005. Determinação de resveratrol em sucos de uva no Brasil. Ciênc Tecnol Aliment 25: 437-442.).
Resveratrol solution was prepared daily in the laboratory by dissolving the content in distilled water and adding 5% refined sugar. Final concentration (40 mg/L) of resveratrol solution was based in previous study, making the proportion of the dose to the weight of the animal (Timmers et al. 2011TIMMERS S ET AL. 2011. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab 14: 612-622.).
Body weight and chow intake of animals were recorded weekly using BioPrecisa® (B15- 0.5, Labmais Ltda, PR, Brazil) precision scale. Beverage consumption (water, whole red grape juice, red wine and resveratrol solution) were measured daily by graduate beaker.
At the end of the experiment, the animals were exposed to vaginal smear to determine their stage of the estrous cycle and to ensure that there was no hormonal intervention in the research at the same physiological moment. Animals in estrus phase are isolated and after 6 hours of fasting anesthetized by intraperitoneal injection of a solution containing 11.50 mg/100 g ketamine body mass and 0.10 mg/100 g xylazine body mass and exsanguinated by cardiac puncture, according to Hem et al. (1998)HEM A, SMITH AJ & SOLBERG P. 1998. Saphenous vein puncture for blood sampling of the mouse, rat, hamster, gerbil, guineapig, ferret and mink. Lab Anim 32: 364-368..
The hepatic tissue was carefully removed, weighed with a precision scale of BioPrecisa ® and the relative weight of the organ, denominated liver index, was calculated according to the equation: liver index = liver Weight (g) X 100/ body Weight (g). After weighing, four fragments of different lobes hepatics were sectioned with a surgical scalpel of each animal and immediately treated for cell cycle analysis, apoptosis assay and histological analysis (Guimarães et al. 2017GUIMARÃES DDAB, DE CASTRO DDSB, OLIVEIRA FLD, NOGUEIRA EM, SILVA MAMD & TEODORO AJ. 2017. Pitaya Extracts Induce Growth Inhibition and Proapoptotic Effects on Human Cell Lines of Breast Cancer via Downregulation of Estrogen Receptor Gene Expression. Oxid Med Cell Longev 2017: 13 p.).
Histological analysis
The livers were fixed in Bouin’s solution for 24 hours and processed in graded of ethanol (70-100%) for 30 minutes each and washed twice in xylene for 15 minutes. Hepatic fragments were then individually embedded in paraffin, the blocks were cross-sected into 4μm thick slices, then stained with hematoxyl and eosin (HE). Photomicrographs were acquired and processed using a Zeiss ® (Oberkochen, Germany) Axioscop 20 microscope equipped with a Canon ® (Tokyo, Japan) G10 digital camera, a 14.7 megapixel JPG and an Image-Pro Plus ® software. Each slide had 10 analyzed fields looking at presence or absence of liver steatosis and portal inflammation, including leukocyte infiltrates in the portal zone. A total of 20 portal triads were evaluated in each slide and a percentage was determined by the number of portal triads containing (yes or not) leukocyte infiltration.
Cell cycle analysis and cell viability
Flow cytometry analysis was performed to measure cell cycle and cell viability of tissue hepatic. The tissue was macerated and the extractions were made with the addition of 0.5 mg/ml collagenase (Sigma®). The cells were washed twice with phosphate buffered saline and resuspended in 500 μL of ice-cold Vindelov solution containing 0.1% Triton X-100, 0.1% citrate and 0.1 mg/mL of RNase and 50 mg/mL of propidium iodide (Sigma Chemical Co., St. Louis, MO) after centrifugation. After incubation for 15 min, the cell suspension was analyzed for DNA content by flow cytometry using a FACS Calibur flow cytometer (Becton Dickinson, Mountain View, CA, USA). The relative proportions of cells with DNA content indicative of apoptosis (<2n), G0/G1 diploid (2n), S (2n < phase < 4n), and G2/M phase (4n) were obtained and analyzed using the CellQuest WinMDI 2.9 program. Considering the experimental conditions that were used in this study, or in any others we were aware of, the fluorescence was not affected by the cell dissociation process. According to Guimarães et al. (2017)GUIMARÃES DDAB, DE CASTRO DDSB, OLIVEIRA FLD, NOGUEIRA EM, SILVA MAMD & TEODORO AJ. 2017. Pitaya Extracts Induce Growth Inhibition and Proapoptotic Effects on Human Cell Lines of Breast Cancer via Downregulation of Estrogen Receptor Gene Expression. Oxid Med Cell Longev 2017: 13 p., the nuclei of viable cells have been gated according to the relationship between FL-2W and FL2-A.
Detection of apoptosis by annexin V-FITC
To measure the rate of apoptosis, the cells were subjected to staining with Annexin V conjugated to FITC. The non-adherent cells were collected, and adherent cells were quickly washed with a calcium/magnesium-free buffered saline solution (BSS) and were detached with 0.125% trypsin/EDTA (Sigma Chemical Co., St. Louis, MO, USA) at room temperature. Subsequently, apoptotic and necrotic cells were stained with Annexin V FITC/Propidium Iodide (PI) (BD Pharmingen, Mountain View, CA,USA) according to the manufacturer’s instructions, quantified with a flow cytometer (FACSCalibur, BD Bioscience, Mountain View, CA, USA), and analyzed using two specific programs, Cell Quest and FlowJo.
Statistical analysis
The data were analyzed using the Windows Graphpad Prism software package. Differences between the groups were analyzed using the Student’s t-test, and the values were considered unpaired and parametric. For means of comparison among the groups, analysis of variance (ANOVA one-way) and Tukey’s post hoc test. The assumption of normality (Gaussian distribution) was verified by Kolmogorov-Smirnov tests to support the use of the statistical methods described above. Results are expressed as mean–standard deviation and the level of significance was set at p<0.05.
RESULTS
During experiment, the different dietary treatments did not affect (p>0.05) the intake of ration and water of animals which were similar among all groups. However, although no difference was observed in the hepatosomatic index, at the end of study the animals that consumed high-fat diet (HG, JG, WG and RG) showed higher (p<0.05) body weight (Table II). The consumption of RS and GJ was higher than the RW group, but despite the difference found in the consumption of beverages, all groups had similar energy consumption when we associated the feed consumption (Table II).
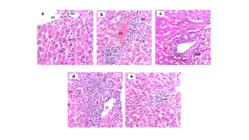
CG showed typical liver histology containing well-organized polygonal hepatocytes with eosinophilic cytoplasm and large central nuclei. Kupffer cells were found attached to endothelial cells in the sinusoids with elongated nuclei and compact chromatin (Figure 2a). On the other hand, the high-fat diet caused significant disruption in the liver architecture of all treated groups (HG, JG, WG, RG). Rats of HG presented severe steatosis (Figure 2b) and the liver of JG was partially affected by high-fat diet protocol, indicating an intermediate score to steatosis (Figure 2c). The WG was similar to HG, indicating that wine treatment induced severe steatosis (Figure 2d). Interestingly, resveratrol protected the liver against damages induced by high-fat diet in 80% of mice (Figure 2e).
Photomicrographs of liver submitted to high-fat diet and distinct treatments. (a) Control group supplemented with control feed. (b) High-fat diet group showing severe steatosis. (c) Grape juice group represents animals submitted to high-fat diet treated with grape juice and partial steatosis in the liver. (d) Red wine group represents animals submitted to high-fat diet treated with red wine and high index of steatosis. (e) Resveratrol group represents animals submitted to high-fat diet treated with resveratrol and low index of liver injury. Hepatocytes (arrowhead); Kupffer Cells (arrow); Hepatocyte ballooning (*); Lipid accumulation (#). Data are representative of three experiments. Magnification: 400X. n=10 animals per group.
The high-fat diet results nonalcoholic steatohepatitis (NASH), which is well characterized by histological findings including liver steatosis and inflammation with hepatocyte injury. Here, high-fat diet (HG - Figure 3b and Figure 4) induced a severe liver inflammation marked by leukocyte infiltrate throughout the lobular and portal zones compared to control animals (CG - Figure 3a and Figure 4). Although JG presented portal triads with partial accumulation of extracellular matrix (Figure 3c and Figure 4), the JG showed significant decreases of NASH properties, such as less lobular inflammation and steatosis than HG. WG presented histological findings compatible to HG, indicating that red wine maintains or aggravates the NASH (Figure 3d and Figure 4). RG showed significant reduction of high-fat diet damages. Resveratrol strongly inhibited the progression of NASH in these animals (Figure 3e and Figure 4).
Photomicrographs of portal triads of liver submitted to high-fat diet. (a) Control group showing classical portal triad: branches of hepatic artery (HA), portal vein (PV) and bile ducts (BD); (b) High-fat diet group with significant portal leukocyte infiltrate; (c) Grape juice group reduced the NASH status, but extracellular matrix (ECM) deposition was found in some portal triads; (d) Red wine group with large leukocyte infiltration (L); (e) Resveratrol group reduced the leukocytes infiltrated in the portal triads. Hepatocytes (arrowhead); Kupffer Cells (arrow); Extracellular matrix deposition (ECM); Leukocytes (L). Data are representative of three experiments. Magnification: 400X. n=10 animals per group.
Effects of grape juice, red wine and resveratrol solution on inflamed of portal triads in liver of rats fed high-fat diet. Means significant difference with different letters (p<0.05). Abbreviations: (CG) Control group supplemented with control feed; (HG) High-fat diet group; (JG) Grape juice group represents animals submitted to high-fat diet treated with grape juice; (WG) Wine group represents animals submitted to high-fat diet treated with red wine; (RG) Resveratrol group represents animals submitted to high-fat diet treated with resveratrol solution isolated. Statistical significance was determined by ANOVA followed by Tukey’s post hoc multiple mean comparison test.
Our results of cell cycle (Table III) and apoptosis (Table IV and Figure 5) are described below. High-fat diet (HG) altered the liver cell cycle of the animals leading to lower (p<0.05) number of viable cells and higher rate of apoptotic cells (Table IV) with an increase (p<0.05) in the number of cells in the G2/M phase (Table III).
Effects of grape juice, red wine and resveratrol solution on rate apoptosis in rats fed high-fat diet. (I) Flow cytometry analysis of groups. (II) Relative increase rate of apoptotic cells of liver (%). Means significant difference with different letters (p<0.05). Abbreviations: (CG) Control group supplemented with control feed; (HG) High-fat diet group; (JG) Grape juice group represents animals submitted to high-fat diet treated with grape juice; (WG) Wine group represents animals submitted to high-fat diet treated with red wine; (RG) Resveratrol group represents animals submitted to high-fat diet treated with resveratrol solution isolated. Statistical significance was determined by ANOVA followed by Tukey’s post hoc multiple mean comparison test.
Effect of grape juice, red wine and resveratrol solution on cell cycle progression of hepatic cells in rats fed high-fat diet.
Effect of grape juice, red wine and resveratrol solution on stages of death process in hepatocytes of different groups (% cells).
Grape juice (JG) was the only drink that reversed the effects of the high-fat diet on liver cells apoptosis. The animals of JG presented cell cycle and apoptosis rate of cells similar CG (Table IV and Figure 5). Even with a high-fat diet, the results show that grape juice protected the liver of the animal by maintaining unchanged percentage of viable cells, apoptotic cells and non-apoptotic cells (Table IV and Figure 5).
The wine consumption (WG) showed to be even more aggressive to the liver tissue. In addition to the alterations already caused by the high-fat diet, WG presented a reduction (p<0.05) in the number of cells in the G0/G1 phase (Table III), lower (p<0.05) viable cell rate and the highest (p<0.05) number of non-apoptotic cells (Table IV).
The animals that consumed resveratrol solution (RG) showed lower viable cell rate, increase of cells in the SubG1 phase and smaller number of G0/G1 and G2/M cells. The isolated compound induced programmed killing of the damaged cells and it promoted increase in the number of cells unsuitable for replication (SubG1) – Table III.
DISCUSSION
Most of the population consume a high-fat diet and the effects of relationship of diet and development of diseases are being evaluated (Fontelles et al. 2018FONTELLES CC, DA CRUZ RS, HILAKIVI-CLARKE L, DE ASSIS S & ONG TP. 2018. Investigation of Paternal Programming of Breast Cancer Risk in Female Offspring in Rodent Models. In Investigations of Early Nutrition Effects on Long-Term Health Humana Press, New York 1735: 207-220., De Oliveira et al. 2016DE OLIVEIRA TK, ALMEIDA FDA, FALCÃO MPM, DE LEMOS-JORDÃO AJJ, RAMOS KR & SILVA JFD. 2016. Analysis of the aqueous extract of Arachis hipoagea L. to reduce dyslipidemia and weight gain in Wistar rats with high fat diet. Pesqui Agropecu Bras 36: 1121-1126.). Previous studies have been carried out on homeostatic tissues, such as the liver (Levy et al. 2011LEVY RB, CLARO RM, MONDINI L, SICHIERI R & MONTEIRO C A. 2011. Distribuição regional e socioeconômica da disponibilidade domiciliar de alimentos no Brasil em 2008-2009. Rev Saúde Públ 46: 6-15., Dani et al. 2007DANI C, OLIBONI LS, VANDERLINDE R, BONATTO D, SALVADOR M & HENRIQUES JAP. 2007. Phenolic content and antioxidant activities of white and purple juices manufactured with organically-or conventionally-produced grapes. Food Chem Toxicol 45: 2574-2580.) and it has been shown that bioactive compounds present in grapes, such as resveratrol, promote hepatoprotective action against high-fat dietary intake (Buchner et al. 2014BUCHNER I, MEDEIROS N, LACERDA D, NORMANN C, GEMELLI T, RIGON P, WANNMACHER CMD, HENRIQUES JAP, DANI C & FUNCHAL C. 2014. Hepatoprotective and antioxidant potential of organic and conventional grape juices in rats fed a high-fat diet. Antioxidants 3: 323-338., Carpene et al. 2015CARPENE C, GOMEZ-ZORITA S, DELERUYELLE S & CARPENE MA. 2015. Novel strategies for preventing diabetes and obesity complications with natural polyphenols. Curr Med Chem 22: 150-164., Farias et al. 2015FARIAS M ET AL. 2015. Effect of grape juice on some biochemical and oxidative stress parameters in serum and liver enzymes of pregnant and lactating rats. Issues Biol Sci Pharm Res 3: 37-46.). However, the source of consumption (juice, wine or compound isolated) has been fundamental for beneficial or deleterious effects to be observed when it comes to the association with high-fat diet (Martins & Nicoletti 2006MARTINS PP & NICOLETTI MA. 2016. Polifenóis no vinho: resveratrol e seus benefícios. Infarma-Ciências Farmacêuticas 28: 216-225., Cardozo et al. 2013CARDOZO MG, MEDEIROS N, DOS SANTOS DL, DE ALMEIDA DC, HENRIQUES JAP, DANI C & FUNCHAL C. 2013. Effect of chronic treatment with conventional and organic purple grape juices (Vitis labrusca) on rats fed with high-fat diet. Cell Mol Neurobiol 33: 1123-1133., Dos Santos Lima et al. 2014DOS SANTOS LIMA M, SILANI IDSV, TOALDO IM, CORRÊA LC, BIASOTO ACT, PEREIRA GE, BORDIGNON-LUIZ AT & NINOW JL. 2014. Phenolic compounds, organic acids and antioxidant activity of grape juices produced from new Brazilian varieties planted in the Northeast Region of Brazil. Food Chem 161: 94-103., Reeves et al. 1993REEVES PG, NIELSEN FH & FAHEY JR GC. 1993. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123: 1939-1951.). Considering that the high-fat diet is a common choice of part of the population and that its deleterious effects have been observed (Fontelles et al. 2018FONTELLES CC, DA CRUZ RS, HILAKIVI-CLARKE L, DE ASSIS S & ONG TP. 2018. Investigation of Paternal Programming of Breast Cancer Risk in Female Offspring in Rodent Models. In Investigations of Early Nutrition Effects on Long-Term Health Humana Press, New York 1735: 207-220., De Oliveira et al. 2016DE OLIVEIRA TK, ALMEIDA FDA, FALCÃO MPM, DE LEMOS-JORDÃO AJJ, RAMOS KR & SILVA JFD. 2016. Analysis of the aqueous extract of Arachis hipoagea L. to reduce dyslipidemia and weight gain in Wistar rats with high fat diet. Pesqui Agropecu Bras 36: 1121-1126.), this study aimed at assessing the effects of grape juice, red wine and isolated resveratrol on rat liver fed a high-fat diet, particularly with regard to the cell cycle, apoptosis and hepatocyte histology.
According to the recommendations of American Institute of Nutrition Recommendations for maintenance adult-rodents (AIN 93M), the daily consumption of lipids for adult rats should be 4% (Reeves et al. 1993REEVES PG, NIELSEN FH & FAHEY JR GC. 1993. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123: 1939-1951.). In our study, the diet contained 20% of total lipids, a concentration five times higher than that recommended, which characterizes a high-fat diet (Wu et al. 2016WU T, GUO Y, LIU R, WANG K & ZHANG M. 2016. Black tea polyphenols and polysaccharides improve body composition, increase fecal fatty acid, and regulate fat metabolism in high-fat diet-induced obese rats. Food Funct 7: 2469-2478.). Studies show that the consumption of a diet with 20-50% of total lipids leads to weight gain and predisposes the individual to various diseases (El Ayed et al. 2018EL AYED M, KADRI S, MABROUK M, AOUANI E & ELKAHOUI S. 2018. Protective effect of grape seed and skin extract against high-fat diet-induced dyshomeostasis of energetic metabolism in rat lung. Lipids Health Dis 17: 109., Nolan et al. 2011NOLAN CJ, DAMM P & PRENTKI M. 2011. Type 2 diabetes across generations: from pathophysiology to prevention and management. The Lancet 378: 169-181., Gonçalves et al. 2018GONÇALVES L, BORTOLATO G, NETO RDB, FRUSCIANTE MR, FUNCHAL C & DANI C. 2018. Grape Juice Consumption with or without High Fat Diet during Pregnancy Reduced the Weight Gain and Improved Lipid Profile and Oxidative Stress Levels in Liver and Serum from Wistar Rats. Beverages 4: 78.). This was also found in experiments with rats where there was a positive association between high-fat diet and animal body weight gain, even without higher caloric intake. (Martins & Nicoletti 2006, Nolan et al. 2011NOLAN CJ, DAMM P & PRENTKI M. 2011. Type 2 diabetes across generations: from pathophysiology to prevention and management. The Lancet 378: 169-181., Cheng et al. 2017CHENG HS, TON SH, PHANG SCW, TAN JBL & KADIR KA. 2017. Increased susceptibility of post-weaning rats on high-fat diet to metabolic syndrome. J Adv Res 8: 743-752., Soltis et al. 2017SOLTIS AR ET AL. 2017. Hepatic dysfunction caused by consumption of a high-fat diet. Cell Rep 21: 3317-3328.). These results corroborate our findings in which high-fat diet played a role in animal body weight gain (HG, JG, WG, RG). However, Gonçalves et al. (2018)GONÇALVES L, BORTOLATO G, NETO RDB, FRUSCIANTE MR, FUNCHAL C & DANI C. 2018. Grape Juice Consumption with or without High Fat Diet during Pregnancy Reduced the Weight Gain and Improved Lipid Profile and Oxidative Stress Levels in Liver and Serum from Wistar Rats. Beverages 4: 78. demonstrated that pregnant rats treated with grape juice and high-fat diet presented lower weight gain when compared to the group that did not consume juice, evidencing that the consumption of grape juice can minimize the gain of gestational bodyweight, as well as Buchner et al. (2014)BUCHNER I, MEDEIROS N, LACERDA D, NORMANN C, GEMELLI T, RIGON P, WANNMACHER CMD, HENRIQUES JAP, DANI C & FUNCHAL C. 2014. Hepatoprotective and antioxidant potential of organic and conventional grape juices in rats fed a high-fat diet. Antioxidants 3: 323-338. and Cardozo et al. (2013)CARDOZO MG, MEDEIROS N, DOS SANTOS DL, DE ALMEIDA DC, HENRIQUES JAP, DANI C & FUNCHAL C. 2013. Effect of chronic treatment with conventional and organic purple grape juices (Vitis labrusca) on rats fed with high-fat diet. Cell Mol Neurobiol 33: 1123-1133. have stated. In this respect, it is worth mentioning that grape juice seems to have reduced the effects of the high-fat diet under bodyweight parameters, as the JG animals showed a trend of lower final weight as well as control animals that consumed a balanced diet.
No difference was found in the experiment between water and chow consumption with respect to the food consumption of the animals, although both were provided ad libitum. Our findings indicate that the animals accepted the manipulated diets well and that daily intake of feed was within the literature reported (Kim et al. 2011KIM S, JIN Y, CHOI Y & PARK T. 2011. Resveratrol exerts anti-obesity effects via mechanisms involving down-regulation of adipogenic and inflammatory processes in mice. Biochem Pharmacol 81: 1343-1351., Szkudelska et al. 2009SZKUDELSKA K, NOGOWSKI L & SZKUDELSKI T. 2009. The inhibitory effect of resveratrol on leptin secretion from rat adipocytes. Eur J Clin Invest 39: 899-905.). Although some studies affirm that there is a lower dietary intake of animals receiving a high-fat diet because they are able to regulate their intake from the energy density of the diet consumed (Inglés et al. 2014INGLÉS M, GAMBINI J, MIGUEL MG, BONET-COSTA V, ABDELAZIZ KM, EL ALAMI M, VIÑA J & BORRÁS C. 2014. PTEN mediates the antioxidant effect of resveratrol at nutritionally relevant concentrations. Biomed Res Int 2014: 6 p., Meng et al. 2017MENG J, SHI TC, SONG S, ZHANG ZW & FANG YL. 2017. Melatonin in grapes and grape-related foodstuffs: A review. Food Chem 231: 185-191.), this effect may occur only in the short term, as there appears to be adaptation after a period of time and consequent normalization of dietary intake (Inglés et al. 2014INGLÉS M, GAMBINI J, MIGUEL MG, BONET-COSTA V, ABDELAZIZ KM, EL ALAMI M, VIÑA J & BORRÁS C. 2014. PTEN mediates the antioxidant effect of resveratrol at nutritionally relevant concentrations. Biomed Res Int 2014: 6 p.). In our study, a trend of lower feed intake was observed in CG and JG. In long term, Kim et al. (2011)KIM S, JIN Y, CHOI Y & PARK T. 2011. Resveratrol exerts anti-obesity effects via mechanisms involving down-regulation of adipogenic and inflammatory processes in mice. Biochem Pharmacol 81: 1343-1351. and Szkudelska et al. (2009)SZKUDELSKA K, NOGOWSKI L & SZKUDELSKI T. 2009. The inhibitory effect of resveratrol on leptin secretion from rat adipocytes. Eur J Clin Invest 39: 899-905. revealed that resveratrol in grape juice was able to decrease leptin secretion in rat adipocytes fed a high-fat diet, reducing their food intake justifying the trend of lower dietary intake of animals consuming grape juice in addition to high-fat diet.
Although the volume of grape juice (GJ), red wine (RW) and resveratrol solution (RS) is different, the doses of consumed beverages are in line with the literature. For whole red grape juice and red wine, the doses usually used range from 5 to 20 ml/day (Martins & Nicoletti 2017, Gu et al. 2016GU J, HU W, SONG ZP, CHEN YG, ZHANG DD & WANG CQ. 2016. Resveratrol-induced autophagy promotes survival and attenuates doxorubicin-induced cardiotoxicity. Int Immunopharmacol 32: 1-7., Scott et al. 2012SCOTT E, STEWARD WP, GESCHER AJ & BROWN K. 2012. Resveratrol in human cancer chemoprevention–choosing the ‘right’ dose. Mol Nutr Food Res 56: 7-13.). The doses used for resveratrol alone show a significant variation, from 1 mg / day to 1.2 g resveratrol / day per animal (Buckton et al. 2018BUCKTON CH, PATTERSON C, HYSENI L, KATIKIREDDI SV, LLOYD-WILLIAMS F, ELLIOTT-GREEN A, CAPEWELL S & HILTON S. 2018. The palatability of sugar-sweetened beverage taxation: A content analysis of newspaper coverage of the UK sugar debate. PLoS ONE 13: e0207576., Mukamal et al. 2016MUKAMAL KJ ET AL. 2016. Moderate alcohol consumption and chronic disease: the case for a long-term trial. Alcohol Clin Exp Res 40: 2283-2291., Savage & Semple 2010SAVAGE DB & SEMPLE RK. 2010. Recent insights into fatty liver, metabolic dyslipidaemia and their links to insulin resistance. Curr Opin Lipidol 21: 329-336., Charbonneau et al. 2007CHARBONNEAU A, UNSON CG & LAVOIE JM. 2007. High-fat diet-induced hepatic steatosis reduces glucagon receptor content in rat hepatocytes: potential interaction with acute exercise. J Physiol 579: 255-267.). The difference found in the consumption of drinks can be explained by the presence of refined sugar in SR and fructose in SU that make these drinks more tasty (Charbonneau et al. 2007CHARBONNEAU A, UNSON CG & LAVOIE JM. 2007. High-fat diet-induced hepatic steatosis reduces glucagon receptor content in rat hepatocytes: potential interaction with acute exercise. J Physiol 579: 255-267.), and the alcoholic content limits their intake in red wine (Deji et al. 2009DEJI N ET AL. 2009. Structural and functional changes in the kidneys of high-fat diet-induced obese mice. Am J Physiol-Renal 296: 118-126.).
The liver is a fundamental organ for the homeostasis of the organism, has a high metabolic rate and is very susceptible to any type of internal or external aggression, such as dietary, exercise or disease stressors (Lozano et al. 2016LOZANO I ET AL. 2016. High-fructose and high-fat diet-induced disorders in rats: impact on diabetes risk, hepatic and vascular complications. Nutr Metab 13: 15., Van et al. 2017). Studies in rats comparing the intake of a high-fat diet (66% lard) and with a balanced diet observed that the liver weight of the animals that consumed high fat did not differ from the control group (Carpene et al. 2015CARPENE C, GOMEZ-ZORITA S, DELERUYELLE S & CARPENE MA. 2015. Novel strategies for preventing diabetes and obesity complications with natural polyphenols. Curr Med Chem 22: 150-164., Meng et al. 2017MENG J, SHI TC, SONG S, ZHANG ZW & FANG YL. 2017. Melatonin in grapes and grape-related foodstuffs: A review. Food Chem 231: 185-191., Ohashi et al. 2018OHASHI T, KATO M, YAMASAKI A, KUWANO A, SUZUKI H, KOHJIMA M & OGAWA Y. 2018. Effects of high fructose intake on liver injury progression in high fat diet induced fatty liver disease in ovariectomized female mice. Food Chem Toxicol 118: 190-197.). These findings corroborate the data from this study, since no difference was observed in the hepatosomatic index of the animals, regardless of the type of diet consumed. It should be noted, however, that the literature shows that the high consumption of saturated fatty acids is associated with inflammatory processes and lipotoxicity of certain organs, such as the liver, causing non-alcoholic fatty liver disease (NAFLD), which would be a possible dietary effect of our study (Carpene et al. 2015CARPENE C, GOMEZ-ZORITA S, DELERUYELLE S & CARPENE MA. 2015. Novel strategies for preventing diabetes and obesity complications with natural polyphenols. Curr Med Chem 22: 150-164., Meng et al. 2017MENG J, SHI TC, SONG S, ZHANG ZW & FANG YL. 2017. Melatonin in grapes and grape-related foodstuffs: A review. Food Chem 231: 185-191., Ohashi et al. 2018OHASHI T, KATO M, YAMASAKI A, KUWANO A, SUZUKI H, KOHJIMA M & OGAWA Y. 2018. Effects of high fructose intake on liver injury progression in high fat diet induced fatty liver disease in ovariectomized female mice. Food Chem Toxicol 118: 190-197., Dixon et al. 2013DIXON LJ, BARNES M, TANG H, PRITCHARD MT & NAGY LE. 2013. Kupffer cells in the liver. Compr Physiol 3: 785-797., Alisi et al. 2017ALISI A, CARPINO G, OLIVEIRA FL, PANERA N, NOBILI & GAUDIO E. 2017. The role of tissue macrophage-mediated inflammation on NAFLD pathogenesis and its clinical implications. Mediat Inflamm, 15 p.).
Although NASH frequently develops to progressive fibrosis, it is poorly observed in high-fat diet experimental condition (Eckert et al. 2015ECKERT C, KLEIN N, KORNEK M & LUKACS-KORNEK V. 2015. The complex myeloid network of the liver with diverse functional capacity at steady state and in inflammation. Front Immunol 6: 179.). Previous studies with animals have shown that high-fat diets induce accumulation of lipids in hepatocytes and Kupffer cells, triggering a process of inflammation (Seki & Schwabe 2015SEKI E & SCHWABE RF. 2015. Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology 61: 1066-1079., Brunt et al. 1999BRUNT EM, JANNEY CG, DI BISCEGLIE AM, NEUSCHWANDER-TETRI BA & BACON BR. 1999. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 94: 2467., Festi et al. 2004FESTI D, COLECCHIA A, SACCO T, BONDI M, RODA E & MARCHESINI G. 2004. Hepatic steatosis in obese patients: clinical aspects and prognostic significance. Obes Rev 5: 27-42., Santos et al. 2013SANTOS J, VALENTIM I, DE ARAÚJO O, ATAIDE T & GOULART M. 2013. Development of nonalcoholic hepatopathy: contributions of oxidative stress and advanced glycation end products. Int J Mol Sci 14: 19846-19866., Farrell et al. 2012FARRELL GC, VAN ROOYEN D, GAN L & CHITTURI S. 2012. NASH is an inflammatory disorder: pathogenic, prognostic and therapeutic implications. Gut Liver 6: 149.). Hepatic steatosis, once established, promotes cellular adaptations to high levels of oxidative stress, which would make the cells able to survive in this adverse environment, but would still keep the cells prone to the process of apoptosis and/or necrosis associated with inflammation (Shono et al. 2011SHONO S, HABU Y, NAKASHIMA M, SATO A, NAKASHIMA H, MIYAZAKI H, KINOSHITA M, TSUMATORI G, SHINOMIYA N & SEKI S. 2011. The immunologic outcome of enhanced function of mouse liver lymphocytes and Kupffer cells by high-fat and high-cholesterol diet. Shock 36: 484-493., Tunali-Akbay et al. 2010TUNALI-AKBAY T, SEHIRLI O, ERCAN F & SENER G. 2010. Resveratrol protects against methotrexate-induced hepatic injury in rats. J Pharm Pharm Sci 13: 303-310.). Santos et al. (2013)SANTOS J, VALENTIM I, DE ARAÚJO O, ATAIDE T & GOULART M. 2013. Development of nonalcoholic hepatopathy: contributions of oxidative stress and advanced glycation end products. Int J Mol Sci 14: 19846-19866. also consider that excessive levels of fatty acids in hepatic tissue can cause damage to cellular proteins and lipids, increase oxidative stress and stimulate receptors associated with inflammatory hepatocellular lesions, defense cell activation, and tissue fibrosis. Our results corroborate these findings and resemble data where the high-fat diet altered the hepatocyte structure with ballooning degeneration and induced macrophage infiltration in rats (De Moura et al. 2016DE MOURA CFG, RIBEIRO FAP, HANDAN BA, AGUIAR O, OSHIMA CTF & RIBEIRO DA. 2016. Grape juice concentrate protects rat liver against cadmium intoxication: histopathology, cytochrome C and metalloproteinases expression. Drug Res 66: 339-344.). Balloon degeneration is considered a form of hepatocyte death in the presence of steatosis and inflammation, characterizing histopathological disorders via cell enlargement (Petyaev 2016PETYAEV IM. 2016. Lycopene deficiency in ageing and cardiovascular disease. Oxid Med Cell Longev 2016: 6 p.). Excess dietary fat can also affect the immune system by activating pro-inflammatory Kupffer cells (M1 subtype) (Petyaev 2016PETYAEV IM. 2016. Lycopene deficiency in ageing and cardiovascular disease. Oxid Med Cell Longev 2016: 6 p.). Kupffer cells are considered liver macrophages and their activation represents a central event in the initiation and progression of hepatic injury in in NASH-developed experimental models (Santos et al. 2013SANTOS J, VALENTIM I, DE ARAÚJO O, ATAIDE T & GOULART M. 2013. Development of nonalcoholic hepatopathy: contributions of oxidative stress and advanced glycation end products. Int J Mol Sci 14: 19846-19866., Tunali-Akbay et al. 2010TUNALI-AKBAY T, SEHIRLI O, ERCAN F & SENER G. 2010. Resveratrol protects against methotrexate-induced hepatic injury in rats. J Pharm Pharm Sci 13: 303-310.).
Petyaev et al. (2016) evaluated the effects of resveratrol alone and within the alcoholic matrix (red wine) on hepatocytes attacked by UV-B radiation as a model of oxidative stress and obtained results contrary to ours. The authors concluded that resveratrol showed synergistic antioxidant effect in wine in both lower and higher dosages to make the other compounds more effective than alone. Another study evaluated trans-resveratrol, red wine and deionized wine supplementation and showed that the three treatments altered the biomarkers of oxidative stress generated by the high-fat diet, but had no effect on the prevention or regression of fat accumulation in the liver (Cheng et al. 2017CHENG HS, TON SH, PHANG SCW, TAN JBL & KADIR KA. 2017. Increased susceptibility of post-weaning rats on high-fat diet to metabolic syndrome. J Adv Res 8: 743-752.). However, more recent studies corroborate our findings when stating that resveratrol alone promotes therapeutic effects against fat infiltration in the liver, induces lower levels of resveratrol, inflammation and modulates the immune system by activating Kupffer cells with phagocytic activity (Chassot et al. 2018CHASSOT LN, SCOLARO B, ROSCHEL GG, COGLIATI B, CAVALCANTI MF, ABDALLA DS & CASTRO IA. 2018. Comparison between red wine and isolated trans-resveratrol on the prevention and regression of atherosclerosis in LDLr (−/−) mice. J Nutr Biochem 61: 48-55., Liu et al. 2016LIU X, YU L, HASSAN W, SUN L, ZHANG L & JIANG Z. 2016. The duality of kupffer cell responses in liver metabolic states. Curr Mol Med 16: 809-819.). Our findings with grape juice corroborate De Moura et al. (2016)DE MOURA CFG, RIBEIRO FAP, HANDAN BA, AGUIAR O, OSHIMA CTF & RIBEIRO DA. 2016. Grape juice concentrate protects rat liver against cadmium intoxication: histopathology, cytochrome C and metalloproteinases expression. Drug Res 66: 339-344. which also found a hepatoprotective effect in grape juice, concluding that juice consumption was able to prevent tissue degeneration in the liver.
Several studies investigate the effects of isolated compounds on cellular parameters and apoptosis to determine your mechanisms of action in tissues. In this sense, however, few studies have investigated the effect of combinations of phytochemicals and diets (Dudley et al. 2011DUDLEY KJ, SLOBODA DM, CONNOR KL, BELTRAND J & VICKERS MH. 2011. Offspring of mothers fed a high fat diet display hepatic cell cycle inhibition and associated changes in gene expression and DNA methylation. PLoS ONE 6: 21662., Fausto et al. 2006FAUSTO N, CAMPBELL JS & RIEHLE KJ. 2006. Liver regeneration. Hepatology 43: S45-S53.).
Tissue homeostasis is maintained in multicellular organisms by the balance between cell proliferation and death of cells (Mitchell & Willenbring 2008MITCHELL C & WILLENBRING H. 2008. A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nat Protoc 3: 1167.). Cell cycle is the process by which genetic material within a cell is replicated and secreted into two new cell compartments, characterizing tissue growth and proliferation (Krysko et al. 2008KRYSKO DV, BERGHE TV, D’HERDE K & VANDENABEELE P. 2008. Apoptosis and necrosis: detection, discrimination and phagocytosis. Methods 44: 205-221.). In contrast to many cell types, hepatocytes are known to maintain constant replication and rejuvenation of their cells (Tarantino 2007TARANTINO G. 2007. Should nonalcoholic fatty liver disease be regarded as a hepatic illness only? WJG 13: 4669., Estrov et al. 2003ESTROV Z, SHISHODIA S, FADERL S, HARRIS D, VAN Q, KANTARJIAN HM, TALPAZ M & AGGARWAL BB. 2003. Resveratrol blocks interleukin-1β–induced activation of the nuclear transcription factor NF-κB, inhibits proliferation, causes S-phase arrest, and induces apoptosis of acute myeloid leukemia cells. Blood 102: 987-995.). However, external factors can interfere in the process of adequate cell proliferation, such as dietary factors, and the defective or inefficient elimination of altered cells may contribute to the development of different pathologies. In this regard, apoptosis and non-apoptotic death represent a continuous process of equilibrium between proliferation and cell death that are required for tissue homeostasis when they occur in a controlled manner (Viola & Soehnlein 2015VIOLA J & SOEHNLEIN O. 2015. Atherosclerosis–a matter of unresolved inflammation. Semin Immunol 27: 184-193.).
Cell changes induced by consumption of a high-fat diet include apoptosis that is considered a common mechanism of hepatic injury and an important point of NAFLD (Yuzefovych et al. 2013YUZEFOVYCH LV, MUSIYENKO SI, WILSON GL & RACHEK LI. 2013. Mitochondrial DNA damage and dysfunction, and oxidative stress are associated with endoplasmic reticulum stress, protein degradation and apoptosis in high fat diet-induced insulin resistance mice. PLoS ONE 8: e54059., Simsa-Maziel & Monsonego-Ornan 2012SIMSA-MAZIEL S & MONSONEGO-ORNAN E. 2012. Interleukin-1β promotes proliferation and inhibits differentiation of chondrocytes through a mechanism involving down-regulation of FGFR-3 and p21. Endocrinology 153: 2296-2310., Han et al. 1995HAN Z, CHATTERJEE D, HE DM, EARLY J, PANTAZIS P, WYCHE JH & HENDRICKSON EA. 1995. Evidence for a G2 checkpoint in p53-independent apoptosis induction by X-irradiation. Mol Cell Biol 15: 5849-5857., Chiang et al. 2014CHIANG WD, SHIBU MA, LEE KI, WU JP, TSAI FJ, PAN LF, HUANG Y & LIN WT. 2014. Lipolysis-stimulating peptide-VHVV ameliorates high fat diet induced hepatocyte apoptosis and fibrosis. J Funct Foods 11: 482-492.). These effects may be linked to liver NAFLD genesis, such as NASH. In this regard, it is essential to identify dietary strategies that can contribute to its prevention and reduction.
The hypothesis is that the excess fat in the diet promoted tissue damage and programmed death of the injured cells. There was a possible attempt of cell proliferation to compensate for the higher rate apoptotic cells, but the cellular changes generated by the diet were identified and induced cell cycle arrest (Koo 2013KOO SH. 2013. Nonalcoholic fatty liver disease: molecular mechanisms for the hepatic steatosis. Clin Mol Hepatol 19: 210.). Any change in the apoptosis process is deleterious and results in tissue damage, which can be generated by dietary inadequacies. Chiang et al. (2014)CHIANG WD, SHIBU MA, LEE KI, WU JP, TSAI FJ, PAN LF, HUANG Y & LIN WT. 2014. Lipolysis-stimulating peptide-VHVV ameliorates high fat diet induced hepatocyte apoptosis and fibrosis. J Funct Foods 11: 482-492. found that the expression of apoptosis and fibrosis had been altered by mice receiving a high protein diet. In excessive fat consumption, fatty acid oxidation and oxidative phosphorylation for adenosine triphosphate (ATP) production are increased. Deregulation of this pathway results in energy deficiencies and/or production of reactive oxygen species responsible for cell damage (Dudley et al. 2011DUDLEY KJ, SLOBODA DM, CONNOR KL, BELTRAND J & VICKERS MH. 2011. Offspring of mothers fed a high fat diet display hepatic cell cycle inhibition and associated changes in gene expression and DNA methylation. PLoS ONE 6: 21662., Valenzuela et al. 2018VALENZUELA M, BASTIAS L, MONTENEGRO I, WERNER E, MADRID A, GODOY P, PÁRRAGA M & VILLENA J. 2018. Autumn Royal and Ribier Grape Juice Extracts Reduced Viability and Metastatic Potential of Colon Cancer Cells. Evid-Based Compl Alt 2018: 7 p., Jung et al. 2006JUNG K J, WALLIG MA & SINGLETARY KW. 2006. Purple grape juice inhibits 7, 12-dimethylbenz [a] anthracene (DMBA)-induced rat mammary tumorigenesis and in vivo DMBA-DNA adduct formation. Cancer Lett 233: 279-288.). This was also evidenced in the study by Dudley et al. (2011)DUDLEY KJ, SLOBODA DM, CONNOR KL, BELTRAND J & VICKERS MH. 2011. Offspring of mothers fed a high fat diet display hepatic cell cycle inhibition and associated changes in gene expression and DNA methylation. PLoS ONE 6: 21662. who reported that consumption of high-fat diet during pregnancy and lactation resulted in epigenetic modifications, with DNA methylation, associated with compromised regulation of cell cycle genes in the offspring’s liver during early postnatal life.
In other studies, the consumption of grape juice had already been protective to the cell cycle of tissues. According study of De Moura et al. (2016)DE MOURA CFG, RIBEIRO FAP, HANDAN BA, AGUIAR O, OSHIMA CTF & RIBEIRO DA. 2016. Grape juice concentrate protects rat liver against cadmium intoxication: histopathology, cytochrome C and metalloproteinases expression. Drug Res 66: 339-344., grape juice prevented changes in the cycle of rat cells liver attacked by cadmium, avoiding tissue degeneration. Previous works showed that grape juice prevented cell proliferation and consequent progression of the disease in cancer cells models (De Moura et al. 2006, Kong et al. 2019KONG B, WANG X, HE B, WEI L, ZHU J, JIN Y & FU Z. 2019. 8:2 fluorotelomer alcohol inhibited proliferation and disturbed the expression of pro-inflammatory cytokines and antigen-presenting genes in murine macrophages. Chemosphere 219: 1052-1060.).
The data suggest that wine consumption inhibited cell growth and that alcoholic consumption has induced non-apoptotic cell death as an additional internal aggressive agent (Ghantous et al. 2018GHANTOUS Y, SCHUSSEL JL & BRAIT M. 2018. Tobacco and alcohol-induced epigenetic changes in oral carcinoma. Curr Opin Oncol 30: 152-158., Araim et al. 2002ARAIM O, BALLANTYNE J, WATERHOUSE AL & SUMPIO BE. 2002. Inhibition of vascular smooth muscle cell proliferation with red wine and red wine polyphenols. J Vasc Surg 35: 1226-1232., Singh et al. 2017SINGH SK, BANERJEE S, ACOSTA EP, LILLARD JW & SINGH R. 2017. Resveratrol induces cell cycle arrest and apoptosis with docetaxel in prostate cancer cells via a p53/p21WAF1/CIP1 and p27KIP1 pathway. Oncotarget 8: 17216.). Normal cells only proliferate in response to cellular development or signs of mitosis that indicate tissue growth, while proliferation of altered cells, such as cancer cells, occurs uncontrolled by stimulus induction internal and/or external aggressors (Bianchini & Vainio 2003BIANCHINI F & VAINIO H. 2003. Wine and resveratrol: mechanisms of cancer prevention? Eur J Cancer Prev 12: 417-425.). These data may be related to the alcohol content of the beverage, as alcohol results in epigenetic changes in the cell cycle and reduces cell viability, as previous studies have shown. (Jesus et al. 2017JESUS LH, CAVAGNI J, CARRARD VC, RÖSING CK & SANT’ANA FILHO M. 2017. Effect of different presentations of resveratrol on cell proliferation and epitelial thickness of the oral mucosa of wistar rats. Clin Biomed Res 37: 175-180., Wang et al. 2017WANG T, ZHANG Y, ZENG Y & QING XU. 2017. Effect of resveratrol on proliferation of liver cancer SMMC-7721 cells and lowering levels of mTOR protein phosphorylation. Chem Pharm Bull 33: 1309-1314.). Furthermore, alcohol was responsible for the disordered increase in cell proliferation of the oral mucosa of wistar rats exposed to various resveratrol presentations in the study by Jesus et al. (2017)JESUS LH, CAVAGNI J, CARRARD VC, RÖSING CK & SANT’ANA FILHO M. 2017. Effect of different presentations of resveratrol on cell proliferation and epitelial thickness of the oral mucosa of wistar rats. Clin Biomed Res 37: 175-180..
Regarding resveratrol, some studies have indicated that the compound would offer benefits in preventing or neutralizing cell damage, aging, cancer, and other cell cycle related diseases (Alarcon de La Lastra & Villegas 2005ALARCON DE LA LASTRA C & VILLEGAS I. 2005. Resveratrol as an anti-inflammatory and anti-aging agent: Mechanisms and clinical implications. Mol Nutr Food Res 49: 405-430., Oliveira et al. 2009OLIVEIRA ACD, VALENTIM IB, GOULART MOF, SILVA CA, BECHARA EJH & TREVISAN MTS. 2009. Fontes vegetais naturais de antioxidantes. Quím Nova 32: 689-702.). However, more recent work has shown that the isolated resveratrol inhibits the enzymatic activity of the two cyclooxygenase forms, leading to cell cycle arrest, G0/G1 phase cell deprivation, inhibition of G2/M cell progression and apoptosis induction (Ghantous et al. 2018GHANTOUS Y, SCHUSSEL JL & BRAIT M. 2018. Tobacco and alcohol-induced epigenetic changes in oral carcinoma. Curr Opin Oncol 30: 152-158., Araim et al. 2002ARAIM O, BALLANTYNE J, WATERHOUSE AL & SUMPIO BE. 2002. Inhibition of vascular smooth muscle cell proliferation with red wine and red wine polyphenols. J Vasc Surg 35: 1226-1232., Singh et al. 2017SINGH SK, BANERJEE S, ACOSTA EP, LILLARD JW & SINGH R. 2017. Resveratrol induces cell cycle arrest and apoptosis with docetaxel in prostate cancer cells via a p53/p21WAF1/CIP1 and p27KIP1 pathway. Oncotarget 8: 17216.). This is consistent with what was found in our analysis when we observed that isolated resveratrol appears to have been cytototoxic, induced the programmed killing of the damaged cells, and promoted an increase in the number of cells unfit to replicate.
CONCLUSIONS
The high-fat diet promoted damage to the liver tissue, altering the cell cycle and histology of the liver. In addition, the diet rich in fat infiltration of liver cells, inflammation and infiltration of leukocytes in the tissue. Excess dietary fat reduced the contents of viable cells and increased apoptosis, even when combined with intake of wine or resveratrol alone. Red wine aggravated the histological damages caused by the high-fat diet, but these lesions were reduced by the intake of grape juice and resveratrol alone. On the other hand, grape juice protected the liver from diet-generated cell damage, showing the cell cycle profile and the viability compatible with the control that consumed a balanced diet.
ACKNOWLEGMENTS
This study was funded by the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) grant numbers 246295, 246296 and 238719. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES) - Finance Code 001.
REFERENCES
- ALARCON DE LA LASTRA C & VILLEGAS I. 2005. Resveratrol as an anti-inflammatory and anti-aging agent: Mechanisms and clinical implications. Mol Nutr Food Res 49: 405-430.
- ALISI A, CARPINO G, OLIVEIRA FL, PANERA N, NOBILI & GAUDIO E. 2017. The role of tissue macrophage-mediated inflammation on NAFLD pathogenesis and its clinical implications. Mediat Inflamm, 15 p.
- ARAIM O, BALLANTYNE J, WATERHOUSE AL & SUMPIO BE. 2002. Inhibition of vascular smooth muscle cell proliferation with red wine and red wine polyphenols. J Vasc Surg 35: 1226-1232.
- BANTEL H. 2012. Mechanisms of cell death in acute liver failure. Front Physiol 3: 79.
- BEDÊ TP, PASCOAL AC, FACÓ LH, DE SALVO CASTRO E, MATTOSO V, DIAS JF & DE AZEREDO VB. 2015. Effect of the intake of liquids rich in polyphenols on blood pressure and fat liver deposition in rats submitted to high-fat diet. Nutr Hosp 31: 2539-2545.
- BIANCHINI F & VAINIO H. 2003. Wine and resveratrol: mechanisms of cancer prevention? Eur J Cancer Prev 12: 417-425.
- BRUNT EM, JANNEY CG, DI BISCEGLIE AM, NEUSCHWANDER-TETRI BA & BACON BR. 1999. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 94: 2467.
- BUCHNER I, MEDEIROS N, LACERDA D, NORMANN C, GEMELLI T, RIGON P, WANNMACHER CMD, HENRIQUES JAP, DANI C & FUNCHAL C. 2014. Hepatoprotective and antioxidant potential of organic and conventional grape juices in rats fed a high-fat diet. Antioxidants 3: 323-338.
- BUCKTON CH, PATTERSON C, HYSENI L, KATIKIREDDI SV, LLOYD-WILLIAMS F, ELLIOTT-GREEN A, CAPEWELL S & HILTON S. 2018. The palatability of sugar-sweetened beverage taxation: A content analysis of newspaper coverage of the UK sugar debate. PLoS ONE 13: e0207576.
- CARDOZO MG, MEDEIROS N, DOS SANTOS DL, DE ALMEIDA DC, HENRIQUES JAP, DANI C & FUNCHAL C. 2013. Effect of chronic treatment with conventional and organic purple grape juices (Vitis labrusca) on rats fed with high-fat diet. Cell Mol Neurobiol 33: 1123-1133.
- CARPENE C, GOMEZ-ZORITA S, DELERUYELLE S & CARPENE MA. 2015. Novel strategies for preventing diabetes and obesity complications with natural polyphenols. Curr Med Chem 22: 150-164.
- CHARBONNEAU A, UNSON CG & LAVOIE JM. 2007. High-fat diet-induced hepatic steatosis reduces glucagon receptor content in rat hepatocytes: potential interaction with acute exercise. J Physiol 579: 255-267.
- CHARRADI K, ELKAHOUI S, LIMA M & AOUANI E. 2013. High-fat diet induced an oxidative stress in white adipose tissue and disturbed plasma transition metals in rat: prevention by grape seed and skin extract. J Physiol Sci 63: 445-455.
- CHASSOT LN, SCOLARO B, ROSCHEL GG, COGLIATI B, CAVALCANTI MF, ABDALLA DS & CASTRO IA. 2018. Comparison between red wine and isolated trans-resveratrol on the prevention and regression of atherosclerosis in LDLr (−/−) mice. J Nutr Biochem 61: 48-55.
- CHENG HS, TON SH, PHANG SCW, TAN JBL & KADIR KA. 2017. Increased susceptibility of post-weaning rats on high-fat diet to metabolic syndrome. J Adv Res 8: 743-752.
- CHIANG WD, SHIBU MA, LEE KI, WU JP, TSAI FJ, PAN LF, HUANG Y & LIN WT. 2014. Lipolysis-stimulating peptide-VHVV ameliorates high fat diet induced hepatocyte apoptosis and fibrosis. J Funct Foods 11: 482-492.
- COHEN JC, HORTON JD & HOBBS HH. 2011. Human fatty liver disease: old questions and new insights. Science 332: 1519-1523.
- CUNHA AL, MOURA KS, BARBOSA JC & DOS SANTOS AF. 2016. Os metabólitos secundários e sua importância para o organismo. Diversitas Journal 1: 175-181.
- CZAJA MJ ET AL. 2013. Functions of autophagy in normal and diseased liver. Autophagy 9: 1131-1158.
- DANI C, OLIBONI LS, VANDERLINDE R, BONATTO D, SALVADOR M & HENRIQUES JAP. 2007. Phenolic content and antioxidant activities of white and purple juices manufactured with organically-or conventionally-produced grapes. Food Chem Toxicol 45: 2574-2580.
- DEJI N ET AL. 2009. Structural and functional changes in the kidneys of high-fat diet-induced obese mice. Am J Physiol-Renal 296: 118-126.
- DE MOURA CFG, RIBEIRO FAP, HANDAN BA, AGUIAR O, OSHIMA CTF & RIBEIRO DA. 2016. Grape juice concentrate protects rat liver against cadmium intoxication: histopathology, cytochrome C and metalloproteinases expression. Drug Res 66: 339-344.
- DE OLIVEIRA TK, ALMEIDA FDA, FALCÃO MPM, DE LEMOS-JORDÃO AJJ, RAMOS KR & SILVA JFD. 2016. Analysis of the aqueous extract of Arachis hipoagea L. to reduce dyslipidemia and weight gain in Wistar rats with high fat diet. Pesqui Agropecu Bras 36: 1121-1126.
- DIXON LJ, BARNES M, TANG H, PRITCHARD MT & NAGY LE. 2013. Kupffer cells in the liver. Compr Physiol 3: 785-797.
- DOS SANTOS LIMA M, SILANI IDSV, TOALDO IM, CORRÊA LC, BIASOTO ACT, PEREIRA GE, BORDIGNON-LUIZ AT & NINOW JL. 2014. Phenolic compounds, organic acids and antioxidant activity of grape juices produced from new Brazilian varieties planted in the Northeast Region of Brazil. Food Chem 161: 94-103.
- DUDLEY KJ, SLOBODA DM, CONNOR KL, BELTRAND J & VICKERS MH. 2011. Offspring of mothers fed a high fat diet display hepatic cell cycle inhibition and associated changes in gene expression and DNA methylation. PLoS ONE 6: 21662.
- ECKERT C, KLEIN N, KORNEK M & LUKACS-KORNEK V. 2015. The complex myeloid network of the liver with diverse functional capacity at steady state and in inflammation. Front Immunol 6: 179.
- EL AYED M, KADRI S, MABROUK M, AOUANI E & ELKAHOUI S. 2018. Protective effect of grape seed and skin extract against high-fat diet-induced dyshomeostasis of energetic metabolism in rat lung. Lipids Health Dis 17: 109.
- ESTROV Z, SHISHODIA S, FADERL S, HARRIS D, VAN Q, KANTARJIAN HM, TALPAZ M & AGGARWAL BB. 2003. Resveratrol blocks interleukin-1β–induced activation of the nuclear transcription factor NF-κB, inhibits proliferation, causes S-phase arrest, and induces apoptosis of acute myeloid leukemia cells. Blood 102: 987-995.
- FARIAS M ET AL. 2015. Effect of grape juice on some biochemical and oxidative stress parameters in serum and liver enzymes of pregnant and lactating rats. Issues Biol Sci Pharm Res 3: 37-46.
- FARRELL GC, VAN ROOYEN D, GAN L & CHITTURI S. 2012. NASH is an inflammatory disorder: pathogenic, prognostic and therapeutic implications. Gut Liver 6: 149.
- FAUSTO N, CAMPBELL JS & RIEHLE KJ. 2006. Liver regeneration. Hepatology 43: S45-S53.
- FESTI D, COLECCHIA A, SACCO T, BONDI M, RODA E & MARCHESINI G. 2004. Hepatic steatosis in obese patients: clinical aspects and prognostic significance. Obes Rev 5: 27-42.
- FONTELLES CC, DA CRUZ RS, HILAKIVI-CLARKE L, DE ASSIS S & ONG TP. 2018. Investigation of Paternal Programming of Breast Cancer Risk in Female Offspring in Rodent Models. In Investigations of Early Nutrition Effects on Long-Term Health Humana Press, New York 1735: 207-220.
- GHANIM H, SIA C L, KORZENIEWSKI K, LOHANO T, ABUAYSHEH S, MARUMGANTI A, CHAUDHRI T & DANDONA P. 2011. A resveratrol and polyphenol preparation suppresses oxidative and inflammatory stress response to a high-fat, high-carbohydrate meal. J Clin Endocrinol Metab 96: 1409-1414.
- GHANTOUS Y, SCHUSSEL JL & BRAIT M. 2018. Tobacco and alcohol-induced epigenetic changes in oral carcinoma. Curr Opin Oncol 30: 152-158.
- GIOVINAZZO G & GRIECO F. 2015. Functional properties of grape and wine polyphenols. Plant Foods Hum Nutr 70: 454-462.
- GONÇALVES L, BORTOLATO G, NETO RDB, FRUSCIANTE MR, FUNCHAL C & DANI C. 2018. Grape Juice Consumption with or without High Fat Diet during Pregnancy Reduced the Weight Gain and Improved Lipid Profile and Oxidative Stress Levels in Liver and Serum from Wistar Rats. Beverages 4: 78.
- GU J, HU W, SONG ZP, CHEN YG, ZHANG DD & WANG CQ. 2016. Resveratrol-induced autophagy promotes survival and attenuates doxorubicin-induced cardiotoxicity. Int Immunopharmacol 32: 1-7.
- GUIMARÃES DDAB, DE CASTRO DDSB, OLIVEIRA FLD, NOGUEIRA EM, SILVA MAMD & TEODORO AJ. 2017. Pitaya Extracts Induce Growth Inhibition and Proapoptotic Effects on Human Cell Lines of Breast Cancer via Downregulation of Estrogen Receptor Gene Expression. Oxid Med Cell Longev 2017: 13 p.
- HAN Z, CHATTERJEE D, HE DM, EARLY J, PANTAZIS P, WYCHE JH & HENDRICKSON EA. 1995. Evidence for a G2 checkpoint in p53-independent apoptosis induction by X-irradiation. Mol Cell Biol 15: 5849-5857.
- HEM A, SMITH AJ & SOLBERG P. 1998. Saphenous vein puncture for blood sampling of the mouse, rat, hamster, gerbil, guineapig, ferret and mink. Lab Anim 32: 364-368.
- INGLÉS M, GAMBINI J, MIGUEL MG, BONET-COSTA V, ABDELAZIZ KM, EL ALAMI M, VIÑA J & BORRÁS C. 2014. PTEN mediates the antioxidant effect of resveratrol at nutritionally relevant concentrations. Biomed Res Int 2014: 6 p.
- JESUS LH, CAVAGNI J, CARRARD VC, RÖSING CK & SANT’ANA FILHO M. 2017. Effect of different presentations of resveratrol on cell proliferation and epitelial thickness of the oral mucosa of wistar rats. Clin Biomed Res 37: 175-180.
- JUNG K J, WALLIG MA & SINGLETARY KW. 2006. Purple grape juice inhibits 7, 12-dimethylbenz [a] anthracene (DMBA)-induced rat mammary tumorigenesis and in vivo DMBA-DNA adduct formation. Cancer Lett 233: 279-288.
- KIM S, JIN Y, CHOI Y & PARK T. 2011. Resveratrol exerts anti-obesity effects via mechanisms involving down-regulation of adipogenic and inflammatory processes in mice. Biochem Pharmacol 81: 1343-1351.
- KONG B, WANG X, HE B, WEI L, ZHU J, JIN Y & FU Z. 2019. 8:2 fluorotelomer alcohol inhibited proliferation and disturbed the expression of pro-inflammatory cytokines and antigen-presenting genes in murine macrophages. Chemosphere 219: 1052-1060.
- KOO SH. 2013. Nonalcoholic fatty liver disease: molecular mechanisms for the hepatic steatosis. Clin Mol Hepatol 19: 210.
- KRYSKO DV, BERGHE TV, D’HERDE K & VANDENABEELE P. 2008. Apoptosis and necrosis: detection, discrimination and phagocytosis. Methods 44: 205-221.
- LALIENA A, MIGUEL B S, CRESPO I, ALVAREZ M, GONZÁLEZ-GALLEGO J & TUÑÓN M J. 2012. Melatonin attenuates inflammation and promotes regeneration in rabbits with fulminant hepatitis of viral origin. J Pineal Res 53: 270-278.
- LEUNG A, TRAC C, DU J, NATARAJAN R & SCHONES D E. 2016. Persistent chromatin modifications induced by high fat diet. J Biol Chem 291: 10446-10455.
- LEVY RB, CLARO RM, MONDINI L, SICHIERI R & MONTEIRO C A. 2011. Distribuição regional e socioeconômica da disponibilidade domiciliar de alimentos no Brasil em 2008-2009. Rev Saúde Públ 46: 6-15.
- LIU X, YU L, HASSAN W, SUN L, ZHANG L & JIANG Z. 2016. The duality of kupffer cell responses in liver metabolic states. Curr Mol Med 16: 809-819.
- LOZANO I ET AL. 2016. High-fructose and high-fat diet-induced disorders in rats: impact on diabetes risk, hepatic and vascular complications. Nutr Metab 13: 15.
- MARTINS PP & NICOLETTI MA. 2016. Polifenóis no vinho: resveratrol e seus benefícios. Infarma-Ciências Farmacêuticas 28: 216-225.
- MENG J, SHI TC, SONG S, ZHANG ZW & FANG YL. 2017. Melatonin in grapes and grape-related foodstuffs: A review. Food Chem 231: 185-191.
- MILIĆ S, LULIĆ D & ŠTIMAC D. 2014. Non-alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations. WJG 20: 9330.
- MITCHELL C & WILLENBRING H. 2008. A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nat Protoc 3: 1167.
- MIZUSHIMA N, LEVINE B, CUERVO AM & KLIONSKY DJ. 2008. Autophagy fights disease through cellular self-digestion. Nature 451: 1069.
- MUKAMAL KJ ET AL. 2016. Moderate alcohol consumption and chronic disease: the case for a long-term trial. Alcohol Clin Exp Res 40: 2283-2291.
- NOLAN CJ, DAMM P & PRENTKI M. 2011. Type 2 diabetes across generations: from pathophysiology to prevention and management. The Lancet 378: 169-181.
- NOUREDDIN M & RINELLA ME. 2015. Nonalcoholic fatty liver disease, diabetes, obesity, and hepatocellular carcinoma. Clin Liver Dis 19: 361-379.
- OARADA M, TSUZUKI T, NIKAWA T, KOHNO S, HIRASAKA K & GONOI T. 2012. Refeeding with a high-protein diet after a 48 h fast causes acute hepatocellular injury in mice. Br J Nutr 107: 1435-1444.
- OHASHI T, KATO M, YAMASAKI A, KUWANO A, SUZUKI H, KOHJIMA M & OGAWA Y. 2018. Effects of high fructose intake on liver injury progression in high fat diet induced fatty liver disease in ovariectomized female mice. Food Chem Toxicol 118: 190-197.
- OLIVEIRA ACD, VALENTIM IB, GOULART MOF, SILVA CA, BECHARA EJH & TREVISAN MTS. 2009. Fontes vegetais naturais de antioxidantes. Quím Nova 32: 689-702.
- PEREIRA RA, DUFFEY KJ, SICHIERI R & POPKIN BM. 2014. Sources of excessive saturated fat, trans fat and sugar consumption in Brazil: an analysis of the first Brazilian nationwide individual dietary survey. Public Health Nutr 17: 113-121.
- PETYAEV IM. 2016. Lycopene deficiency in ageing and cardiovascular disease. Oxid Med Cell Longev 2016: 6 p.
- POLJSAK B. 2011. Strategies for reducing or preventing the generation of oxidative stress. Oxid Med Cell Longev 2011: 15 p.
- RAHAL A, KUMAR A, SINGH V, YADAV B, TIWARI R, CHAKRABORTY S & DHAMA K. 2014. Oxidative stress, prooxidants, and antioxidants: the interplay. Biomed Res Int 2014: 19 p.
- REEVES PG, NIELSEN FH & FAHEY JR GC. 1993. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123: 1939-1951.
- REYNÉS B, PALOU M & PALOU A. 2017. Gene expression modulation of lipid and central energetic metabolism related genes by high-fat diet intake in the main homeostatic tissues. Food Funct 8: 629-650.
- RINDLER PM, PLAFKER SM, SZWEDA L & KINTER M. 2013. High dietary fat selectively increases catalase expression within cardiac mitochondria. J Biol Chem 288: 1979-1990.
- ROESLER R, MALTA LG, CARRASCO LC, HOLANDA RB, SOUSA CAS & PASTORE GM. 2007. Atividade antioxidante de frutas do cerrado. Food Sci Technol 27: 53-60.
- SANTOS J, VALENTIM I, DE ARAÚJO O, ATAIDE T & GOULART M. 2013. Development of nonalcoholic hepatopathy: contributions of oxidative stress and advanced glycation end products. Int J Mol Sci 14: 19846-19866.
- SAUTTER CK, DENARDIN S, ALVES AO, MALLMANN CA, PENNA NG & HECKTHEUER L H. 2005. Determinação de resveratrol em sucos de uva no Brasil. Ciênc Tecnol Aliment 25: 437-442.
- SAVAGE DB & SEMPLE RK. 2010. Recent insights into fatty liver, metabolic dyslipidaemia and their links to insulin resistance. Curr Opin Lipidol 21: 329-336.
- SCOTT E, STEWARD WP, GESCHER AJ & BROWN K. 2012. Resveratrol in human cancer chemoprevention–choosing the ‘right’ dose. Mol Nutr Food Res 56: 7-13.
- SEKI E & SCHWABE RF. 2015. Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology 61: 1066-1079.
- SHONO S, HABU Y, NAKASHIMA M, SATO A, NAKASHIMA H, MIYAZAKI H, KINOSHITA M, TSUMATORI G, SHINOMIYA N & SEKI S. 2011. The immunologic outcome of enhanced function of mouse liver lymphocytes and Kupffer cells by high-fat and high-cholesterol diet. Shock 36: 484-493.
- SIMSA-MAZIEL S & MONSONEGO-ORNAN E. 2012. Interleukin-1β promotes proliferation and inhibits differentiation of chondrocytes through a mechanism involving down-regulation of FGFR-3 and p21. Endocrinology 153: 2296-2310.
- SINGH CK, SIDDIQUI IA, EL-ABD S, MUKHTAR H & AHMAD N. 2016. Combination chemoprevention with grape antioxidants. Mol Nutr Food Res 60: 1406-1415.
- SINGH SK, BANERJEE S, ACOSTA EP, LILLARD JW & SINGH R. 2017. Resveratrol induces cell cycle arrest and apoptosis with docetaxel in prostate cancer cells via a p53/p21WAF1/CIP1 and p27KIP1 pathway. Oncotarget 8: 17216.
- SOLTIS AR ET AL. 2017. Hepatic dysfunction caused by consumption of a high-fat diet. Cell Rep 21: 3317-3328.
- SZKUDELSKA K, NOGOWSKI L & SZKUDELSKI T. 2009. The inhibitory effect of resveratrol on leptin secretion from rat adipocytes. Eur J Clin Invest 39: 899-905.
- TARANTINO G. 2007. Should nonalcoholic fatty liver disease be regarded as a hepatic illness only? WJG 13: 4669.
- TIMMERS S ET AL. 2011. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab 14: 612-622.
- TUNALI-AKBAY T, SEHIRLI O, ERCAN F & SENER G. 2010. Resveratrol protects against methotrexate-induced hepatic injury in rats. J Pharm Pharm Sci 13: 303-310.
- VALENZUELA M, BASTIAS L, MONTENEGRO I, WERNER E, MADRID A, GODOY P, PÁRRAGA M & VILLENA J. 2018. Autumn Royal and Ribier Grape Juice Extracts Reduced Viability and Metastatic Potential of Colon Cancer Cells. Evid-Based Compl Alt 2018: 7 p.
- VAN HERCK M, VONGHIA L & FRANCQUE S. 2017. Animal models of nonalcoholic fatty liver disease—a starter’s guide. Nutrients 9: 1072.
- VIOLA J & SOEHNLEIN O. 2015. Atherosclerosis–a matter of unresolved inflammation. Semin Immunol 27: 184-193.
- WANG T, ZHANG Y, ZENG Y & QING XU. 2017. Effect of resveratrol on proliferation of liver cancer SMMC-7721 cells and lowering levels of mTOR protein phosphorylation. Chem Pharm Bull 33: 1309-1314.
- WELLEN KE & THOMPSON CB. 2010. Cellular metabolic stress: considering how cells respond to nutrient excess. Mol Cell 40: 323-332.
- WU T, GUO Y, LIU R, WANG K & ZHANG M. 2016. Black tea polyphenols and polysaccharides improve body composition, increase fecal fatty acid, and regulate fat metabolism in high-fat diet-induced obese rats. Food Funct 7: 2469-2478.
- YUZEFOVYCH LV, MUSIYENKO SI, WILSON GL & RACHEK LI. 2013. Mitochondrial DNA damage and dysfunction, and oxidative stress are associated with endoplasmic reticulum stress, protein degradation and apoptosis in high fat diet-induced insulin resistance mice. PLoS ONE 8: e54059.
Publication Dates
-
Publication in this collection
10 Aug 2020 -
Date of issue
2020
History
-
Received
13 Oct 2019 -
Accepted
7 Nov 2019