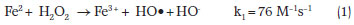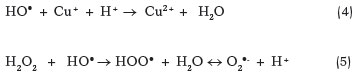Abstract
The addition of Cu2+ ions to the classical Fenton reaction (Fe2+ plus H2O2 at pH 3) is found to accelerate the degradation of organic compounds. This synergic effect causes an approximately 15 % additional reduction of the total organic carbon (TOC), representing an overall improvement of the efficiency of the mineralization of phenol. Although Fe2+ exhibits a high initial rate of degradation, the degradation is not complete due to the formation of compounds refractory to the hydroxyl radical. The interference of copper ions on the degradation of phenol by the Fenton reaction was investigated. In the presence of Cu2+, the degradation is slower, but results in a greater reduction of TOC at the end of the reaction (t = 120 min). In the final stages of the reaction, when the Fe3+ in the solution is complexed in the form of ferrioxalate, the copper ions assume the role of the main catalyst of the degradation
AGRICULTURAL ENGINEERING
Interference of inorganic ions on phenol degradation by the Fenton reaction
Leidi Cecilia FriedrichI,* * Corresponding author < leidi@iq.usp.br> ; Carmem Lúcia de Paiva e Silva ZantaII; Amilcar Machulek Jr.III; Volnir de Oliveira SilvaI; Frank Herbert QuinaI
IUSP/Instituto de Química, Av. Professor Lineu Prestes, 580 - Bloco 04 - 05508-001 - São Paulo, SP - Brasil
IIUFAL/Instituto de Química e Biotecnologia, Campus A. C. Simões, Av. Lourival Melo Mota, s/n - Tabuleiro do Martins - 57072-970 - Maceió, AL - Brasil
IIIUFMS/Centro de Ciências Exatas e Tecnologia - Depto. de Química, Cidade Universitária, Av. Filinto Müller, 1555 - 79074-460 - Campo Grande, MS - Brasil
ABSTRACT
The addition of Cu2+ ions to the classical Fenton reaction (Fe2+ plus H2O2 at pH 3) is found to accelerate the degradation of organic compounds. This synergic effect causes an approximately 15 % additional reduction of the total organic carbon (TOC), representing an overall improvement of the efficiency of the mineralization of phenol. Although Fe2+ exhibits a high initial rate of degradation, the degradation is not complete due to the formation of compounds refractory to the hydroxyl radical. The interference of copper ions on the degradation of phenol by the Fenton reaction was investigated. In the presence of Cu2+, the degradation is slower, but results in a greater reduction of TOC at the end of the reaction (t = 120 min). In the final stages of the reaction, when the Fe3+ in the solution is complexed in the form of ferrioxalate, the copper ions assume the role of the main catalyst of the degradation.
Introduction
Currently, there is great concern with the environment, particularly regarding the pollution of water resources, leading to a search for technologies to treat domestic and industrial waste. Among these new technologies, advanced oxidation processes (AOP) are potentially the most promising due to their high efficiency and versatility. Thus, a variety of classes of compounds can be completely mineralized by existing AOP, including compounds refractory to conventional biological treatment processes (Kiwi et al., 2000; Pérez et al., 2002; Pignatello et al., 2006).
The AOP are typically based on the generation of highly oxidizing species such as the hydroxyl radical (HO), which has a high oxidation potential (Eº = + 2.80 V vs. NHE) and is capable of initiating reactions that can often lead to the total degradation of organic matter. Among the AOP, the Fenton reaction is perhaps the most promising as an alternative method for wastewater treatment due to its high oxidative power, very fast reaction rate, relatively low cost and ease of operation and maintenance (Walling and Camaioni, 1975).
The classic mechanism of the Fenton reaction is a simple redox reaction in which Fe2+ ions are oxidized to Fe3+ and H2O2 is reduced to a hydroxyl radical and hydroxide ion (Equation 1).
Copper also acts as a catalyst in the decomposition of H2O2 (cupro-Fenton reaction), similar to iron (Aguiar et al., 2007). Both transition metals react with H2O2 to form intermediate complexes that then decompose, forming the radical HO. Organic peroxides (RO2H) are decomposed by these metals to form alkoxyl radicals (RO) and organic peroxyl radicals (RO2). In the cupro-Fenton reaction, the complex formed between H2O2 and the metal ion has been reported to be more stable than the analogous complex in the ferrous Fenton reaction (Johnson et al., 1985).
The oxidizing activity of Fe3+ and Cu2+ ions has been evaluated for various phenolic compounds, mainly derivatives of catechol. Catechol is oxidized by Cu2+, generating a semiquinone that is subsequently oxidized to form a quinone. Molecular oxygen (O2) can also act as the electron acceptor, being reduced by the semiquinone radical to superoxide/hydroperoxide, O2-/HO2, which can be converted into H2O2. The mechanism proposed by Aguiar et al. (2007) for the reduction of Fe3+ or Cu2+ by catechol is outlined in Figure 1. Therefore, there are several pathways involving phenolic compounds and metal ions that can potentially increase the efficiency of the Fenton process (Hamilton et al., 1966; Chen and Pignatello, 1997).
In this study, we report an investigation of the interference of copper ions on the degradation of phenol by the Fenton reaction.
Materials and Methods
The following reagents were used: Ferrous sulfate heptahydrate (FeSO4.7 H2O, Synth), copper sulfate pentahydrate (CuSO4.5 H2O, Synth), sodium sulfate (Na2SO4, Vetec), hydrogen peroxide (H2O2, 30 %, Synth), sulfuric acid (H2SO4, Synth), sodium hydroxide (NaOH, Merck), phenol (Aldrich), catechol (Acros), acetic acid (Merck), formic acid (Aldrich), oxalic acid (Aldrich) and acetonitrile (JT Baker), all reagent grade or superior, were used as received.
The solutions of phenol were prepared by directly dissolving the desired amount of phenol in aqueous solution. The reactor used for the degradation of phenol was a batch reactor with an internal volume of 1.0 L (Figure 2), protected from incident light in order to minimize the effect of photochemical reactions. The solution temperature was controlled at 30 ºC using a thermostatic bath and magnetic stirring.
Initially, a solution containing 10 mM phenol, 0.5 mM Fe2+ and different concentrations of Cu2+ at pH = 3.0 was added to the reactor. Aqueous hydrogen peroxide was added slowly (3.33 mM min-1), with the aid of a peristaltic pump, during the first 60 minutes of reaction to minimize the formation of the hydroperoxyl radical (Equation 2) (Kang et al., 2002).
At selected time intervals, 5 mL of sample solution were collected and 2 drops of a solution of 2.0 M NaOH immediately added, raising the pH to ~ 12. The increase in pH precipitated Fe3+, stopping the reaction. After filtration, the sample was reacidified to a pH around 3.0 to maintain the same conditions as in the reactor and total organic carbon (TOC) was analyzed with a Shimadzu Model TOC-5000A analyzer.
Phenol and the main degradation products (hydroxyaromatics and aliphatic acids) were identified and quantified by high performance liquid chromatography (for hydroxyaromatics - Shimadzu 2010A LC-MS, and for aliphatic acids - Shimazdu 20AD HPLC) using standard compounds for identification and calibration. For the determination of the aromatic intermediates, a Shim-pack C18 reverse phase column (5 µm, 4.6 × 150 mm) was used with detection at 270 nm. The mobile phase consisted of an aqueous solution containing 0.2 % acetic acid (A) and acetonitrile containing 0.2 % acetic acid (B). During 3 min, the mobile phase (flow rate 0.7 mL min-1) was 18 % B: 82 % A, followed by a gradient of 18-58 % B over 10 min and completing the analysis by reverting for 1 min to 18 % B: 82 % A. To determine the intermediate acids, a Hamilton PRP brand-X300 ion exchange column was used with detection at 220 nm. The mobile phase consisted of an aqueous solution of H2SO4 (pH 2.00 ± 0.02), at a flow rate of 1 mL min-1, with the column temperature maintained at 30 ºC. Before being injected onto the ion exchange column, the samples were filtered through a C18 cartridge previously activated with methanol. This step removed the remaining aromatic compounds, necessary since these compounds are strongly retained on the ion exchange column, resulting in broad bands that hamper analysis (Machuleck et al., 2007).
Results and Discussion
The addition of Cu2+ affected the rate of oxidation of phenol (p < 0.05l), and an increase in the concentration of Cu2+ increased the rate of reaction (Figure 3). In the presence of only Fe2+ (Fenton reaction), the net decrease of the TOC is only 40 % after 120 min. However, upon addition of sufficient Cu2+ (> 5.0 mM) to the Fenton reaction, the degradation is almost complete by 60 min, implicated in the reduction of organic material, enhancing the degradation of the pollutant, in this case the phenol.
Figure 4 further illustrates the synergy between Fe2+ and Cu2+ in the degradation of phenol (TOC reduction). Thus, little or no mineralization is observed in the presence of 0.50 mM Cu2+ alone. In the presence of Fe2+ ion alone, the mineralization reaches a maximum of ca. 40-45 % after 30 minutes of reaction. Combining the two ions, in a cupro-Fenton reaction, the mineralization reaches 60 % at the end of the reaction. It was initially analyzed the concentration of phenol as a function of reaction time (Figure 5). Phenol was completely degraded in 15 min in both the Fenton and cupro-Fenton reactions, but only at a much slower rate by Cu2+ alone in the absence of Fe2+.
The two intermediates initially formed in the degradation of phenol are catechol and hydroquinone, both of which can act as catalysts for the Fenton (Zanta et al., 2010) and cupro-Fenton reactions (Valenzuela et al., 2008) due to the regeneration of Fe2+ and Cu1+. In the presence of Cu2+, the amount of catechol generated was much lower (Figure 6), explaining the much lower catalytic effect on phenol degradation (Figure 5).
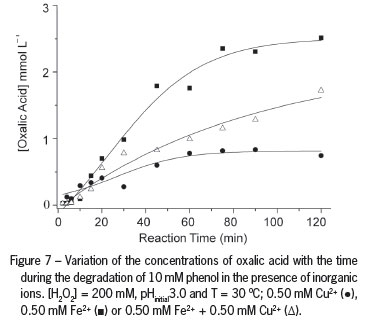
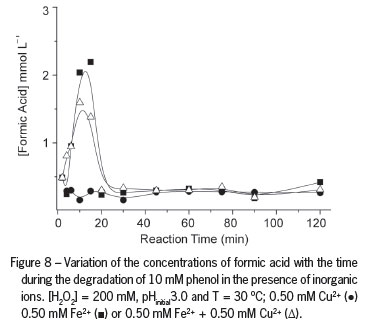
The analysis of the organic compounds remaining at the end of the reaction showed the formation of oxalic acid (OA) (Figure 7) and formic acid (FA) (Figure 8). OA is not degraded in the Fenton reaction due to formation of the ferrioxalate complex, which removes free iron from the solution and stops the reaction. Acetic acid was also detected, but it was quickly oxidized to CO2 and water. Oxalic acid and formic acid proved to be the two most refractory intermediates and could be detected up until the end of the reaction, in agreement with other authors (Kwon et al., 1999). In the presence of H2O2, Cu2+ is regenerated by the Fenton reaction between Cu1+ and H2O2 (Equation 3) under experimental conditions in which HO● radicals are formed.
The study of the primary reactions of the thermal step of the Fenton reaction in the presence of copper ions suggests the possible role of copper ions. Catechol and/or hydroquinone formed by the Fenton oxidation of phenol can reduce Cu2+ to Cu+ (Figure 1). The reaction of copper (I) ions with H2O2 can regenerate Cu2+ via a reaction similar to the Fenton reaction, forming HO● radicals (Equation 3).
These HO● radicals can oxidize Cu1+ to form Cu2+ (Equation 4) or, in the presence of excess H2O2, be consumed by another molecule of H2O2 to produce HOO● and O2●- (Equation 5) (Liu et al., 2005).
In the final stages of the reaction, when iron is complexed in the form of ferrioxalate, copper ions continue to degrade intermediates in the oxidation process, resulting an increase in the overall mineralization of phenol.
Conclusions
In the conventional Fenton reaction with Fe2+/Fe3+ and H2O2, aliphatic organic acids formed in the degradation of phenol (mainly oxalic acid) make it difficult to obtain complete mineralization, resulting in a final TOC value that is quite high. The combination of the Fenton reaction with a cupro-Fenton reaction can increase the overall efficiency of the process, a synergistic effect that occurs mainly in the final stages of the degradation and mineralization.
Acknowledgements
L.C.F. thanks the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brasília, for graduate research fellowship support. C.L.P.S.Z. thanks FAPEAL, A.M. Jr. thanks FUNDECT for financial support of the work at UFMS and F.H.Q. thank the CNPq and INCT- Catalysis for fellowships and funding.
Received January 20, 2012
Accepted March 25, 2012
Edited by: Daniel Scherer de Moura
- Aguiar, A.; Ferraz, A.; Contreras, D.; Rodríguez, J. 2007. Mechanism and apllications of the Fenton reaction assisted by iron-reducing phenolic compounds. Química Nova 30: 623-628 (in Portuguese, with abstract in English).
- Chen, R.; Pignatello, J.J. 1997. Role of quinone intermediates as electron shuttles in Fenton and photoassisted Fenton oxidations of aromatic compounds. Environmental Science & Technology 31:2399-2406.
- Hamilton, G.A.; Friedman, J.P.; Campbell, P.M. 1966. The hydroxylation of anisole by hydrogen peroxide in the presence of catalytic amounts of ferric ion and catechol: scope, requirements and kinetic studies. Journal of the American Chemical Society 88:5266-5268.
- Johnson, G.R.A.; Nazhat, N.B.; Saadallanazhat, R.A. 1985. Reaction of the aquocopper(I) ion with hydrogen peroxide: Evidence against hydroxyl free radical formation. Journal of the Chemical Society 7:407-408.
- Kang, N.; Lee, D.S.; Yoon, J. 2002. Kinetic modeling of Fenton oxidation of phenol and monochlorophenols. Chemosphere 47:915-921.
- Kiwi, J.; Lopez, A.; Nadtochenko, V. 2000. Mechanism and kinetics of the OH-radical intervention during Fenton oxidation in the presence of a significant amount of radical scavenger (Cl-). Environmental Science & Technology 34:2162-2168.
- Kwon, B.G.; Lee, D.S.; Kang, N.; Yoon, J.Y. 1999. Characteristics of p-chlorophenol oxidation by Fenton's reagent. Water Research 33:210-218.
- Liu, R.; Goodell, B.; Jellison, J.; Amirbahman, A. 2005. Electrochemical study of 2, 3-dihydroxybenzoic acid and its interaction with Cu (II) an H2O2 in aqueous solutions: implications for wood decay. Environmental Science & Technology 39:175-180.
- Machuleck Jr., A; Moraes, J.F.; Vautier-Giongo, C.; Silverio, C.A.; Friedrich, L.C.; Nascimento, C.A.O.; Gonzalez, M.C.; Quina, F.H. 2007. Abatement of the inhibitory effect of chloride anions on the photo-Fenton process. Environmental Science & Technology 41:8459-8463.
- Pérez, M.; Torrades, F.; Doménech, X.; Peral, J. 2002. Fenton and photo-Fenton oxidation of textile effluents. Water Research 36:2703-2710.
- Pignatello, J.J.; Oliveros, S.E.; Mackay, A. 2006. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry: critical reviews. Environmental Science & Technology 36:1-84.
- Valenzuela, R.; Contreras, D.; Oviedo, C.; Freer, J.; Rodriguez, J. 2008. Copper catechol-driven Fenton reactions and their potential role in wood degradation. International Biodeterioration & Biodegradation 61:345-350.
- Walling, C.; Camaioni, D.M. 1975. Aromatic hydroxylation by peroxydisulfate. Journal of the American Chemical Society 97:1603-1604.
- Zanta, C.L.P.S.; Friedrich, L.C.; Machulek Jr., A.; Higa, K.M.; Quina, F.H. 2010. Surfactant degradation by a catechol-driven Fenton reaction. Journal of Hazardous Materials 178:258-263.
Publication Dates
-
Publication in this collection
14 Nov 2012 -
Date of issue
Dec 2012
History
-
Received
20 Jan 2012 -
Accepted
25 Mar 2012