ABSTRACT
To establish a transfusion-associated graft-versus-host disease (TA-GVHD) mouse model with busulfan and fludarabine for effective treatment evaluation.
BALB/c (H-2d) mice were injected with busulfan (15 mg/kg) and fludarabine (30 mg/kg) twice a day for 4 days. The mice were transfused with 106 T cell-depleted bone marrow (TCD-BM )and cells in different groups 3 days after chemotherapy: syngeneic BALB/c, MHC minor mismatch DBA/2 (H-2d), or MHC major mismatch C57BL/6(H2-b). Recipient BALB/c mice were injected with either blood only or blood+splenocyte. TA-GVHD was monitored in terms of body weight loss, clinical scores, and survival. Dexamethasone (50 mg/kg), cyclophosphamide (50 mg/kg), cyclosporine A (30 mg/kg), and anti-CD3 (1 mg/kg) were injected to each group to examine the treatments.
Blood transfusion alone is insufficient to induce TA-GVHD in a chemotherapy-based mouse model. A MHC-mismatched TA-GVHD model can be induced by splenocyte and blood transfusion. This MHC-mismatched TA-GVHD model was resistant to dexamethasone treatment. Treatment based on anti-CD3 monoclonal antibody slightly ameliorated TA-GVHD. Treatment effectiveness was associated with T-cell depletion following activation by anti-CD3.
Busulfan and fludarabine chemotherapy regimen can be used to establish a TA-GVHD mouse model. Anti-CD3 monoclonal antibody is a potential alternative to treat TA-GVHD.
Key words:
transfusion-associated graft-versus-host disease; fludarabine; busulfan; animal model; major histocompatibility complex
INTRODUCTION
Transfusion-associated graft-versus-host disease (TA-GVHD) is a fatal complication of blood transfusion and is accounted for 90% to 100% mortality 11. Kopolovic I, Ostro J, Tsubota H, Lin Y, Cserti-Gazdewich CM, Messner HA, Keir AK, DenHollander N, Dzik WS, Callum J. A systematic review of transfusion-associated graft-versus-host disease. Blood. 2015 Jul 16;126(3):406-14.. TA-GVHD is a syndrome similar to GVHD induced by bone marrow transplantation. Immunosuppressive patients who receive blood transfusion containing immune-active lymphocytes are prone to serious clinical syndromes 22. Hummon D, Zantek ND, Sumstad D, Miller JS, McKenna DH. Transfusion-associated graft-versus-host disease: a perspective from a cell therapy laboratory. Transfusion. 2009 May;49(5):1018-9.. With extensive applications of immunosuppressive nucleotide analogs, such as fludarabine, the incidence of TA-GVHD increases annually 33. Jawa RS, Young DH, Stothert JC, Kulaylat MN, Landmark JD. Transfusion -associated graft versus host disease in the immunocompetent patient: an ongoing problem.J Intensive Care Med. 2015 Mar;30(3):123-30..
TA-GVHD usually occurs in immunosuppressive and immunodeficient patients. The pathogenesis and mechanism of TA-GVHD are complex and likely similar to GVHD in patients who have undergone bone marrow transplantation. Infused donor lymphocytes can be activated , undergo proliferation and differentiation; these lymphocytes then attack GVHD-targeted organs and thus cause TA-GVHD (44. Schroeder ML. Transfusion -associated graft-versus-host disease. Br J Haematol 2002;117:275-87.. The severity of TA-GHVD is correlated with the number and immune activity of T lymphocytes in blood components. TA-GVHD generally occurs 2 weeks after blood transfusion 55. Williamson LM, Stainsby D, Jones H, Love E, Chapman CE,Navarrete C, Lucas G, Beatty C, Casbard A, Cohen H. The impact of universal leukodepletion of the blood supply on hemovigilance reports of posttransfusion purpura and transfusion- associated graft-versus-host disease. Transfusion 2007;47:1455-67.. The main possible targets of TA-GVHD include the skin, digestive system, bone marrow, and lungs. However, the clinical manifestations of this disease are complex and atypical.
TA-GVHD progresses rapidly and presents poor prognosis. With the increasing application of immunosuppression therapy, the incidence of TA-GVHD has also significantly increased 33. Jawa RS, Young DH, Stothert JC, Kulaylat MN, Landmark JD. Transfusion -associated graft versus host disease in the immunocompetent patient: an ongoing problem.J Intensive Care Med. 2015 Mar;30(3):123-30.. This disease is commonly treated with large doses of dexamethasone (Dex), antithymocyte globulin (ATG), and immune inhibitors, such as cyclosporine A (CsA) and cyclophosphamide (CTX). However, none of these treatments can effectively reduce mortality 22. Hummon D, Zantek ND, Sumstad D, Miller JS, McKenna DH. Transfusion-associated graft-versus-host disease: a perspective from a cell therapy laboratory. Transfusion. 2009 May;49(5):1018-9.. Therefore, the development of effective treatments is of high clinical value. For instance, some mouse models have been established by directly transfusing human cells into immunodeficient mice. However, these models may be unsuitable for clinical simulations. With high mortality but limited cases in clinics, mouse models are necessary to investigate TA-GVHD.
Anti-CD3 monoclonal antibody (mAb) has provided many applications in clinical practices, including treatments for renal transplantation rejection, graft-versus-host disease, and type 1 diabetes (66. Carpenter PA, Lowder J, Johnston L, Frangoul H, Khoury H, Parker P, Jerome KR, McCune JS, Storer B, Martin P, Appelbaum F, Abonour R, Westervelt P, Anasetti C. A phase II multicenter study of visilizumab, humanized anti-CD3 antibody, to treat steroid-refractory acute graft-versus-host disease. Biol Blood Marrow Transplant. 2005;11:465-471.. Anti-CD3 mAb pretreatment can effectively separate graft verus leukemia (GVL) from GVHD 77. Li N, Zhao D, Kirschbaum M, Zhang C, Lin CL, Todorov I, Kandeel F, Forman S, Zeng D. HDAC inhibitor reduces cytokine storm and facilitates induction of chimerism that reverses lupus in anti-CD3 conditioning regimen. Proc Natl Acad Sci U S A. 2008;105:4796-4801.,88. Liang Y, Huang T, Zhang C, Todorov I, Atkinson M, Kandeel F, Forman S, Zeng D. Donor CD8+ T cells facilitate induction of chimerism and tolerance without GVHD in autoimmune NOD mice conditioned with anti-CD3 mAb. Blood . 2005;105:2180-2188.. In this study, treatments with anti-CD3 antibodies and other drugs, including CsA, CTX, and Dex, were examined by using a mouse model of TA-GVHD with busulfan and fludarabine chemotherapy after splenocyte and blood transfusion was conducted.
MATERIALS AND METHODS
1. Reagents: Busulfan was purchased from Kyowa Hakko Kylin Pharmaceutical Company. Fludarabine was obtained from Guangdong South of the Five Ridges Pharmaceutical Company. RPMI 1640 medium, fetal bovine serum, and 1× PBS were purchased from HyClone Company. Heparin was obtained from Changzhou Qianhong Technology Company. Red blood cell lysate was purchased from Beijing Soledad Bao Technology Company. Trypan Blue was obtained from Sigma Company. CD3 bead antibody was purchased from Miltenyi Company. Dexamethasone was obtained from Jinan Limin Pharmaceutical Co., Ltd., and cyclophosphamide was purchased from Shanxi Pude Pharmaceutical Company.
2. Mice: Six- to ten-week-old male C57BL/6 (H-2Kb) &DBA/2 (H-2Kd) and eight- to twelve-week-old male BALB/c mice were purchased from Shanghai Silaike Experimental Animal Company. These mice were maintained in pathogen-free rooms in the Animal Experimental Center of Fujian Medical University (FMU). Animal use protocols were approved by the FMU institutional review committee and performed in accordance with animal ethical standards.
3. Transfusion cell preparation: C57BL/6 or DBA/2 mice were euthanatized. In each mouse, spleen was harvested, and blood from the heart was collected. The spleen was meshed to a single-cell suspension. Blood from the heart was pretreated with heparin and rinsed five times with transfusion buffer (10% FBS in PBS). Splenocytes were counted using Trypan Blue. Cell suspension (500 µl) was transfused into the mice via tail vein injection.
4. T-cell depleted bone marrow (TCD-BM): C57BL/6 or DBA/2 mice were euthanized, and bone marrow samples were harvested from the tibia, femur, and humerus. Anti-CD3 antibody magnetic beads (Miltenyi) were used to negatively select or remove T lymphocytes from the bone marrow.
5. Chemotherapy: The mice were injected intraperitoneally with the required dose of busulfan and fludarabine (total volume of 500 µl) on the basis of their weights.
6. Scoring of TA-GVHD: After transfusion was completed, the mice were monitored in terms of TA-GVHD occurrence and survival. In general, the clinical evidence of TA-GVHD includes weight loss, poor posture, low activity, poor fur texture, poor skin integrity, and diarrhea. The assessment of clinical TA-GVHD was scored on a scale from 0 (none) to 2, as described in our previous publication (99. Li X, Deng R, He W, Liu C, Wang M, Young J, Meng Z, Du C, Huang W, Chen L, Chen Y, Martin P, Forman S, Zeng D. Loss of B7-H1 expression by recipient parenchymal cells leads to expansion of infiltrating donor CD8+ T cells and persistence of graft-versus-host disease. J Immunol. 2012 Jan 15;188(2):724-34.. The scales are defined as follows: for weight loss, 0=less than 10%, 1=10% to 25%, and 2=more than 25%; for posture, 0=normal, 1=hunching noted only at rest, and 2=severe hunching impairs movement; for activity, 0=normal, 1=mild to moderately decreased, and 2=stationary unless stimulated; for fur texture, 0=normal, 1=mild to moderate ruffling, and 2=severe ruffling/poor grooming; for skin integrity, 0=normal, 1=scaling of paws/tail, and 2=obvious areas of denuded skin; for diarrhea, 0=normal, 1=mild (occurred for only 1 day), and 2=persistent diarrhea (lasted for more than 3 days).
7. Pathology scoring: Ten days after transplantation, GVHD target organs (liver, colon, lungs, and skin) were fixed with formaldehyde, dehydrated, embedded in paraffin, sliced, and stained with H&E. The assessment was scored as previously described (1010. Cooke KR, Kobzik L, Martin TR, Brewer J, Delmonte J Jr, Crawford JM, Ferrara JL. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood , 1996. 88(8): p. 3230-9.,1111. Kaplan DH, Anderson BE, McNiff JM, Jain D, Shlomchik MJ, Shlomchik WD. Target antigens determine graft-versus-host disease phenotype. J Immunol, 2004. 173(9): p. 5467-75..
8. Measurement of serum cytokine: Serum cytokine concentration was measured using ELISA kits (BD Biosciences Pharmingen) 77. Li N, Zhao D, Kirschbaum M, Zhang C, Lin CL, Todorov I, Kandeel F, Forman S, Zeng D. HDAC inhibitor reduces cytokine storm and facilitates induction of chimerism that reverses lupus in anti-CD3 conditioning regimen. Proc Natl Acad Sci U S A. 2008;105:4796-4801..
9. Flow cytometry: The following anti-mouse mAbs were purchased from BD Biosciences Pharmingen (San Jose, CA), eBioscience (San Diego, CA), BioLegend (San Diego, CA), or R&D Systems (Minneapolis, MN): TCRβ (H57-597), CD11b/Mac-1 (M1/70), H-2b (AF6-88.5), B220 (RA3-6B2), and Thy1.2(30-H12). Annexin kit was purchased from Roche Company. Cell surface staining was measured using a 4-laser MoFlo Immunocytometry System (Dako, Glostrup, Denmark). Data were analyzed using FlowJo software (Tree Star, Ashland, OR).
10. Statistical analysis: Survival was evaluated by log-rank test with GraphPad Prism version 4.0 (GraphPad). Means were compared by unpaired two-tailed Student’s t-test.
RESULTS
Establishment of MHC-mismatched TA-GVHD model by splenocyte and blood transfusion
Most reports on TA-GVHD cases indicated that patients have received whole blood transfusion in clinics. TA-GVHD patients have usually presented immunosuppression and/or immunodeficiency under chemotherapy. TA-GVHD occurs more often after treatment with nucleotide analogs. To establish a mouse model relevant to clinical practice, we combined the chemo-reagent busulfan and the nucleotide analog fludarabine, which are common drugs used to condition patients undergoing bone marrow transplantation. Afterward, we tested whether transfused blood alone can induce TA-GVHD in mouse models. The mice were injected with busulfan (15 mg/kg) and fludarabine (30 mg/kg) twice a day for 4 days. Three days after chemotherapy, the mice were transfused with 1 ml of whole blood and1066. Carpenter PA, Lowder J, Johnston L, Frangoul H, Khoury H, Parker P, Jerome KR, McCune JS, Storer B, Martin P, Appelbaum F, Abonour R, Westervelt P, Anasetti C. A phase II multicenter study of visilizumab, humanized anti-CD3 antibody, to treat steroid-refractory acute graft-versus-host disease. Biol Blood Marrow Transplant. 2005;11:465-471. TCD-BM from C57BL/6 (MHC-mismatched model) or with 1 ml of whole blood and106 TCD-BM from DBA/2 (MHC-matched model). We observed the mice for 80 days and did not find any signs of GVHD (Fig. 1A). All the mice survived for more than 80 days (Fig. 1B). These results indicated that blood transfusion alone in a mouse model cannot effectively induce TA-GVHD after chemotherapy.
Recipients were transfused 1ml whole blood +10M TCD-BM from C57BL/6 or DBA/2 three days after chemotherapy (busulfan and fludarabine) treatment. (A) % body weigh(B)% survival after transfusion (n=6).
TA-GVHD and GVHD share many similarities with each other. TA-GVHD requires activation of transfused lymphocytes to proliferate and attack target organs, such as the lungs intestines. We highly suspected that blood transfusion alone did not carry enough lymphocytes to induce observable TA-GVHD. Thus, we transfused splenocytes and blood to the chemotherapy-treated mice to establish a suitable model. Titration doses of splenocytes, blood, and TCD-BM from C57BL/6 were injected. Busulfan- and fludarabine-treated mice were then transfused with different doses of splenocytes and blood (Group A at a dose of 50×106 splenocytes and 1000 µl of blood; Group B at a dose of 25×106 splenocytes and 250 µl of blood; Group C at a dose of 12.5×106 splenocytes and 250 µl of blood; Group D at a dose of 6.25×106 splenocytes and 125 µl of blood). We found that all the mice developed TA-GVHD. The severity of TA-GVHD was determined in a dose-dependent manner. The doses of Group A and Group B mice induced the lethal severity of TA-GVHD. The mice developed severe clinical TA-GVHD, and all the recipients died immediately after transfusion. By contrast, Group C and Group D developed less severe TA-GVHD, and most of the mice survived after transfusion (Figs. 2A and 2B). Histopathology in Group D showed slight lymphocyte infiltration and low pathological score. Group A presented the highest clinical score and shortest survival. Histopathology showed vast lymphocyte infiltration and the highest pathological score (Fig. 2C). Interestingly, the mice transfused with 1 ml of whole blood and 50×106 splenocytes and 106 TCD-BM from DBA/2 (MHC-matched model) did not show severe TA-GVHD, and all the mice survived after 80 days (Fig. 2D). These results indicated that we can induce a MHC-mismatched TA-GVHD model by splenocyte and blood transfusion.
Chemotherapy (busulfan and fludarabine) treated mice were transfused with different dose of splenocyte and blood (Group A at a dose of 50X106splenocyte&1000ul blood; Group B at a dose of 25X106splenocyte&250ul blood; Group C at a dose of 12.5X106splenocyte&250ul blood; Group D at a dose of 6.25X106splenocyte&125ul blood).After transfusion, recipients were monitored for clinical TA- GVHD 1-2 times a week. (A) Clinical score (mean ± SE, N=6). (B)%Survival (n=6).(C) pathology score (n=4). (D) %Survival of DBA/2 model(n=6)
Treatment for TA-GVHD in the established mouse model
Given that mice in Group A died immediately, we decided to use Group B for further testing of TA-GVHD treatment. Gamma irradiation for blood components effectively prevented TA-GVHD 1212. Rühl H, Bein G, Sachs UJ. Transfusion -associated graft-versus-host disease. Transfus Med Rev. 2009 Jan;23(1):62-71. Review.. However, gamma irradiation is time consuming and costly. In some developing countries, few patients at high risk of developing TA-GVHD, such as those who underwent bone marrow transplantation, have received blood components through gamma irradiation. Increasing studies on TA-GVHD have been reported in recent years, but the treatment for this disease remains an obstacle. Therefore, we tested the treatment of TA-GVHD in the established mouse model. In clinical practice, large doses of glucocorticoids and immune inhibitors (such as CsA and CTX) were used to treat TA-GVHD. Recently, anti-CD3-based conditioning regimen was reported to separate GVL from GVHD in animal models 1313. Li N, Chen Y, He W, Yi T, Zhao D, Zhang C, Lin CL, Todorov I, Kandeel F, Forman S, Zeng D. Anti-CD3 preconditioning separates GVL from GVHD via modulating host dendritic cell and donor T-cell migration in recipients conditioned with TBI. Blood . 2009;113:953-962.. Subsequently, we tested these treatments. Mitogenic anti-CD3 can cause cytokine storm 1414 Chatenoud L, Bluestone JA. CD3-specific antibodies: a portal to the treatment of autoimmunity. Nat Rev Immunol. 2007 Aug;7(8):622-32.. To avoid such side effects and determine the safe dose to treat TA-GVHD, we titrated down the dose from 5 mg/kg as described in animal models 1313. Li N, Chen Y, He W, Yi T, Zhao D, Zhang C, Lin CL, Todorov I, Kandeel F, Forman S, Zeng D. Anti-CD3 preconditioning separates GVL from GVHD via modulating host dendritic cell and donor T-cell migration in recipients conditioned with TBI. Blood . 2009;113:953-962.. Serum concentrations of cytokines (IL-2, IL-6, TNF-α, and IFN-γ) were measured at 0.5, 1, 4, and 24 h after treatments. We found that the peak concentration was dependent on the dosage. When anti-CD3 was injected at 1 mg/kg, the peak concentration markedly decreased (Fig. 3A). Accordingly, we used anti-CD3 (1 mg/kg) to treat TA-GVHD. Dexamethasone (Dex, 50 mg/kg) was also used to compare the treatment effects.
Afterward, the mouse models were set up as described in Group B (Fig. 2). All the mice were randomized into PBS group, Dex group, or anti-CD3 group. One week after transfusion, when the mice began to present TA-GVHD, PBS (0.5 ml), Dex (50 mg/kg), or anti-CD3 (1 mg/kg) was injected to treat the disease. All the groups were followed up for 80 days. We found that dexamethasone treatment slightly affected the mouse model, and this finding indicated that this model was steroid refractory. A significant difference existed between the survival of animals treated with anti-CD3 and Dex, and anti-CD3-treated mice showed slightly prolonged survival compared with Dex/PBS controls (p < 0.05, Fig. 3B). Recipients treated with anti-CD3 developed milder TA-GVHD, and about 30% of the mice survived for more than 80 days; by contrast, recipients treated with PBS developed severe TA-GVHD, and majority of the mice died 80 days after transfusion (p < 0.05, Fig. 3B). These results indicated that anti-CD3-mAb treatment ameliorated TA-GVHD in a chemotherapy-based MHC-mismatched transfusion mouse model.
(A)BALB/c mice were injected with different doses of anti-CD3 (5mg/kg,3mg/kg,1mg/kg) or PBS. The serum concentrations of cytokines (IL-2,IL-6,TNF-α,IFN-γ) were shown(n=3); (B)The mice were treated with busulfan&fludarabine and transfused with 25X106splenocyte&250ul blood as described in Group B. All the mice were randomized to PBS group or anti-CD3 group. One week after the transfusion, 0.5ml PBS, Dex (50mg/kg) or anti-CD3 (1mg/kg) were injected i.v. to treat TA-GVHD. All the groups were monitored for clinical TA- GVHD 1-2 times a week and followed up for 80days. %Survival was shown (n=24). (C)The mice were treated with busulfan&fludarabine and transfused with 25X106splenocyte&250ul blood as described in Group B. All the mice were randomized different groups. One week after the transfusion, when the mice began to show up TA-GVHD, CTX&Dex(50mg/kg) ,CsA(30mg/kg)&Dex(50mg/kg), CTX(50mg/kg) &Anti-CD3 (1mg/kg) or CsA(30mg/kg)&Anti-CD3 (1mg/kg) were injected to treat the disease. All the groups were monitored for clinical TA- GVHD 1-2 times a week and followed up for 80days. %Survival was shown (n=20-24).
Considering that the use of anti-CD3 antibody slightly improved the model, we further examined whether anti-CD3 antibodies can improve the treatment effect of cyclosporin A (CsA) and cyclophosphamide (CTX), which are drugs used to treat TA-GVHD. Given the created steroid refractory model of TA-GVHD, all the mice were randomized into different groups. CTX and Dex (50 mg/kg), CsA (30 mg/kg) and Dex (50 mg/kg), CTX (50 mg/kg) and Anti-CD3 (1 mg/kg), or CsA (30 mg/kg) and Anti-CD3 (1 mg/kg) were injected as treatments one week after transfusion or when the mice manifested symptoms of TA-GVHD. All the groups were followed up for 80 days. Anti-CD3-treated animals showed prolonged survival compared with Dex-treated mice (p < 0.05, Fig. 3C). However, CsA showed no additive effect to other drugs on this mouse model.
Anti-CD3 mAb treatment of TA-GVHD is associated with apoptosis and T-cell receptor internalization of donor T cells
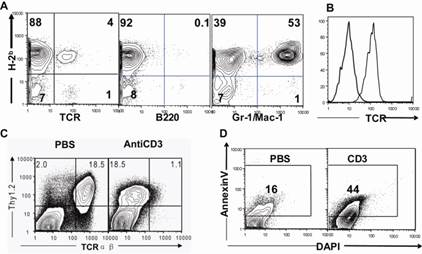
Given that the treatment effect of anti-CD3 was detected in a chemotherapy-based MHC-mismatched transfusion mouse model, we should determine whether the donor or recipient T cells bound to the antibodies. Thereafter, we checked the chimeric status on the day we injected anti-CD3 (d7) by using several markers (TCR as a T-cell marker; B220 as a B cell marker; Gr-1/Mac1 as a myeloid marker). We found that majority of the T cells, B cells, and myeloid cells were H2-b-positive donor type (Fig. 4A). At 24 h after anti-CD3 injection, T-cell receptor (TCR) internalization was found on the T cells (Figs. 4B and 4C). The T cells also underwent apoptosis in ex vivo culture (Fig. 4D). Overall, anti-CD3 mAb treatment of TA-GVHD is associated with T-cell depletion following activation with anti-CD3. This finding indicated the immunosuppression mechanism of anti-CD3 in treating TA-GVHD by blocking T cells.
Anti-CD3mAb treatment of TA-GVHD is associated with the apoptosis and TCRs internalization of donor T cells
(A) The mice were treated with busulfan&fludarabine and transfused with 25X106 splenocyte&250ul blood as described in Group B. One week after the transfusion, mononuclear cells(MNCs) from peripheral blood (Orbital blood) were stained for H-2Kb (donor marker), TCR, B220 and Mac-1/Gr-1. One representative FACS pattern from 3 recipients is shown. (B&C)The mice were injected with anti-CD3. (B) mononuclear cells(MNCs) from peripheral blood (Orbital blood) were stained for Thy1.2 (T cell marker) and TCR. One representative FACS pattern of TCRs internalization is shown. (C)Mean fluorescence of anti-TCR-FITC on T cells was measured at 24 hours after antibody injection. One representative FACS pattern of TCRs internalization is shown. (D) Spleen cells (0.5×106/well) were stimulated with anti-CD3 in a U-bottom 96-well plate for 48 hours. Cell apoptosis as measured by staining of DAPI versus Annexin V. One representative FACS pattern is shown.
DISCUSSION
Transfusion-associated graft-versus-host disease (TA-GVHD) is a rare but almost universally fatal complication of blood transfusion. The risk factors of this disease include the number and immunoreactivity of contaminating lymphocytes in transfusion components. The dominant mechanism of TA-GVHD in immunocompetent and compromised hosts is exposure to viable donor lymphocytes that are responsive against recipients but are not recognized as foreign substances 33. Jawa RS, Young DH, Stothert JC, Kulaylat MN, Landmark JD. Transfusion -associated graft versus host disease in the immunocompetent patient: an ongoing problem.J Intensive Care Med. 2015 Mar;30(3):123-30.,1515. Raina A, Chaudhary G, Dogra TD, Khandelwal D, Balayan A, Jain V, Kanga U, Seth T. Benefit of STR-based chimerism analysis to identify TA-GVHD as a cause of death: Utility of various biological specimens. Med Sci Law. 2015 Apr 6.. Similar to GVHD, TA-GVHD requires an immunoactive component, difference in MHC between a donor and a recipient, and susceptibility of a patient’s immune system to the engraftment of donor lymphocytes. TA-GVHD is unresponsive to immunosuppressive therapy, and the associated mortality exceeds 90%. Leukoreduction cannot completely eliminate the risk of TA-GVHD. Irradiation (with a preferred irradiation dose of 2500 cGy) of blood components, which inhibit the proliferation of donor lymphocytes, is necessary to prevent TA-GVHD. Therefore, gamma irradiation for blood components remains the main strategy to prevent TA-GVHD in clinical practice 1616. Ohto H and Anderson K. Are guidelines for use of gamma irradiation for the prevention of transfusion-associated graft-versus-host disease adequate for newborns Transfus Med 1997;7:172-3.. Whole blood treatment with riboflavin and ultraviolet light can also be an alternative to gamma irradiation 1717. Fast LD, Nevola M, Tavares J, Treatment of whole blood with riboflavin plus ultraviolet light , an alternative to gamma irradiation in the prevention of transfusion-associated graft-versus-host disease? Transfusion . 2013; 53(2):373-81.
Animal models should be established to investigate diseases because of high mortality, low incidence, and unsatisfactory treatment. In several mouse models, human lymphocytes have been transfused into NOD/SCID/IL-2Rgnull mice 1616. Ohto H and Anderson K. Are guidelines for use of gamma irradiation for the prevention of transfusion-associated graft-versus-host disease adequate for newborns Transfus Med 1997;7:172-3.. TA-GVHD exhibits similar clinical features to GVHD after allogeneic hematopoietic stem cell transplantation, which is characterized by a multisystem and cutaneous involvement. In the current study, we established an animal model by transfusion of MHC-mismatched splenocytes and blood to recipients treated with combination of busulfan and fludarabine. The mice presented hunch back appearance, diarrhea, hair loss, skin damage, weight loss, and other typical symptoms of GVHD. These observations are partly consistent with those of TA-GVHD patients suffering from rashes, fever, liver dysfunction, and gastrointestinal disorders.
Interestingly, blood transfusion alone is insufficient to induce TA-GVHD in the MHC-mismatched mouse model. The mice developed TA-GVHD after the addition of splenocytes. This finding might result from the lymphocyte insufficiency in peripheral blood, probably because that donor lymphocytes were easier to be rejected as foreign bodies (1818. Lundqvist A, McCoy JP, Samsel L, Childs R. Reduction of GVHD and enhanced antitumor effects after adoptive infusion of alloreactive ly49-mismatched NK cells from MHCmatched donors. Blood 2007;109:3603-6.. Immunosuppressive regimens involving corticosteroids, antithymocyte globulin (ATG), and immune inhibitors (CsA, CTX) have yielded poor results with few documented survivors 1919. Malladi SV, Paul R, Chandra N, Rao NM, Raju SY.TA-GVHD, a Fatal Complication Following Blood Transfusion from a First-Degree Relative.J Obstet Gynaecol India. 2013 Oct;63(5):344-6.,2020. Sebnem Kilic S, Kavurt S, Balaban Adim S. Transfusion -associated graft-versus-host disease in severe combined immunodeficiency.J Investig Allergol Clin Immunol. 2010;20(2):153-6.. Some scholars even believe that “there is currently no treatment” 22. Hummon D, Zantek ND, Sumstad D, Miller JS, McKenna DH. Transfusion-associated graft-versus-host disease: a perspective from a cell therapy laboratory. Transfusion. 2009 May;49(5):1018-9.. In our study, we found that anti-CD3 antibody treatment ameliorated TA-GVHD in the established mouse model. A significant difference existed between the anti-CD3 group and the PBS group, and this finding was consistent with the observation on human patients 2121. Carpenter PA, Appelbaum FR, Corey L, Deeg HJ, Doney K, Gooley T, Krueger J, Martin P, Pavlovic S, Sanders J, Slattery J, Levitt D, Storb R, Woolfrey A, Anasetti C. A humanized non-FcR-binding anti-CD3 antibody, visilizumab, for treatment of steroid-refractory acute graft-versus-host disease. Blood . 2002 Apr 15;99(8):2712-9. We hypothesized that the mechanism by which anti-CD3 ameliorated TA-GVHD is immunosuppression. Still, some other mechanisms might also play a role. Anti-CD3 can regulate the expression of chemokine receptors, as well as target organ protective molecules, such as B7H1 and indoleamine 2,3-dioxygenase 99. Li X, Deng R, He W, Liu C, Wang M, Young J, Meng Z, Du C, Huang W, Chen L, Chen Y, Martin P, Forman S, Zeng D. Loss of B7-H1 expression by recipient parenchymal cells leads to expansion of infiltrating donor CD8+ T cells and persistence of graft-versus-host disease. J Immunol. 2012 Jan 15;188(2):724-34.,1313. Li N, Chen Y, He W, Yi T, Zhao D, Zhang C, Lin CL, Todorov I, Kandeel F, Forman S, Zeng D. Anti-CD3 preconditioning separates GVL from GVHD via modulating host dendritic cell and donor T-cell migration in recipients conditioned with TBI. Blood . 2009;113:953-962.. Further research is necessary to determine the mechanisms underlying the improvement of TA-GVHD.
In our study, a classical anti-CD3 (145-2C11) ameliorated TA-GVHD in a chemotherapy-based MHC-mismatched transfusion mouse model. The classical anti-CD3 causes cytokine storm, which can affect the clinical progression of TA-GVHD. In our study, 1 mg/kg anti-CD3 did not cause a clinically observable cytokine storm in TA-GVHD mice. This cytokine storm issue can also be addressed and settled by testing non-mitogenic anti-CD3 mAbs, which have been extensively reported.
In our study, one antibody injection was administered, and the anti-CD3 treatment effect is limited. The survival rate of the anti-CD3 group was increased from 10% to 30% in the mouse model. As a consequence, the outcome of TA-GVHD remains very poor. Nevertheless, several injections may enhance the treatment effect. Autologous peripheral blood progenitor cell infusion is also applied in clinical practice 2222. Hutchinson K, Kopko PM, Muto KN, Tuscano J, O'Donnell RT, Holland PV, Richman C, Paglieroni TG, Wun T.Early diagnosis and successful treatment of a patient with transfusion-associated GVHD with autologous peripheral blood progenitor cell transplantation. Transfusion . 2002 Dec;42(12):1567-72. In our future study, this therapy combined with anti-CD3 will be used to determine the optimal and clinically applicable regimen and thus improve the survival of patients with TA-GVHD.
CONCLUSION
Busulfan and fludarabine chemotherapy regimen can be used to establish a TA-GVHD mouse model. Anti-CD3 monoclonal antibody is a potential alternative to treat TA-GVHD.
ACKNOWLEDGMENTS
We thank Dr. Defu Zeng and the staff at City of Hope National Medical Center(USA) for kindly providing the anti-CD3 mAb (145-2C11). We thank Dr. Jianda Hu and the staff at animal experimental center of Fujian Medical University (China) for excellent technical assistance. We also thank National and Fujian Provincial Key Clinical Specialty Discipline Construction Program (China) for the payment of publication fee.
REFERENCE
-
1Kopolovic I, Ostro J, Tsubota H, Lin Y, Cserti-Gazdewich CM, Messner HA, Keir AK, DenHollander N, Dzik WS, Callum J. A systematic review of transfusion-associated graft-versus-host disease. Blood. 2015 Jul 16;126(3):406-14.
-
2Hummon D, Zantek ND, Sumstad D, Miller JS, McKenna DH. Transfusion-associated graft-versus-host disease: a perspective from a cell therapy laboratory. Transfusion. 2009 May;49(5):1018-9.
-
3Jawa RS, Young DH, Stothert JC, Kulaylat MN, Landmark JD. Transfusion -associated graft versus host disease in the immunocompetent patient: an ongoing problem.J Intensive Care Med. 2015 Mar;30(3):123-30.
-
4Schroeder ML. Transfusion -associated graft-versus-host disease. Br J Haematol 2002;117:275-87.
-
5Williamson LM, Stainsby D, Jones H, Love E, Chapman CE,Navarrete C, Lucas G, Beatty C, Casbard A, Cohen H. The impact of universal leukodepletion of the blood supply on hemovigilance reports of posttransfusion purpura and transfusion- associated graft-versus-host disease. Transfusion 2007;47:1455-67.
-
6Carpenter PA, Lowder J, Johnston L, Frangoul H, Khoury H, Parker P, Jerome KR, McCune JS, Storer B, Martin P, Appelbaum F, Abonour R, Westervelt P, Anasetti C. A phase II multicenter study of visilizumab, humanized anti-CD3 antibody, to treat steroid-refractory acute graft-versus-host disease. Biol Blood Marrow Transplant. 2005;11:465-471.
-
7Li N, Zhao D, Kirschbaum M, Zhang C, Lin CL, Todorov I, Kandeel F, Forman S, Zeng D. HDAC inhibitor reduces cytokine storm and facilitates induction of chimerism that reverses lupus in anti-CD3 conditioning regimen. Proc Natl Acad Sci U S A. 2008;105:4796-4801.
-
8Liang Y, Huang T, Zhang C, Todorov I, Atkinson M, Kandeel F, Forman S, Zeng D. Donor CD8+ T cells facilitate induction of chimerism and tolerance without GVHD in autoimmune NOD mice conditioned with anti-CD3 mAb. Blood . 2005;105:2180-2188.
-
9Li X, Deng R, He W, Liu C, Wang M, Young J, Meng Z, Du C, Huang W, Chen L, Chen Y, Martin P, Forman S, Zeng D. Loss of B7-H1 expression by recipient parenchymal cells leads to expansion of infiltrating donor CD8+ T cells and persistence of graft-versus-host disease. J Immunol. 2012 Jan 15;188(2):724-34.
-
10Cooke KR, Kobzik L, Martin TR, Brewer J, Delmonte J Jr, Crawford JM, Ferrara JL. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood , 1996. 88(8): p. 3230-9.
-
11Kaplan DH, Anderson BE, McNiff JM, Jain D, Shlomchik MJ, Shlomchik WD. Target antigens determine graft-versus-host disease phenotype. J Immunol, 2004. 173(9): p. 5467-75.
-
12Rühl H, Bein G, Sachs UJ. Transfusion -associated graft-versus-host disease. Transfus Med Rev. 2009 Jan;23(1):62-71. Review.
-
13Li N, Chen Y, He W, Yi T, Zhao D, Zhang C, Lin CL, Todorov I, Kandeel F, Forman S, Zeng D. Anti-CD3 preconditioning separates GVL from GVHD via modulating host dendritic cell and donor T-cell migration in recipients conditioned with TBI. Blood . 2009;113:953-962.
-
14Chatenoud L, Bluestone JA. CD3-specific antibodies: a portal to the treatment of autoimmunity. Nat Rev Immunol. 2007 Aug;7(8):622-32.
-
15Raina A, Chaudhary G, Dogra TD, Khandelwal D, Balayan A, Jain V, Kanga U, Seth T. Benefit of STR-based chimerism analysis to identify TA-GVHD as a cause of death: Utility of various biological specimens. Med Sci Law. 2015 Apr 6.
-
16Ohto H and Anderson K. Are guidelines for use of gamma irradiation for the prevention of transfusion-associated graft-versus-host disease adequate for newborns Transfus Med 1997;7:172-3.
-
17Fast LD, Nevola M, Tavares J, Treatment of whole blood with riboflavin plus ultraviolet light , an alternative to gamma irradiation in the prevention of transfusion-associated graft-versus-host disease? Transfusion . 2013; 53(2):373-81
-
18Lundqvist A, McCoy JP, Samsel L, Childs R. Reduction of GVHD and enhanced antitumor effects after adoptive infusion of alloreactive ly49-mismatched NK cells from MHCmatched donors. Blood 2007;109:3603-6.
-
19Malladi SV, Paul R, Chandra N, Rao NM, Raju SY.TA-GVHD, a Fatal Complication Following Blood Transfusion from a First-Degree Relative.J Obstet Gynaecol India. 2013 Oct;63(5):344-6.
-
20Sebnem Kilic S, Kavurt S, Balaban Adim S. Transfusion -associated graft-versus-host disease in severe combined immunodeficiency.J Investig Allergol Clin Immunol. 2010;20(2):153-6.
-
21Carpenter PA, Appelbaum FR, Corey L, Deeg HJ, Doney K, Gooley T, Krueger J, Martin P, Pavlovic S, Sanders J, Slattery J, Levitt D, Storb R, Woolfrey A, Anasetti C. A humanized non-FcR-binding anti-CD3 antibody, visilizumab, for treatment of steroid-refractory acute graft-versus-host disease. Blood . 2002 Apr 15;99(8):2712-9
-
22Hutchinson K, Kopko PM, Muto KN, Tuscano J, O'Donnell RT, Holland PV, Richman C, Paglieroni TG, Wun T.Early diagnosis and successful treatment of a patient with transfusion-associated GVHD with autologous peripheral blood progenitor cell transplantation. Transfusion . 2002 Dec;42(12):1567-72
-
1
Compliance with Ethical Standards Ethical approval: All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted
-
2
Disclosures The authors have no financial conflicts of interest.
Publication Dates
-
Publication in this collection
2017
History
-
Received
03 Feb 2016 -
Accepted
14 July 2016