ABSTRACT
The increasing concern over the spread of diseases has lead to a high consumption of antimicrobial additives in the medical and industrial fields. Since these particles can lixiviate from loaded materials, the contact between this additive and mammalian cells can occur during manufacture, use and disposal of the products. Silver on fumed silica (AgNP_SiO2) and titanium dioxide (TiO2) can be used as antimicrobial additives that are applied in polymeric formulation. While these additives can inhibit bacteria, fungus and virus proliferation; they may also be harmful to humans. Standard toxicological studies were undertaken using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide), CBPI (cytokinesis-block proliferation index) and micronucleus assay using different sets of additive concentrations. The nanosize of the samples evaluated was confirmed by transmission electronic microscopy. No significant micronucleus frequency increase or cell viability reduction were observed with the exposure of L-929 murine fibroblast cells to AgNP_SiO2 and TiO2 particles at any of the tested concentrations. The non toxic effect of the analyzed particles can be explained by considering its agglomeration tendency, composition, and crystalline form. Further investigations should be done to understand the interference of agglomeration and how it affects the toxicological study.
Key words:
titanium dioxide; silver; silica; toxicology; mammalian cell
INTRODUCTION
For a vast array of commercial and medical applications, nanoscale silver (AgNP) and titanium dioxide (TiO2) particles are produced and used in different sizes, shapes, crystalline forms, morphology, surface coating and so forth11 Dankovic D, Kuempel E, Wheeler M. An approach to risk assessment for TiO2. Inhal Toxicol. 2007; 19:205-212.,22 Gatoo MA, Naseem S, Arfat MY, Dar AM, Qasim K, Zubair S. Physicochemical properties of nanomaterials: implication in associated toxic manifestations. Biomed Res Int. 2014; 2014: 498420.. AgNP is the most used nanoparticle in products manufactured for the marketplace 33 Brunetti G, Donner E, Laera G, Sekine R, Scheckel KG, Khaksar M, Vasilev K, Mastro GD, Lombi E. Fate of zinc and silver engineered nanoparticles in sewerage networks. Water Res. 2015; 77:72-84.,44 -Vance ME, Kuiken T, Vejerano EP, McGinnis SP, Hochella MFJ, Rejeski D, Hull MS. Nanotechnology in the real world: redeveloping the nanomaterial consumer products inventory. Beilstein J Nanotechnol. 2015; 6: 1769-1780.. In dermatologic applications (such as aerosols, suspensions and emulsions), TiO2 nanoparticles are the most widely used55 Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006; 311:622-627.,66 Natarajan V, Wilson CL, Hayward SL, Kidambi S. Titanium dioxide nanoparticles trigger loss of function and perturbation of mitochondrial dynamics in primary hepatocytes. Plos One. 2015; 10: e0134541.. The growing consumption of these particles is mainly due to their biocide properties77 Chernousova S, Epple M. Silver as antibacterial agent: ion, nanoparticle, and metal. Angew Chem Int. 2013; 52:1636-1653.. Such widespread use and the production of novel engineered materials raises concerns that the release and exposure to nano and microparticles from loaded materials may pose a risk to human health88 Zhang R, Bai Y, Zhang B, Chen L, Yan B. The potential health risk of titania nanoparticles. J Hazard Mater.2012; 211- 212: 404- 413. and to the environment99 Beer C, Foldbjerg R, Hayashi Y, Sutherland DS, Autrup H. Toxicity of silver nanoparticles - Nanoparticle or silver ion? Toxicol Lett. 2012; 208:286-92..
Once the incorporation of nano and micro-sized particles has been widely applied within consumer products, the toxicological evaluation of the potential risks of its chemicals, mainly during the early stages of product development is of interest. Particle characteristics such as shape, chemical composition, capping agent, carrier composition, crystalline form, and surface energy may have an impact on the interaction with cells1010 Navya, PN, Daima, HK. Rational engineering of physicochemical properties of nanomaterials for biomedical applications with nanotoxicological perspectives. Nano Convergence. 2016; 3:1. and influence the toxic potential22 Gatoo MA, Naseem S, Arfat MY, Dar AM, Qasim K, Zubair S. Physicochemical properties of nanomaterials: implication in associated toxic manifestations. Biomed Res Int. 2014; 2014: 498420.. For nanoparticles, as size decreases; increases the ion conductivity on the surface1111 Tamaki T, Nakanishi N, Ohashi H, Yamaguchi T. The effect of particle size and surface area on the ion conductivity of layered double hydroxide. Electrochem Commun. 2012; 25:50-53.. Also, because of their small size, these particles may enter plant and animals cells and reach critical areas in organelles tissues and organs1212 Asharani PV, Mun GLK, Hande MP, Valiyaveettil S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2009; 3:279-90.. Not nanoscale TiO2 is considered to be a non-toxic biocide1313 Bermudez E, Mangum JB, Asgharian B, Wong BA, Reverdy EE, Janszen DB. Long-term pulmonary responses of three laboratory rodent species to subchronic inhalation of pigmentary titanium dioxide particles. Toxicol Sci. 2002; 70:86-97.,1414 Rosa ELS. Kinetic effects of TiO2 fine particles and nanoparticles aggregates on the nano mechanical properties of human neutrophils assessed by force spectroscopy. BMC Biophys. 2013; 6:11. and its rutile crystal form is recommended as safe to use in cosmetic and pharmaceutical applications1515 Turci F, Peira E, Corazzari I, Fenoglio I, Trotta M, Fubini B. Crystalline phase modulates the potency of nanometric TiO2 to adhere to and perturb the stratum corneum of porcine skin under indoor light. Chem Res Toxicol. 2013; 26: 1579-1590..
Reckoning with the above discussions, a range of studies has been performed in normal and cancerous cell trials to determine the role of different particles in toxicity22 Gatoo MA, Naseem S, Arfat MY, Dar AM, Qasim K, Zubair S. Physicochemical properties of nanomaterials: implication in associated toxic manifestations. Biomed Res Int. 2014; 2014: 498420.,1616 Barkhordari A, Barzegar S, Hekmatimoghaddam H, Jebali A, Moghadam SR, Khanjani N. The toxic effects of silver nanoparticles on blood mononuclear cells. Int J Occup Environ Health. 2014; 5:164-168.,1717 Zhang T, Wang L, Chen Q, Chen C. Cytotoxic potential of silver nanoparticles. Yonsei Med J. 2014; 55:283-291.,1818 Kulandaivelu B, Gothandam KM. Cytotoxic Effect on Cancerous Cell Lines by Biologically Synthesized Silver Nanoparticles. Braz Arch Biol Technol. 2016;59: e16150529.. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide) is a metabolic status assay that evaluates reductive capacity of cells1919 Abe K, Matsuki N. Measurement of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction activity and lactate dehydrogenase release using MTT. Neurosci Res. 2000; 38:325-29.. In micronucleus assay the genetic damage is reported by the number of cells containing micronuclei2020 Kirsch-Volders M, Decordier I, Elhajouji A, Plas G, Aardema MJ, Fenech M. In vitro genotoxicity testing using the micronucleus assay in cell lines, human lymphocytes and 3D human skin models. Mutagenesis. 2011; 26:177-184.. These pre-screening measurements are useful to provide evidence of the adverse cytotoxicity effects that certain chemicals can cause to living organisms2121 Dechsakulthorn F, Hayes A, Bakand S, Joeng L, Winder C. In vitro cytotoxicity assessment of selected nanoparticles using human skin fibroblastos. AATEX 2007;14: 397-400..
The aim of this study is to evaluate the citotoxicity and genotoxicity of silver nanoparticles adsorbed on fumed silica (AgNP_SiO2) and comercial titanium dioxide (TiO2) to mammalian cells.
MATERIALS AND METHODS
Particle characterization
The additives nanosilver adsorbed on fumed silica (AgNP_925-SiO, silicon dioxide 98.0-99.4% and silver 0.1-2.0%), supplied by TNS Nanotecnologia Ltda., referred to herein as “AgNP_SiO2” and commercial rutile dioxide titanium (TiPure R-103), supplied by DuPont referred to herein as “TiO2” were used in this study.
The qualitative analysis of mineral composition was determined by X-ray diffraction in a Pan Analytical X’pert PRO and X’PertHigh Score software. Particle size distribution was determined by laser diffraction, and the equipment used was a CILAS 1180 particle size analyzer, with scanning ranging from 0.04 μm to 2500 μm. AgNP_SiO2 and TiO2 were predispersed in deionized water using ultrasound for 60 s.
To perform morphological analysis by scanning electron microscopy (SEM) the samples were deposited in a carbon type stuck to stub and metalized with gold. For image acquisition was used a SEM of field emission (SEM-FEG) (Inspect F50, FEI) with 20 kV, spot 3 and working distance (WD) of 10 mm. Transmission electron microscopy (TEM) was performed at Tecnai, G2 T20 at a voltage of 200 kV. The samples were prepared by mounting a drop of the ethanol suspension containing the particles on a 300 mesh copper grid carbon film. The average particle diameter and size distribution were calculated using Image J version 1.40g software.
L-929 murine cell line
L-929 murine fibroblast cell line were purchased from cell bank from Rio de Janeiro - Cell Bank, and were grown in the presence of the cell culture medium containing Dulbecco's Modified Eagle's Medium (DMEM- Sigma-Aldrich) (89%) supplemented with 1% penicillin/streptomycin (Cultilab) and 10% fetal calf serum (FCS - Cultilab). The cell was derived from normal subcutaneous areolar and adipose tissue of a 100 day old male mouse.
MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) and cytokinesis-block proliferation index (CBPI) assays were performed to identify cytotoxicity effects in L-929 murine fibroblast cells exposed to silver nanoparticle (AgNP_SiO2) and titanium dioxide (TiO2).
Considering the literature reports connecting the particle size to triggering detrimental effects on mammalian cells and the differences in toxicity values between AgNP and TiO22222 Sambale F, Wagner S, Stahl F, Khaydarov RR, Scheper T, Bahnemann D. Investigations of the toxic effect of silver nanoparticles on mammalian cell lines. J Nanomater. 2015; 2015: 136765.,2323 Uboldi C, Urbán P, Gilliland D, Bajaka E, Valsami-Jones E, Ponti J, Rossi F. Role of the crystalline form of titanium dioxide nanoparticles: Rutile, and not anatase, induces toxic effects in Balb/3T3 mouse fibroblasts. Toxicol In Vitro. 2016; 31:137-145., different sets of concentration were tested for AgNP_SiO2 and TiO2 in MTT assay.
MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay
The MTT cell proliferation assay was performed according to ISO 10993-5 to evaluate the cytotoxicity of AgNP_SiO2 and TiO2 particles. The incubation of AgNP_SiO2 and TiO2 were performed with Mus musculus murine fibroblasts cell line L-929. The particle suspensions were performed in seven concentrations (15, 30, 60, 120, 250, 500, 1000 ppm/plate) of AgNP_SiO2 and of TiO2 dispersed in DMEM (Sigma-Aldrich). For each particle and concentration, the assay was performed in nine replicates and repeated on two independent experiments. Cells were also treated with doxorubicin (Sigma-Aldrich) in nine different concentrations (5; 2.5; 1.25; 0.62; 0.31; 0.15; 0.07; 0.03 and 0.01 μg/mL as a positive control. Cell suspensions were inoculated onto 96-well cell culture plates at 1 x 105 cells/mL, incubated in an humidified atmosphere of 5% CO2/95% air at 37 °C for 24 hours to allow for cell adhesion and sedimentation. At the end of period, test metal particles and positive control doxorubicin (Sigma-Aldrich) were incubated with the cells for 24 hours. After the end of the exposure period, a 50 μL MTT mixture (0.5 mg/mL) was added into each well. They were again incubated in a humidified atmosphere of 5% CO2/95% air at 37°C for 3 hours. Then the MTT mixture was removed and the precipitated formazan was dissolved in isopropyl ethanol.
Absorbance of the formazan product from each well was measured by spectrophotometer (SPECTRA max PLUS 384 Microplate reader, Molecular Devices) at 570 nm. Three controls were set up for each experiment: (1) blank - quality check of assay (phosphate buffered saline - PBS), (2) negative control (cells + DMEM) consisting of 100% cell viability2424 Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assay. J Immunol Methods. 1983; 65: 55-63. and (3) positive control consisting of doxorubicin (Sigma-Aldrich).
In viable cells the yellow tetrazolium salt is reduced by mitochondrial enzymes (succinate dehydrogenase) to a blue water-insoluble formazan product2424 Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assay. J Immunol Methods. 1983; 65: 55-63.. Citotoxicity values were obtained from the absorbance average (570 nm) of the replicates in each treatment (concentration) deducted from PBS values and compared to negative control values. Cytotoxicity was categorized according to Dahl et al.2525 Dahl JE, Frangou-Polyzois MJ, Polyzois GL. In vitro biocompatibility of denture relining materials. Gerodontology. 2006; 23:17-22., as follow:
-
- More than 90% cell viability: not cytotoxic
-
- 60-90% cell viability: slightly cytotoxic
-
- 30-59% cell viability: moderately cytotoxic
-
- Less than 30% cell viability: severely cytotoxic
Cytokinesis-block proliferation index (CBPI) and micronucleus assay
Genotoxicity of AgNP_SiO2 and TiO2 particles to murine fibroblasts cell was evaluated based on OECD 487 cytokinesis-block proliferation index (CBPI) assay. The CBPI test was performed with Mus musculus murine fibroblasts cell line L-929. Three distinctive concentrations of AgNP_SiO2 and TiO2 particles (15, 30, 60 ppm/plate) were tested in CBPI and micronucleus assay. For each particle and concentration, two independent experiments were conducted, each of which performed in triplicate. The dispersion procedure was the same as that used for the MTT assay. Two controls were set up for each experiment: (1) negative control consisting of DMEM medium; and (2) positive control consisting of methyl methanesulfonate (MMS). Incubation was performed with 0.7 x 105 cells/mL in a 5 mL suspension containing DMEM medium (Sigma-Aldrich) (89%), 1% penicillin/streptomycin (Cultilab) and 10% fetal calf serum (Cultilab) in a humidified atmosphere of 5% CO2/95% air at 37°C for 24 hours.
At the end of the period, the test particles and the positive control MMS (4 x 10-5 M) were incubated for 24 and 6 hours respectively. After the end of exposure period, the cells were incubated with cytochalasin B (Sigma-Aldrich) (3 µg/mL) for 42 hours. Then the cells were treated with a hypotonic solution of KCl 0.075M (37°C) for 15 minutes and fixed in ethanol:acetic acid solution (3:1) at 4 °C. The slides were covered with the acridine orange staining reagent (50µg/mL) and examined with fluorescence microscopy (Nikon Eclipse Ni H600L) with a blue light excitation filter (488 nm) and yellow light emission/barrier (575 nm) using oil immersion objective.
According to Fenech2626 Fenech M. Cytokinesis-block micronucleus cytome assay. Nat Protoc. 2007; 2: 1084-1104., only binucleated cells (2000 cells/spot) with intact nuclei, approximately equal scales, and with the same pattern of staining were analyzed. For cytotoxicity evaluation the cytokinesis-block proliferation index (CBPI) was used, which indicates the number of cells with 1, 2, 3 or more nuclei in 500 viable cells, calculated in 2 independent assays per treatment.
Genotoxicity was expressed as the average ± standard error of the percent of micronucleus events per 2000 cells; calculated in two independent assays per treatment.
Statistics analysis
The results were expressed by average ± standard error of the mean and significance was obtained through statistical analysis of variance (ANOVA) following the Tukey's test if applicable. For all groups, p <0.05 was considered as being statistically significant.
RESULTS
Particle characterization
The results of the X-ray diffraction detected the presence of SiO2 and Ag in the AgNP_SiO2 sample (Figure 1A). In accordance with diffractogram results, the SiO2 in this sample was considered amorphous due to no diffraction peaks and a spreading halo with intensity of about 2Ѳ = 22 ° (characteristic of amorphous materials)2727 Barazani B. Investigação sobre a sinterização de sílica vítrea por plasma pulsado. São Paulo: Escola Politécnica da Universidade de São Paulo; 2011.,2828 Zanoteli K, Freitas JCC, Silva, PRND. Estudo de catalisadores de níquel suportados em cinza de casca de arroz na reforma de metano com dióxido de carbono visando a produção de hidrogênio e gás de síntese [Study of nickel catalysts supported on rice husk ash in reforming of methane with carbon dioxide in order to produce hydrogen and synthesis gas]. Quim Nova. 2014; 37:1657-1662.. The TiO2 sample was confirmed as pure rutile (Figure 1B).
Values of average diameters D10, D50 and D90 determined by granulometric analysis are shown in Table 1.
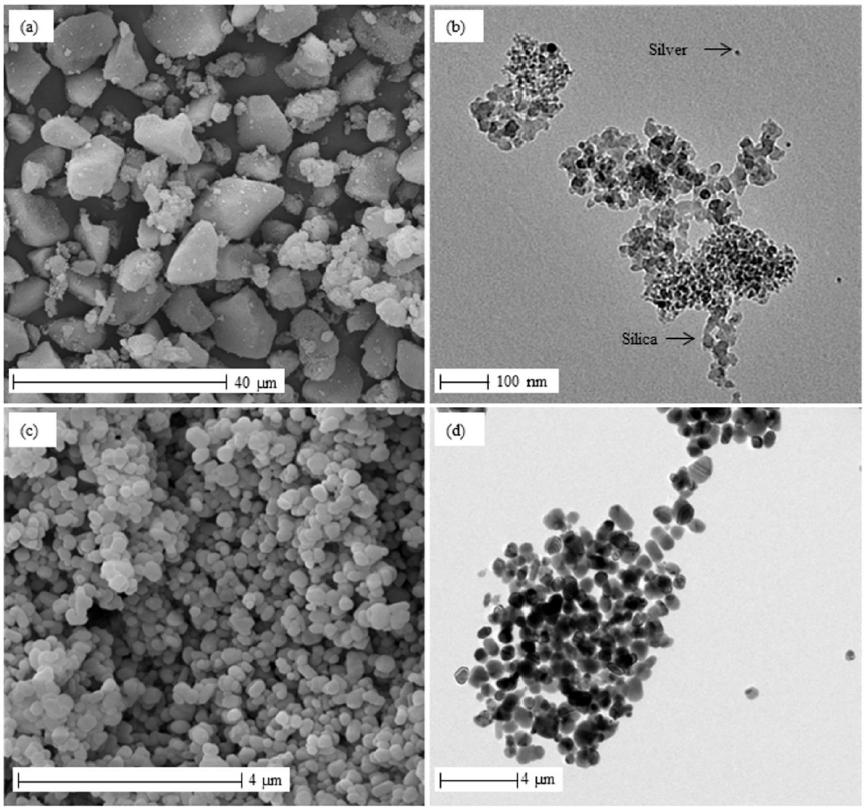
It was noticed that the average size of the AgNP_SiO2 (12.97µm) showed a value above the nanoscale (Table 1). This result reflects the size of the aggregates, as observed in SEM and TEM images (Figure 2A and Figure 2B). On SEM images, AgNP_SiO2 was observed in the form of blocks with irregular geometry and size, with the same size determined by laser diffraction (between 5 and 30 µm). However, by observing TEM images it was possible to confirm nanoscale of this additive, with nanoparticles of silica (20 nm) and silver (10 nm), both in spherical forms (Figure 2B).
The TiO2 particles have an average size of 0.29 µm, but as seen in SEM images (Figure 2C), more particles have nanoscale, and the size determined by laser diffraction reflects the size of agglomerates. As shown in TEM images in Figure 2D, TiO2 is of spherical form and average size of 90 nm.
The results found in laser diffraction and micrograph demonstrated that both additives have nanoscale and high tendency to agglomeration.
Micrographs of the SEM (left) and TEM (right) additives for (A, B) AgNP_SiO2 and (C, D) TiO2.
MTT data
Viability assay expound the cellular reaction to a toxicant1212 Asharani PV, Mun GLK, Hande MP, Valiyaveettil S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2009; 3:279-90.. The relation between test chemical (AgNP_SiO2 and TiO2), concentration (ppm) and cell viability (%) after 24 hour exposures using the MTT assay are presented in Figure 3.
Cell viability of L-929 murine fibroblast after 24 h of exposure to distinctive concentrations of (A) AgNP_SiO2 and (B) TiO2 by MTT method. Each point represents the mean and standard deviation in nine replicates.
The viability of the cells exposed to AgNP_SiO2 was significantly different (p<0.05) between the tested additive concentrations. In cell exposed to TiO2 particles there was no difference between the concentrations of the additives. Since cell viability values were between 95.83 - 97.53 in the presence of AgNP_SiO2 (Figure 3A) and 94.47 - 96.54 in cells treated with TiO2 (Figure 3B), according to cell viability classification and the experimental conditions performed in this study, the AgNP_SiO2 and TiO2 particles were classified as non cytotoxic.
CBPI and micronucleus data
The experimental data for CBPI values and micronucleus frequency are summarized in Table 2. With data presented as mean values ± standard deviations. The experiment was conducted following 24 h exposure time.
No significant cell viability reduction was observed on murine cells treated with AgNP_SiO2 and TiO2. There were no concentration-dependent increases in micronucleus frequency on murine cells treated with AgNP_SiO2 and TiO2 (Table 2).
DISCUSSION
The viability of L929 murine cell was found to be greater than 90%, even in the highest concentrations used in NpAg_SiO2 (average size - 10-20 nm; 1000 ppm) and TiO2 (average size - 90 nm;10000 ppm) tests. In contrast to our findings, cytotoxic and genotoxic studies have shown harmful effects of nanosized (~0.1 to 100 nm) Ag and TiO2 particles to mammals’ cells1212 Asharani PV, Mun GLK, Hande MP, Valiyaveettil S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2009; 3:279-90.. A variety of scales and concentrations have been assigned to the AgNP2222 Sambale F, Wagner S, Stahl F, Khaydarov RR, Scheper T, Bahnemann D. Investigations of the toxic effect of silver nanoparticles on mammalian cell lines. J Nanomater. 2015; 2015: 136765.,2929 Haase A, Arlinghaus HF, Tentschert J, Jungnickel H, Graf P, Mantion A, Draude F, Galla S, Plendl J, Goetz ME, Masic A, Meier W, Thüunemann AF, Taubert A, Luch A. Application of laser postionization secondary neutral mass spectrometry/time-of-flight secondary ion mass spectrometry in nanotoxicology: visualization of nanosilver in human macrophages and cellular responses. ACSNano. 2011; 5:3059-3068.,3030 Hackenberg S, Scherzed A, Kessler M, Hummel S, Technau A, Froelich K, Ginzkey C, Koehler C, Hagen R, Kleinsasser N. Silver nanoparticles: Evaluation of DNA damage, toxicity and functional impairment in human mesenchymal stem cells. Toxicol Lett. 2011; 195: 9-14. and TiO2 toxicity values2323 Uboldi C, Urbán P, Gilliland D, Bajaka E, Valsami-Jones E, Ponti J, Rossi F. Role of the crystalline form of titanium dioxide nanoparticles: Rutile, and not anatase, induces toxic effects in Balb/3T3 mouse fibroblasts. Toxicol In Vitro. 2016; 31:137-145.,3131 Trouiller B, Reliene R, Westbrook A, Solaimani P, Schiestl RH. Titanium dioxide nanoparticles induce DNA damage and genetic instability in vivo in mice. Cancer Res. 2009; 69: 8784-8789.,3232 Ghosh M, Chakraborty A, Mukherjee A. Cytotoxic, genotoxic and the hemolytic effect of titanium dioxide (TiO2) nanoparticles on human erythrocyte and lymphocyte cells in vitro. J Appl Toxicol. 2013; 33: 1097-1110.. In a cytokinesis blocked micronucleus assay, DNA damage and significant numbers of micronuclei were observed in human fibroblast and human cancer cells after the treatment with AgNP (6-20 nm)1212 Asharani PV, Mun GLK, Hande MP, Valiyaveettil S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2009; 3:279-90.. In a research conducted by Li et al.3333 Li Y, Chen DH, Yan J, Chen Y, Mittelstaedt RA, Zhang Y, Biris AS, Heflich RH, Chen T. Genotoxicity of silver nanoparticles evaluated using the Ames test and in vitro micronucleus assay. Mutat Res. 2012; 745:4-10. the AgNP (5 nm) particle induced significant increase in micronucleus frequency over the control. Carlson et al.3434 Carlson C, Hussain SM, Schrand AM, Braydich-Stolle LK, Hess KL, Jones RL, Schlager JJ. Unique cellular interaction of silver nanoparticles: size-dependent generation of reactive oxygen species. J Phys Chem B. 2008; 112:13608-13619. observed in 24 h MTT study a decrease of 88.66% in mitochondrial function of alveolar macrophages exposed to AgNP (15 nm) at concentrations of 50 µg/mL. However, bigger AgNP (55 nm) exhibit a viability decrease of 33.89% at the highest dose (75 ppm) and AgNPs (5-40 nm) in a concentration up to 100 ppm can be used as a drug delivery1818 Kulandaivelu B, Gothandam KM. Cytotoxic Effect on Cancerous Cell Lines by Biologically Synthesized Silver Nanoparticles. Braz Arch Biol Technol. 2016;59: e16150529.. These above reports showed a variable degree of AgNP and TiO2 toxicity, highlighting the complexity of the study of nanomaterials, emphasized by the diversity of the evaluated cells.
Concerns associated to nanoparticles are the metal ions released from the material99 Beer C, Foldbjerg R, Hayashi Y, Sutherland DS, Autrup H. Toxicity of silver nanoparticles - Nanoparticle or silver ion? Toxicol Lett. 2012; 208:286-92., as well as their larger surface area, that enhance the interaction with cells3535 Kalbassi MR, Johari SA, Soltani M, Yu IJ. Particle size and agglomeration affect the toxicity levels of silver nanoparticle types in aquatic environment. Ecopersia. 2013; 1: 273-290., and the small size, that allows for easier penetration into the cells3636 Mudunkotuwa IA, Grassian VH. The devil is in the details (or the surface): impact of surface structure and surface energetics on understanding the behavior of nanomaterials in the environment. J Environ Monit. 2011; 13: 1135-44.,3737 Bian SW, Mudunkotuwa IA, Rupasinghe T, Grassian VH. Aggregation and dissolution of 4 nm ZnO nanoparticles in aqueous environments: influence of pH, ionic strength, size, and adsorption of humic acid. Langmuir. 2011; 27:6059-68.. Although the engineering of NPs internalization into cells is considered as non-specific according to studies with microcopy images3838 Gao H, Yang Z, Zhang S, Cao S, Shen S, Pang Z, Jiang X. Ligand modified nanoparticles increases cell uptake, alters endocytosis and elevates glioma distribution and internalization. Scientific reports. 2013; 3:2534., endocytosis is an important mechanism to Ag3939 Wang H, Wu L, Reinhard BM. Scavenger Receptor Mediated Endocytosis of Silver Nanoparticles into J774A.1 Macrophages is Heterogeneous. ACS Nano. 2012; 28; 6(8):7122-7132. and TiO2 nanoparticles4040 Jin C-Y, Zhu B-S, Wang X-F, Lu Q-H. Cytotoxicity of Titanium Dioxide Nanoparticles in Mouse Fibroblast Cells. Chem. Res. Toxicol. 2008; 21:1871-1877. cell intake. However, particle agglomeration can mitigate the toxic effects of particle size, which may vary depending on cell type and internalization mechanisms4141 Albanese A, Chan WCW. Effect of gold nanoparticle aggregation on cell uptake and toxicity. ACS Nano. 2011; 5:5478-89.,4242 Bernier MC, Kirat KE, Besse M, Morandat S, Vayssade M. Preosteoblasts and fibroblasts respond differently to anatase titanium dioxide nanoparticles: A cytotoxicity and inflammation study. Colloids Surf B: Biointerfaces. 2012; 90:68-74.. In this study, micrographs and granulometric analysis show that AgNP_SiO2 and TiO2 present a tendency to agglomerate, with a set size of 12.97 µm and 0.29 µm, respectively. It is recognized that at the cellular level, size will have an impact on particle uptake4343 Huang X, Young NP, Townley HE. Characterization and comparison of mesoporous silica particles for optimized drug delivery. Nanomater Nanotechno. 2014; 4:2.. In this way, as reported by Sambale et al.2222 Sambale F, Wagner S, Stahl F, Khaydarov RR, Scheper T, Bahnemann D. Investigations of the toxic effect of silver nanoparticles on mammalian cell lines. J Nanomater. 2015; 2015: 136765.non nanomaterial (above 0.1 µm) may prevent the cell apoptosis by diminishing access and penetration of particles into the cytoplasm, also the microparticles do not dissolve as rapidly as nanoscale ones, providing a slow release of metal, particularly around the nucleus of the cell4444 Papageorgiou I, Brown C, Schins R, Singh S, Newson R, Davis S, Fisher J, Ingham E, Case CP. The effect of nano- and micron-sized particles of cobalt-chromium alloy on human fibroblasts in vitro. Biomaterials. 2007; 28:2946-58..
The AgNP_SiO2 presents the silica covering a range of 98.0-99.4% of the total particles (data provided by the suppliers). According to X-Ray diffraction, this silica is amorphous. Silica on amorphous structure is considered non toxic4545 Brunner TJ, Wick P, Manser P, Spohn P, Grass RN, Limbach LK, Bruinink A, Stark WJ. In vitro cytotoxicity of oxide nanoparticles: comparison to asbestos, silica, and the effect of particle solubility. Environ. Sci. Technol. 2006, 40, 4374-4381.,4646 Barnes CA, Elsaesser A, Arkusz J, Smok A, Palus J, Lesniak A, Salvati A, Hanrahan JP, Jong WH, Dziubaltowska E, Stepnik M, Rydzynski K, McKerr G, Lynch I, Dawson KA, Howard CV. Reproducible comet assay of amorphous silica nanoparticles detects no genotoxicity. Nano Lett. 2008; 8:3060-3074., also when silver nanoparticles are deposited on a silica carrier, the hydrophilicity characteristics of silica reduces the nonspecific binding of proteins4747 Hu L, Fawcett JP, Gu J. Protein target discovery of drug and its reactive intermediate metabolite by using proteomic strategy. Acta Pharm Sin B. 2012; 2:126-136. and the release of AgNP4848 Agnihotri S, Mukherji S, Mukherji S. Immobilized silver nanoparticles enhance contact killing and show highest efficacy: elucidation of the mechanism of bactericidal action of silver. Nanoscale. 2013; 5:7328-40. leading to lower toxicity4949 Hu SH, Liu TY, Huang HY, Liu DM, Chen SY. Magnetic-sensitive silica nanospheres for controlled drug release. Langmuir. 2008; 24: 239-244.. In a previous study, cell culture condition was shown to influence the toxicity of amorphous silica. Drescher and coworkers5050 Drescher D, Orts-Gil G, Laube G, Natte K, Veh RW, Österle W, Kneipp J. Toxicity of amorphous silica nanoparticles on eukaryotic cell model is determined by particle agglomeration and serum protein adsorption effects. Anal Bioanal Chem. 2011; 400:1367-73. observed an agglomeration tendency of silica nanoparticles in all FCS-containing medium, the same supplement used in this study, and this condition caused a decrease in toxicity. Such behavior may be explained by the organic components of treatment media5151 Lynch I, Dawson KA. Protein-nanoparticle interactions. Nanotoday. 2008; 3: 40-47., considering that the adsorption of serum proteins into silica surface can change particle compatibility, membrane contact and uptake into the cells5252 Stayton I, Winiarz J, Shannon K, Ma Y. Study of uptake and loss of silica nanoparticles in living human lung epithelial cells at single cell level. Anal Bioanal Chem. 2009; 394:1595-1608.. Although micronucleus (MN) test has been considered more appropriate for evaluating the genotoxicity of the AgNP3333 Li Y, Chen DH, Yan J, Chen Y, Mittelstaedt RA, Zhang Y, Biris AS, Heflich RH, Chen T. Genotoxicity of silver nanoparticles evaluated using the Ames test and in vitro micronucleus assay. Mutat Res. 2012; 745:4-10., there is an issue pertaining the use of cytochalasin B in MN assay. The addition of this chemical could mask the real cytotoxicity of a tested substance4444 Papageorgiou I, Brown C, Schins R, Singh S, Newson R, Davis S, Fisher J, Ingham E, Case CP. The effect of nano- and micron-sized particles of cobalt-chromium alloy on human fibroblasts in vitro. Biomaterials. 2007; 28:2946-58.,5353 Kim HR, Kim MJ, Lee SY, Oh SM, Chung KH. Genotoxic effects of silver nanoparticles stimulated by oxidative stress in human normal bronchial epithelial (BEAS-2B) cells. Mutat Res. 2011; 726:129-135.,5454 Kim HR, Park YJ, Shin DY, Oh SM, Chung KH. Appropriate in vitro methods for genotoxicity testing of silver nanoparticles. Environ Health Toxicol. 2013; 28: e2013003., by restricting particle uptake due the phagocytosis inhibition5555 Falck GCM, Lindberg HK, Suhonen S, Vippola M, Vanhala E, Catalán J, Savolainen K, Norppa H. Genotoxic effects of nanosized and fine TiO2. Hum Exp Toxicol. 2009; 28: 339-52.. The alternative assays to access genotoxicity of nano and microparticles would be the Ames test and Comet assay5656 Woodruff RS, Li Y, Yan J, Bishop M, Jones MY, Watanabe F, Biris AS, Rice P, Zhouf T, Chenb T. Genotoxicity evaluation of titanium dioxide nanoparticles using the Ames test and Comet assay. J. Appl. Toxicol. 2012; 32: 934-943.. However, in the case of this study, that evaluated the toxicity of known antimicrobial particles, the Ames test may not be a suitable option because its test uses bacterial cells for determining mutagenicity5757 Clift MJD, Raemy DO, EndesC, Ali Z, Lehmann AD, Brandenberger C, Petri-Fink A, Wick P, Parak WJ, Gehr P, Schins RPF, Rothen-Rutishauser B. Can the Ames test provide an insight into nano-object mutagenicity? Investigating the interaction between nano-objects and bacteria. Nanotechnology. 2013; 7(8): 1373-1385..
The uptake of small clusters by cells is more efficient than the uptake of larger clusters5858 Chithrani BD, Ghazani AA, Chan WCW. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006; 6:662-8. and the presence of FCS in the MTT assay and cytochalasin B in MN test was shown to influence the formation of aggregates and therefore, may have an impact on the dispersion and bioavailability of the particles 5959 Murdock RC, Braydich-Stolle L, Schrand AM, Schlager JJ, Hussain SM. Characterization of nanomaterial dispersion in solution prior to in vitro exposure using dynamic light scattering technique. Toxicol Sci. 2008; 101:239-253.. Thus, the non toxic effect of AgNP_SiO2 and TiO2 observed in our experimental conditions could be related to the agglomeration tendency of the tested particles5959 Murdock RC, Braydich-Stolle L, Schrand AM, Schlager JJ, Hussain SM. Characterization of nanomaterial dispersion in solution prior to in vitro exposure using dynamic light scattering technique. Toxicol Sci. 2008; 101:239-253.,6060 Ates M, Daniels J, Arslan Z, Farah IO. Effects of aqueous suspensions of titanium dioxide nanoparticles on Artemia salina: assessment of nanoparticle aggregation, accumulation, and toxicity. Environ Monit Assess. 2013; 185:3339-48., amplified by the contribution of treatment conditions used in the assay on the agglomeration and cell input mechanisms. Moreover, the TiO2 rutile morphology is described as a less photocatalyst6161 Kakinoki K, Yamane K, Teraoka R, Otsuka M, Matsuda Y. Effect of relative humidity on the photocatalytic activity of titanium dioxide and photostability of famotidine. J Pharm Sci. 2004; 93: 582-589. and non-cytotoxic1515 Turci F, Peira E, Corazzari I, Fenoglio I, Trotta M, Fubini B. Crystalline phase modulates the potency of nanometric TiO2 to adhere to and perturb the stratum corneum of porcine skin under indoor light. Chem Res Toxicol. 2013; 26: 1579-1590.,6262 Sayes CM, Wahi R, Kurian PA, Liu Y, West JL, Ausman KD, Warheit DB, Colvin VL. Correlating nanoscale titania structure with toxicity: a cytotoxicity and inflammatory response study with human dermal fibroblasts and human lung epithelial cells. Toxicol Sci. 2006; 92:174-185.form of TiO2 crystal6363 Warheit DB, Webb TR, Reed KL, Frerichs S, Sayes CM. Pulmonary toxicity study in rats with three forms of ultrafine-TiO2 particles: Differential responses related to surface properties. Toxicology. 2007; 230: 90-104..
The murine fibroblast cell line L-929 used in this study is a suitable cell type for the investigation of in vitro toxicity of the metal particles, being routinely used for this purpose due its biological response, reproducible growth rates and facility in the cell culture conditions6464 Schedlel A, Samorapoompichit P, Rausch-Fan XH, Franz A, Fureder W, Sperr WR, Sperr W, Ellingerl A, Slavicek R, Boltz-Nitulescu G, Valent P. Response of L-929 Fibroblasts, Human Gingival Fibroblasts, and Human Tissue Mast Cells to Various Metal Cations. J. Dent. Res. 1995; 74(8): 1513-1520.,6565 International Organization for Standardization. ISO 10993-5: Biological evaluation of medical devices - Part 5: Tests for in vitro cytotoxicity. 2009, Switzerland.. However, with respect to the sensitivity, this cell line were found to be resistant to plant extracts6666 Nascimento FG, Faqueti A, Wilhelm JF, Wittkowski C, Tomczak FD, Borges SL, Yunes RA, Franchi Jr. GC, Nowill AE, Filho VC, Machado MS, Freitas RA, Malheiros A. Seasonal influence and cytotoxicity of extracts, fractions and major compounds from Allamanda schottii. Rev. Bras. Farmacogn. 2014; 24: 545-552., but also sensitive to other substances6464 Schedlel A, Samorapoompichit P, Rausch-Fan XH, Franz A, Fureder W, Sperr WR, Sperr W, Ellingerl A, Slavicek R, Boltz-Nitulescu G, Valent P. Response of L-929 Fibroblasts, Human Gingival Fibroblasts, and Human Tissue Mast Cells to Various Metal Cations. J. Dent. Res. 1995; 74(8): 1513-1520.,6767 Thonemann B, Schmalz G, Hiller KA, Schweik H. Responses of L929 mouse fibroblasts, primary and immortalized bovine dental papilla-derived cell lines to dental resin components. Dent. Mater. 2002; 18(4): 318-323.. In summary, it seems that apart of cell line, the elements that control nanoparticles cytotoxic responses vary depending on the concentration, size and type of material tested as well as the assay system. Mahmoudi and coworkers (2009)6868 Mahmoudi M, Simchi A, Vali H, Imani M, Shokrgozar MA, Azadmanesh K, Azari F. Cytotoxicity and Cell Cycle Effects of Bare and Poly(vinylalcohol)-Coated Iron Oxide Nanoparticles in Mouse Fibroblasts. Adv. Eng. Mater. 2009; 11(12): B243-B250. reported enhancement of mitosis and apoptosis phenomena in G0/G1 phase after the exposure of fibroblast cell to iron oxide nanoparticles. Also, cellular damage and DNA deterioration was reported upon the exposure to TiO24040 Jin C-Y, Zhu B-S, Wang X-F, Lu Q-H. Cytotoxicity of Titanium Dioxide Nanoparticles in Mouse Fibroblast Cells. Chem. Res. Toxicol. 2008; 21:1871-1877. and Ag nanoparticles6969 Ahamed M, Karns M, Goodson M, Rowe J, Hussain SM, Schlager JJ, Hong Y. DNA damage response to different surface chemistry of silver nanoparticles in mammalian cells. Toxicol. Appl. Pharm. 2008; 233: 404-410., and the concentration of these nanoparticles was reported inside mitochondria, nucleus and nucleolus1212 Asharani PV, Mun GLK, Hande MP, Valiyaveettil S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2009; 3:279-90..
At this point, it can be speculated that the manner in which the particle is presented to the cell in terms of supporting elements (e.g. SiO2), crystal form, particle and agglomerate sizes, as well as the treatment medium conditions could be the factors handling this toxicological study. Additionally, the comparison of our results with others was hampered due to miscellaneous methods, concentrations, cell types and mainly the characteristics of the materials used in previous toxicological inventories. Due to the diversity of structural components, the committee on emerging and newly identified health risks - SCENIHR7070 SCENIHR. (Scientific Committee on Emerging and Newly Identified Health Risks). Risk assessment of products of nanotechnologies. Brussels: European Commission, 2009. Available at http://ec.europa.eu/health/ph_risk/committees/04_ scenihr/docs/scenihr_ o_023.pdf. Accessed 8/Dec/2015. advocates that the toxicological assessment of novel materials (such as AgNP_SiO2) should be done on a case-by-case basis.
CONCLUSION
The particles AgNP_SiO2 and TiO2 did not cause citotoxicity and genotoxicity detectable by MTT and CBPI tests in L-929 murine fibroblast cell at any tested concentration. The non toxic effect of the analyzed particles can be explained by considering its agglomeration tendency, supporting element, and crystalline form, together with their interaction in culture media. Clearly, further investigations are needed to better understand the cyto/genotoxicity mechanisms of AgNP_SiO2 and TiO2 in cultured mammalian cells.
REFERENCES
-
1Dankovic D, Kuempel E, Wheeler M. An approach to risk assessment for TiO2. Inhal Toxicol. 2007; 19:205-212.
-
2Gatoo MA, Naseem S, Arfat MY, Dar AM, Qasim K, Zubair S. Physicochemical properties of nanomaterials: implication in associated toxic manifestations. Biomed Res Int. 2014; 2014: 498420.
-
3Brunetti G, Donner E, Laera G, Sekine R, Scheckel KG, Khaksar M, Vasilev K, Mastro GD, Lombi E. Fate of zinc and silver engineered nanoparticles in sewerage networks. Water Res. 2015; 77:72-84.
-
4-Vance ME, Kuiken T, Vejerano EP, McGinnis SP, Hochella MFJ, Rejeski D, Hull MS. Nanotechnology in the real world: redeveloping the nanomaterial consumer products inventory. Beilstein J Nanotechnol. 2015; 6: 1769-1780.
-
5Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006; 311:622-627.
-
6Natarajan V, Wilson CL, Hayward SL, Kidambi S. Titanium dioxide nanoparticles trigger loss of function and perturbation of mitochondrial dynamics in primary hepatocytes. Plos One. 2015; 10: e0134541.
-
7Chernousova S, Epple M. Silver as antibacterial agent: ion, nanoparticle, and metal. Angew Chem Int. 2013; 52:1636-1653.
-
8Zhang R, Bai Y, Zhang B, Chen L, Yan B. The potential health risk of titania nanoparticles. J Hazard Mater.2012; 211- 212: 404- 413.
-
9Beer C, Foldbjerg R, Hayashi Y, Sutherland DS, Autrup H. Toxicity of silver nanoparticles - Nanoparticle or silver ion? Toxicol Lett. 2012; 208:286-92.
-
10Navya, PN, Daima, HK. Rational engineering of physicochemical properties of nanomaterials for biomedical applications with nanotoxicological perspectives. Nano Convergence. 2016; 3:1.
-
11Tamaki T, Nakanishi N, Ohashi H, Yamaguchi T. The effect of particle size and surface area on the ion conductivity of layered double hydroxide. Electrochem Commun. 2012; 25:50-53.
-
12Asharani PV, Mun GLK, Hande MP, Valiyaveettil S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2009; 3:279-90.
-
13Bermudez E, Mangum JB, Asgharian B, Wong BA, Reverdy EE, Janszen DB. Long-term pulmonary responses of three laboratory rodent species to subchronic inhalation of pigmentary titanium dioxide particles. Toxicol Sci. 2002; 70:86-97.
-
14Rosa ELS. Kinetic effects of TiO2 fine particles and nanoparticles aggregates on the nano mechanical properties of human neutrophils assessed by force spectroscopy. BMC Biophys. 2013; 6:11.
-
15Turci F, Peira E, Corazzari I, Fenoglio I, Trotta M, Fubini B. Crystalline phase modulates the potency of nanometric TiO2 to adhere to and perturb the stratum corneum of porcine skin under indoor light. Chem Res Toxicol. 2013; 26: 1579-1590.
-
16Barkhordari A, Barzegar S, Hekmatimoghaddam H, Jebali A, Moghadam SR, Khanjani N. The toxic effects of silver nanoparticles on blood mononuclear cells. Int J Occup Environ Health. 2014; 5:164-168.
-
17Zhang T, Wang L, Chen Q, Chen C. Cytotoxic potential of silver nanoparticles. Yonsei Med J. 2014; 55:283-291.
-
18Kulandaivelu B, Gothandam KM. Cytotoxic Effect on Cancerous Cell Lines by Biologically Synthesized Silver Nanoparticles. Braz Arch Biol Technol. 2016;59: e16150529.
-
19Abe K, Matsuki N. Measurement of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction activity and lactate dehydrogenase release using MTT. Neurosci Res. 2000; 38:325-29.
-
20Kirsch-Volders M, Decordier I, Elhajouji A, Plas G, Aardema MJ, Fenech M. In vitro genotoxicity testing using the micronucleus assay in cell lines, human lymphocytes and 3D human skin models. Mutagenesis. 2011; 26:177-184.
-
21Dechsakulthorn F, Hayes A, Bakand S, Joeng L, Winder C. In vitro cytotoxicity assessment of selected nanoparticles using human skin fibroblastos. AATEX 2007;14: 397-400.
-
22Sambale F, Wagner S, Stahl F, Khaydarov RR, Scheper T, Bahnemann D. Investigations of the toxic effect of silver nanoparticles on mammalian cell lines. J Nanomater. 2015; 2015: 136765.
-
23Uboldi C, Urbán P, Gilliland D, Bajaka E, Valsami-Jones E, Ponti J, Rossi F. Role of the crystalline form of titanium dioxide nanoparticles: Rutile, and not anatase, induces toxic effects in Balb/3T3 mouse fibroblasts. Toxicol In Vitro. 2016; 31:137-145.
-
24Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assay. J Immunol Methods. 1983; 65: 55-63.
-
25Dahl JE, Frangou-Polyzois MJ, Polyzois GL. In vitro biocompatibility of denture relining materials. Gerodontology. 2006; 23:17-22.
-
26Fenech M. Cytokinesis-block micronucleus cytome assay. Nat Protoc. 2007; 2: 1084-1104.
-
27Barazani B. Investigação sobre a sinterização de sílica vítrea por plasma pulsado. São Paulo: Escola Politécnica da Universidade de São Paulo; 2011.
-
28Zanoteli K, Freitas JCC, Silva, PRND. Estudo de catalisadores de níquel suportados em cinza de casca de arroz na reforma de metano com dióxido de carbono visando a produção de hidrogênio e gás de síntese [Study of nickel catalysts supported on rice husk ash in reforming of methane with carbon dioxide in order to produce hydrogen and synthesis gas]. Quim Nova. 2014; 37:1657-1662.
-
29Haase A, Arlinghaus HF, Tentschert J, Jungnickel H, Graf P, Mantion A, Draude F, Galla S, Plendl J, Goetz ME, Masic A, Meier W, Thüunemann AF, Taubert A, Luch A. Application of laser postionization secondary neutral mass spectrometry/time-of-flight secondary ion mass spectrometry in nanotoxicology: visualization of nanosilver in human macrophages and cellular responses. ACSNano. 2011; 5:3059-3068.
-
30Hackenberg S, Scherzed A, Kessler M, Hummel S, Technau A, Froelich K, Ginzkey C, Koehler C, Hagen R, Kleinsasser N. Silver nanoparticles: Evaluation of DNA damage, toxicity and functional impairment in human mesenchymal stem cells. Toxicol Lett. 2011; 195: 9-14.
-
31Trouiller B, Reliene R, Westbrook A, Solaimani P, Schiestl RH. Titanium dioxide nanoparticles induce DNA damage and genetic instability in vivo in mice. Cancer Res. 2009; 69: 8784-8789.
-
32Ghosh M, Chakraborty A, Mukherjee A. Cytotoxic, genotoxic and the hemolytic effect of titanium dioxide (TiO2) nanoparticles on human erythrocyte and lymphocyte cells in vitro. J Appl Toxicol. 2013; 33: 1097-1110.
-
33Li Y, Chen DH, Yan J, Chen Y, Mittelstaedt RA, Zhang Y, Biris AS, Heflich RH, Chen T. Genotoxicity of silver nanoparticles evaluated using the Ames test and in vitro micronucleus assay. Mutat Res. 2012; 745:4-10.
-
34Carlson C, Hussain SM, Schrand AM, Braydich-Stolle LK, Hess KL, Jones RL, Schlager JJ. Unique cellular interaction of silver nanoparticles: size-dependent generation of reactive oxygen species. J Phys Chem B. 2008; 112:13608-13619.
-
35Kalbassi MR, Johari SA, Soltani M, Yu IJ. Particle size and agglomeration affect the toxicity levels of silver nanoparticle types in aquatic environment. Ecopersia. 2013; 1: 273-290.
-
36Mudunkotuwa IA, Grassian VH. The devil is in the details (or the surface): impact of surface structure and surface energetics on understanding the behavior of nanomaterials in the environment. J Environ Monit. 2011; 13: 1135-44.
-
37Bian SW, Mudunkotuwa IA, Rupasinghe T, Grassian VH. Aggregation and dissolution of 4 nm ZnO nanoparticles in aqueous environments: influence of pH, ionic strength, size, and adsorption of humic acid. Langmuir. 2011; 27:6059-68.
-
38Gao H, Yang Z, Zhang S, Cao S, Shen S, Pang Z, Jiang X. Ligand modified nanoparticles increases cell uptake, alters endocytosis and elevates glioma distribution and internalization. Scientific reports. 2013; 3:2534.
-
39Wang H, Wu L, Reinhard BM. Scavenger Receptor Mediated Endocytosis of Silver Nanoparticles into J774A.1 Macrophages is Heterogeneous. ACS Nano. 2012; 28; 6(8):7122-7132.
-
40Jin C-Y, Zhu B-S, Wang X-F, Lu Q-H. Cytotoxicity of Titanium Dioxide Nanoparticles in Mouse Fibroblast Cells. Chem. Res. Toxicol. 2008; 21:1871-1877.
-
41Albanese A, Chan WCW. Effect of gold nanoparticle aggregation on cell uptake and toxicity. ACS Nano. 2011; 5:5478-89.
-
42Bernier MC, Kirat KE, Besse M, Morandat S, Vayssade M. Preosteoblasts and fibroblasts respond differently to anatase titanium dioxide nanoparticles: A cytotoxicity and inflammation study. Colloids Surf B: Biointerfaces. 2012; 90:68-74.
-
43Huang X, Young NP, Townley HE. Characterization and comparison of mesoporous silica particles for optimized drug delivery. Nanomater Nanotechno. 2014; 4:2.
-
44Papageorgiou I, Brown C, Schins R, Singh S, Newson R, Davis S, Fisher J, Ingham E, Case CP. The effect of nano- and micron-sized particles of cobalt-chromium alloy on human fibroblasts in vitro. Biomaterials. 2007; 28:2946-58.
-
45Brunner TJ, Wick P, Manser P, Spohn P, Grass RN, Limbach LK, Bruinink A, Stark WJ. In vitro cytotoxicity of oxide nanoparticles: comparison to asbestos, silica, and the effect of particle solubility. Environ. Sci. Technol. 2006, 40, 4374-4381.
-
46Barnes CA, Elsaesser A, Arkusz J, Smok A, Palus J, Lesniak A, Salvati A, Hanrahan JP, Jong WH, Dziubaltowska E, Stepnik M, Rydzynski K, McKerr G, Lynch I, Dawson KA, Howard CV. Reproducible comet assay of amorphous silica nanoparticles detects no genotoxicity. Nano Lett. 2008; 8:3060-3074.
-
47Hu L, Fawcett JP, Gu J. Protein target discovery of drug and its reactive intermediate metabolite by using proteomic strategy. Acta Pharm Sin B. 2012; 2:126-136.
-
48Agnihotri S, Mukherji S, Mukherji S. Immobilized silver nanoparticles enhance contact killing and show highest efficacy: elucidation of the mechanism of bactericidal action of silver. Nanoscale. 2013; 5:7328-40.
-
49Hu SH, Liu TY, Huang HY, Liu DM, Chen SY. Magnetic-sensitive silica nanospheres for controlled drug release. Langmuir. 2008; 24: 239-244.
-
50Drescher D, Orts-Gil G, Laube G, Natte K, Veh RW, Österle W, Kneipp J. Toxicity of amorphous silica nanoparticles on eukaryotic cell model is determined by particle agglomeration and serum protein adsorption effects. Anal Bioanal Chem. 2011; 400:1367-73.
-
51Lynch I, Dawson KA. Protein-nanoparticle interactions. Nanotoday. 2008; 3: 40-47.
-
52Stayton I, Winiarz J, Shannon K, Ma Y. Study of uptake and loss of silica nanoparticles in living human lung epithelial cells at single cell level. Anal Bioanal Chem. 2009; 394:1595-1608.
-
53Kim HR, Kim MJ, Lee SY, Oh SM, Chung KH. Genotoxic effects of silver nanoparticles stimulated by oxidative stress in human normal bronchial epithelial (BEAS-2B) cells. Mutat Res. 2011; 726:129-135.
-
54Kim HR, Park YJ, Shin DY, Oh SM, Chung KH. Appropriate in vitro methods for genotoxicity testing of silver nanoparticles. Environ Health Toxicol. 2013; 28: e2013003.
-
55Falck GCM, Lindberg HK, Suhonen S, Vippola M, Vanhala E, Catalán J, Savolainen K, Norppa H. Genotoxic effects of nanosized and fine TiO2. Hum Exp Toxicol. 2009; 28: 339-52.
-
56Woodruff RS, Li Y, Yan J, Bishop M, Jones MY, Watanabe F, Biris AS, Rice P, Zhouf T, Chenb T. Genotoxicity evaluation of titanium dioxide nanoparticles using the Ames test and Comet assay. J. Appl. Toxicol. 2012; 32: 934-943.
-
57Clift MJD, Raemy DO, EndesC, Ali Z, Lehmann AD, Brandenberger C, Petri-Fink A, Wick P, Parak WJ, Gehr P, Schins RPF, Rothen-Rutishauser B. Can the Ames test provide an insight into nano-object mutagenicity? Investigating the interaction between nano-objects and bacteria. Nanotechnology. 2013; 7(8): 1373-1385.
-
58Chithrani BD, Ghazani AA, Chan WCW. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006; 6:662-8.
-
59Murdock RC, Braydich-Stolle L, Schrand AM, Schlager JJ, Hussain SM. Characterization of nanomaterial dispersion in solution prior to in vitro exposure using dynamic light scattering technique. Toxicol Sci. 2008; 101:239-253.
-
60Ates M, Daniels J, Arslan Z, Farah IO. Effects of aqueous suspensions of titanium dioxide nanoparticles on Artemia salina: assessment of nanoparticle aggregation, accumulation, and toxicity. Environ Monit Assess. 2013; 185:3339-48.
-
61Kakinoki K, Yamane K, Teraoka R, Otsuka M, Matsuda Y. Effect of relative humidity on the photocatalytic activity of titanium dioxide and photostability of famotidine. J Pharm Sci. 2004; 93: 582-589.
-
62Sayes CM, Wahi R, Kurian PA, Liu Y, West JL, Ausman KD, Warheit DB, Colvin VL. Correlating nanoscale titania structure with toxicity: a cytotoxicity and inflammatory response study with human dermal fibroblasts and human lung epithelial cells. Toxicol Sci. 2006; 92:174-185.
-
63Warheit DB, Webb TR, Reed KL, Frerichs S, Sayes CM. Pulmonary toxicity study in rats with three forms of ultrafine-TiO2 particles: Differential responses related to surface properties. Toxicology. 2007; 230: 90-104.
-
64Schedlel A, Samorapoompichit P, Rausch-Fan XH, Franz A, Fureder W, Sperr WR, Sperr W, Ellingerl A, Slavicek R, Boltz-Nitulescu G, Valent P. Response of L-929 Fibroblasts, Human Gingival Fibroblasts, and Human Tissue Mast Cells to Various Metal Cations. J. Dent. Res. 1995; 74(8): 1513-1520.
-
65International Organization for Standardization. ISO 10993-5: Biological evaluation of medical devices - Part 5: Tests for in vitro cytotoxicity. 2009, Switzerland.
-
66Nascimento FG, Faqueti A, Wilhelm JF, Wittkowski C, Tomczak FD, Borges SL, Yunes RA, Franchi Jr. GC, Nowill AE, Filho VC, Machado MS, Freitas RA, Malheiros A. Seasonal influence and cytotoxicity of extracts, fractions and major compounds from Allamanda schottii. Rev. Bras. Farmacogn. 2014; 24: 545-552.
-
67Thonemann B, Schmalz G, Hiller KA, Schweik H. Responses of L929 mouse fibroblasts, primary and immortalized bovine dental papilla-derived cell lines to dental resin components. Dent. Mater. 2002; 18(4): 318-323.
-
68Mahmoudi M, Simchi A, Vali H, Imani M, Shokrgozar MA, Azadmanesh K, Azari F. Cytotoxicity and Cell Cycle Effects of Bare and Poly(vinylalcohol)-Coated Iron Oxide Nanoparticles in Mouse Fibroblasts. Adv. Eng. Mater. 2009; 11(12): B243-B250.
-
69Ahamed M, Karns M, Goodson M, Rowe J, Hussain SM, Schlager JJ, Hong Y. DNA damage response to different surface chemistry of silver nanoparticles in mammalian cells. Toxicol. Appl. Pharm. 2008; 233: 404-410.
-
70SCENIHR. (Scientific Committee on Emerging and Newly Identified Health Risks). Risk assessment of products of nanotechnologies. Brussels: European Commission, 2009. Available at http://ec.europa.eu/health/ph_risk/committees/04_ scenihr/docs/scenihr_ o_023.pdf. Accessed 8/Dec/2015.
Publication Dates
-
Publication in this collection
2018
History
-
Received
31 Oct 2016 -
Accepted
20 Aug 2018