Abstract
Presence of the relatively new sulfonylurea herbicide monosulfuron-ester at 0.03-300 nmol/L affected the growth of two non-target nitrogen-fixing cyanobacteria (Anabaena flos-aquae and Anabaena azotica) and substantially inhibited in vitro Acetolactate synthase activity, with IC50 of 3.3 and 101.3 nmol/L for A. flos-aquae and A. azotica, respectively. Presenting in 30-300 nmol/L, it inhibited protein synthesis of the cyanobacteria with less amino acids produced as its concentration increased. Our findings support the view that monosulfuron-ester toxicity in both nitrogen-fixing cyanobacteria is due to its interference with protein metabolism via inhibition of branch-chain amino acid biosynthesis, and particularly Acetolactate synthase activity.
Keywords:
Acetolactate synthase; Amino acids; Cyanobacteria; Monosulfuron-ester
Introduction
Monosulfuron-ester is a relatively new sulfonylurea herbicide that was developed by the National Pesticide Engineering Research Center in Tianjin, China.11 Li ZM, Jia GF, Wang LX. Sulfonylurea compounds and its herbicide usage. Chinese patent CNI: ZL94118793. 1994. This herbicide is very effective at post-emergence rates of 30-60 nmol/L (active ingredients) in a wide range of crops including corn (Zea mays L.), wheat (Triticum aestivum L.), rice (Oryza sativa L.), and millet (Panicum miliaceum L.).22 Wang W. Application of new sulfonylurea herbicides in rice direct seedling fields. Shanghai Agric Sci. 2006;:12-16. The mode of action of sulfonylurea is inhibition of acetolactate synthase (ALS).33 Riar DS, Norsworthy JK, Srivastava V, et al. Physiological and molecular basis of acetolactate synthase-inhibiting herbicide resistance in barnyardgrass (Echinochloa crus-galli). J Agric Food Chem. 2012;61(2):278-289. The ALS is present in all plants as well as in bacteria, fungi, and cyanobacteria,44 Yu Q, Nelson JK, Zheng MQ, et al. Molecular characterisation of resistance to ALS-inhibiting herbicides in Hordeum leporinum, biotypes. Pest Manage Sci. 2007;63(9):918-927. but not present in mammals. Because of their relatively low toxicity to mammals, high efficacy and environmental safety,55 Reddy KN, Whiting K. Weed control and economic comparisons of glyphosate-resistant, sulfonylurea-tolerant, and conventional soybean (Glycine max) systems. Weed Technol. 2014;14(1):204-211. the use of sulfonylurea herbicides has increased rapidly. However, application of selective herbicides such as sulfonylurea in cropping systems can substantially reduce soil microbial diversity if they are overused or misused.
Cyanobacteria are one of the largest and most important groups of algae on earth. In particular, nitrogen-fixing cyanobacteria are vital photosynthetic microorganisms that contribute to soil fertility by fixing atmospheric nitrogen, releasing small quantities of the major fertilizing product, ammonia, as well as other small nitrogenous polypeptides during active growth,66 Grewe CB, Pulz O. The Biotechnology of Cyanobacteria. Ecology of Cyanobacteria II; 2012:707–739. and also because they maintain ecosystem stability.77 Shen JY, Lu YT. Effects of herbicides on biodiversity of rice fields in China. In: Impact Assessment of Farm Chemicals Run Off from Paddy Conference. Japan: National Institute for Agro-Environmental Sciences, Japan (NIAES)/National Institute of Agricultural Science and Technology, Korea (NIAST); 2005:56–67.Anabaena azotica Ley and Anabaena flos-aquae (Lyngb) Breb are two of the most common filamentous nitrogen-fixing cyanobacteria in China agricultural fields.88 Vijai KA. Anabaena flos-aquae. Crit Rev Environ Sci Technol. 2014;44(18):1995-2037. In general, cyanobacteria are very sensitive to herbicides because they possess many characteristics of higher plants.99 Dai G, Deblois CP, Liu S, et al. Differential sensitivity of five cyanobacterial strains to ammonium toxicity and its inhibitory mechanism on the photosynthesis of rice-field cyanobacterium Ge-Xian-Mi (Nostoc). Aquat Toxicol. 2008;89(2):113-121. Previous study showed that cyanobacteria had different degrees of sensitivity to herbicides.1010 Shen JY, Lu YT, Cheng G. Effects of chemical herbicides on toxicity of non-target nitrogen-fixing Cyanobacteria in paddy fields. In: 20th Asian-Pacific Weed Sci. Conf. Vietnam: Weed Sci. Soc. Asian-Pacific. 2005:665–670. However, the effect of monosulfuron-ester on nitrogen-fixing cyanobacteria that may play a role in enhancing the fertility of agricultural fields has not been reported in the literature. Therefore, the objectives of this research were to determine the effects of a range of monosulfuron-ester concentrations on growth, protein content, in vitro and extractable activity of ALS, and amino acid production of both nitrogen-fixing cyanobacteria. Findings from this work should provide a better understanding of the toxicity and its mode of action of monosulfuron-ester in the two ubiquitous nitrogen-fixing cyanobacteria.
Materials and methods
Monosulfuron-ester of 99.4% purity was synthesized at the National Pesticide Engineering Research Center in Tianjin, China. Monosulfuron-ester was dissolved in the mixture of organic solvent dimethylformamide (DMF, N,N-dimethylformamide, Jianshan Chemicals Ltd., China) and surfactant TritonX-100 (4-(1,1,3,3-tetramethylbutyl)phenyl-polyethylene glycol) (Jibishi Gene Technology Ltd., China) to form the herbicide concentrate below the concentration of first effect, i.e., DMF 0.5% (v/v) and TritonX-100 0.005% (v/v). The fresh concentrate was filtered and sterilized and then added to the culture medium to prepare the test solutions of the desired monosulfuron-ester doses.
To measure monosulfuron-ester concentrations in the medium and in algal cells a HPLC analysis procedure was developed employing a Agilent 1100 HPLC (Agilent Inc.) equipped with UV-200 detector, ODS Diamonsi (5 µm) column with 250 ×4.6 mm. Operating conditions for the HPLC analysis were: mobile phase-methanol/water (containing H3PO4, pH4.0) (53/47, v/v); mobile phase flow rate 1.0 mL/min; sample injection volume 10 µL; wavelength 236 nm; and column temperature 40 °C. Under these conditions the resulting calibration curve correlating the monosulfuron-ester concentration to the apex area was y = 91302x + 26735 (R2 = 0.9953) with an average recovery of 95-103%.
Cultures of A. azotica (FACHB-181) and A. flos-aquae (FACHB-245) two nitrogen-fixing algal species of the Nostocaceae, were obtained from the Institute of Hydrobiology of the Chinese Academy of Sciences in Wuhan, China. Axenic cultures were grown in a sterilized HB-111 liquid medium (0.075 g/L K2HPO4, 0.125 g/L MgSO4, 0.1 g/L CaCO3, 0.005 g/L FeC6H5O7, 0.005 g/L C6H8O7, 0.0025 g/L MoO3·H2O, 0.01 g/L NaOH) at 30 ± 2 °C under constant fluorescent light of 36.2 µmol/m2/s. The experimental cultures were first grown in 250 mL flasks containing 100 mL of the medium with 0.5-1 million cells per milliliters under the same conditions. At the exponential growth phase of the algal cultures, small aliquots of the concentrate were added to the culture medium flasks to result in 0.003, 0.03, 0.3, 3, 30, and 300 nmol/L of monosulfuron-ester in the test samples. Sterilized water was added instead to the same culture media to produce the control samples. Samples were collected 6 d after the herbicide addition to determine the protein and amino acid contents, and the ALS activity.
Growth rates of cyanobacteria were measured by recording light absorbance of the culture at 448 nm using a spectrophotometer (Shanghai Biotechnic Ltd.). Standard curves relating Abs448 nm with cell numbers were developed for continuous cultures of A. azotica and A. flos-aquae. During the experimental period, samples were withdrawn after the herbicide treatment at 1, 2, 3, 4, 5, and 6 days for growth rate measurements. The growth rate (µ) of the cyanobacteria was calculated using the following Eq. (1).
where X1 and X0 are Abs448 nm measured at times T1 and T0. To estimate the cell's dry weight, the cultures were centrifuged, and the solid mass was washed with distilled water three times, dried at 105 °C for 8 h; the cell's dry weight was the average of three weight measurements.
The protein content of the cultures was determined by using bovine serum albumin (BSA) as a standard described by Bradford (1976).1111 Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-254. One-hundred nanomoles of Coomassie Brilliant Blue G-250 was first dissolved in 50 mL of 95% ethanol and then 100 mL of 85% (w/v) phosphoric acid was added to this solution. The resulting solution was diluted to a final volume of 1 L. BSA was diluted in five concentrations (0.2, 0.4, 0.6, 0.8, 1 mg/L) and their absorbance at the 595 nm wavelength were measured after 2 min at 20 °C. 5 mL liquid sample was taken and centrifuged (8000 rpm) 10 minutes at 4 °C to collect algal cells. Add a small amount of phosphate buffer (pH 7.8), repeated freezing and thawing until the algae fluid became blue, then centrifuged the sample at 8000 rmp for 30 min at 4 °C and the resulting supernatant was used to determine protein content. 0.1 mL supernatant was taken to determine the protein content according the regression equation of the straight line of protein relating Abs595 nm.
The amino acid components were analyzed using an amino acid automatic analyzer (Hitachi) with an ion exchange column after the cyanobacterial sample was hydrolyzed in 6 mol/L HCl at 110 ± 1 °C for 24 h. The absorbance of the sample was measured at 570 nm except 440 nm for proline after color development of the ninhydrin reaction at 100 °C.
ALS was extracted from 6 d cultivated nitrogen-fixing cyanobacteria of the two test species. The cyanobacteria were centrifuged at 10,000 × g for 30 min at 4 °C (Sorvall Centrifuge, DJB Labcare Ltd.) in buffer or medium after cells were sonicated using an ultrasonic cell disruptor (250 mv, 3 s, 3 s) in the dark at 4 °C. The protein in the supernatant was precipitated with ammonium sulphate, and then centrifuged at 10,000 × g for 30 min at 4 °Cafter 2 h incubation at 0 °C. The pellet was dissolved in phosphate buffer (pH = 7.5) containing 200 mmol/L sodium pyruvate, 5 mmol/L MgCl2, 10 mmol/L thiamine pyrophosphate (TPP Sigma Company), 200 µmol/L flavin adenine dinucleotide (FAD Sigma Company), and 1 mol/L dl-dithiothreitol (DTT GL Biochem Ltd.). This enzyme solution was kept in ice/water in the dark. The enzyme solution was dissolved in 50 mmol/L phosphate buffer (pH = 7.5) containing 20 mmol/L sodium pyruvate and 0.5 mmol/L MgCl2. A volume of 0.1 mL of monosulfuron-ester was added to 0.4 mL of enzyme and 0.5 mL of enzyme reaction solution. The mixture was subsequently incubated at 37 ± 1 °C in a water bath for 1 h in the dark. After adding 0.1 mL of 3 mmol/L sulfuric acid, the mixture was incubated at 60 ± 1 °C for 15 min to decarboxylate the acetolactate to acetoin. 0.5 mL of freshly prepared 5.0% α-naphthol in 2.5 mol/L sodium hydroxide and 0.5 mL of 0.5% creatine were then added to the mixture and incubated at 60 ± 1 °C for 15 min for color development. The mixture was then cooled to the room temperature (25 °C) in a water bath and absorbance was measured at 525 nm using a spectrophotometer. The specific activity of ALS was expressed as nmol of acetoin/nmol of protein/h.1212 Fan ZJ, Ai YW, Qian CF, Li ZM. Herbicide activity of Monosulfuron-ester and its mode of action. J Environ Sci (China). 2005;17:399-403.
To determine the extractable ALS activity, 0.1 mL of monosulfuron-ester and 0.4 mL of the enzyme were substituted with 0.5 mL of the enzyme solution extracted from nitrogen-fixing cyanobacteria that had been cultivated in the culture medium containing monosulfuron-ester. Six concentrations of monosulfuron-ester were used as outlined previously and the specific activity of ALS was determined and expressed as described above.
A completely randomized design with three replications was used in all duplicated experiments. Analyses of variance (ANOVA) were performed on the non-transformed data. Significant differences were determined using Duncan's test at p = 0.05 level of significance (PROC GLM, SAS Institute, 2001). The values of IC50 were calculated with the program OriginPro7.5SR1 (OriginLab Corporation, USA).
Results
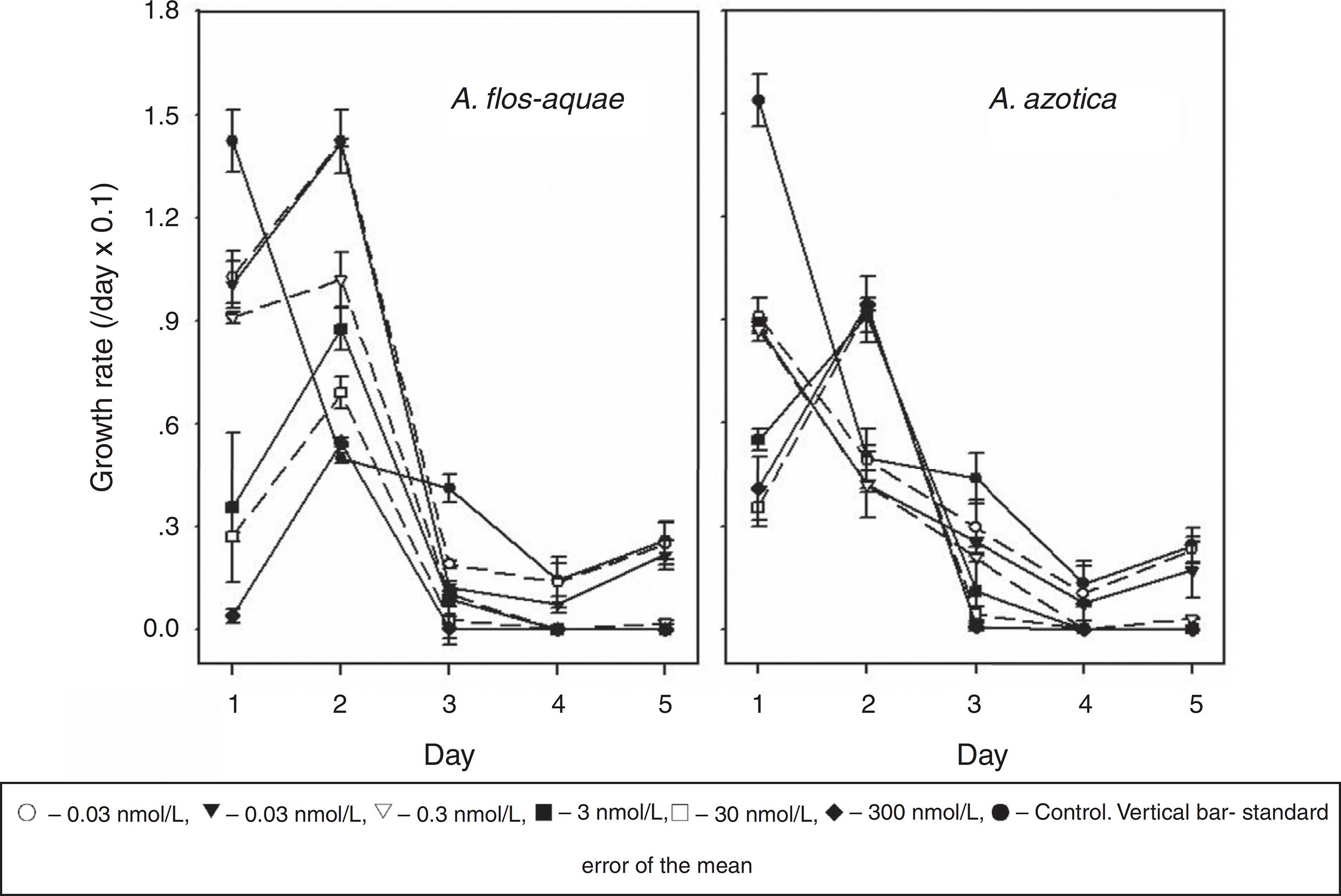
In general, the growth rates of A. flos-aquae and A. azotica of the control decreased gradually as the culturing time increased; however, the growth rates of both cyanobacteria treated with monosulfuron-ester (0.03-300 nmol/L) exhibited a wave pattern of initial decline followed by gradual increases (Fig. 1).
Effect of monosulfuron-ester concentration on the growth rate profile: A. flos-aquae and A. azotica.
The growth rates of A. flos-aquae and A. azotica were reduced by 28-97% and 41-73% at 0.03-300 nmol/L, after 1 day, respectively, relative to the control; the monosulfuron-ester treatment then transient stimulated the growth on second day. For A. flos-aquae, the growth rates at 0.03, 0.3, 3, 30, and 300 nmol/L increased by 185, 184, 104, 75, 38, and 9%, respectively relative to the control treatment and the growth rate increase declined with higher monosulfuron-ester concentration; however, for A. azotica, the growth rates increased by 83-90% at 3-300 nmol/L. It can be seen that both cyanobacteria had the different responses to monosulfuron-ester. A. flos-aquae was more sensitivity to this herbicide than A. azotica.
Fig. 2 illustrates the effect of monosulfuron-ester concentration on the protein content in the two cyanobacteria. Monosulfuron-ester applied at low concentrations of 0.03-0.3 nmol/L stimulated the production of proteins in both cyanobacteria, but the reverse was observed at higher concentrations (i.e. 30-300 nmol/L). The protein content of A. flos-aquae cell cultures treated with monosulfuron-ester at 0.03 and 0.3 nmol/L increased 101 and 89%, respectively relative to the control (p < 0.05); its protein content decreased sharply as monosulfuron-ester concentration increased, for example, the protein content was 9-16% lower with 30-300 nmol/L monosulfuron-ester. The monosulfuron-ester treatment did not markedly have effect on protein content in A. azotica, its protein content increased by only 0.8% with monosulfuron-ester 0.03 nmol/L. It is thus clear that A. flos-aquae exhibited greater sensitivity to monosulfuron-ester.
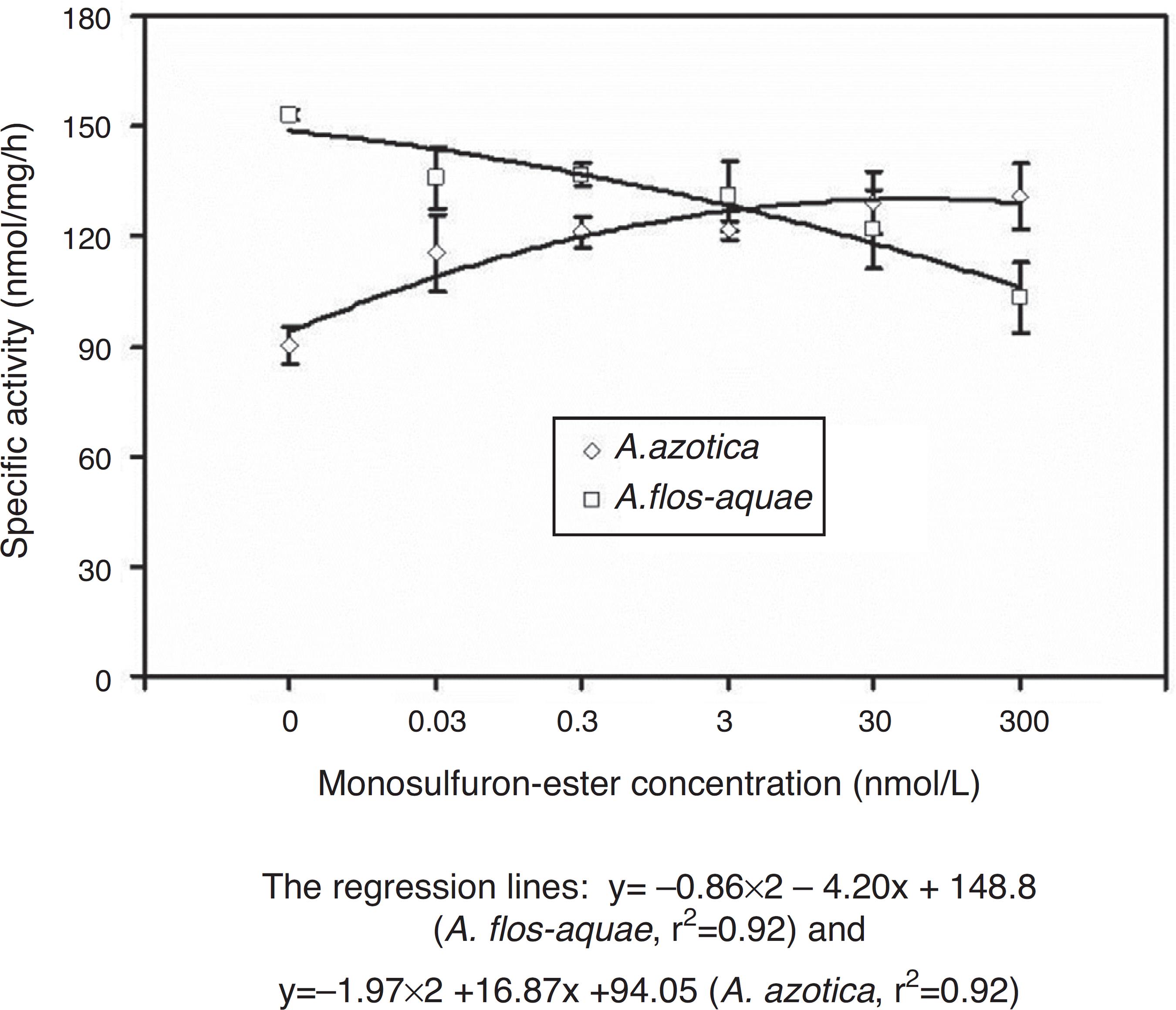
The in vitro ALS activity varied between the two cyanobacterial species following monosulfuron-ester application (Fig. 3). For A. flos-aquae, the activity of ALS was reduced in the range of 0.8-92% as the concentration of monosulfuron-ester increased from 0.003 to 300 nmol/L, and there was a significant difference in activity between each concentration (p < 0.05). The ALS activity of A. azotica at low monosulfuron-ester concentrations (0.003-0.3 nmol/L) did not differ significantly from the same of the control; it declined notably, by 19-75% relative to the control, as the concentration of monosulfuron-ester increased from 3 to 300 nmol/L. Calculated IC50 values for A. flos-aquae and A. azotica were 3.3 and 101.3 nmol/L respectively. These results indicate that monosulfuron-ester does inhibit ALS activity in the two nitrogen-fixing cyanobacteria, particularly at higher concentrations.
Interestingly, the extractable ALS activity in the two cyanobacteria responded differently to monosulfuron-ester in 0.03-300 nmol/L (Fig. 4); relative to the control, the extractable ALS activity in A. azotica increased 28-45% while the same in A. flos-aquae declined by 11-33%. There was a further evidence that A. flos-aquae were more sensitive to this herbicide than A. azotica.
Table 1 presents the effect of monosulfuron-ester concentration on amino acids produced in the two cyanobacteria. Amino acid production in cells of A. flos-aquae and A. azotica was clearly inhibited by monosulfuron-ester in the characteristic dose-dependent response; namely, increased inhibition of amino acid production with increasing monosulfuron-ester concentration. The quantity of branch-chain amino acids valine, isoleucine, leucine in cells of A. flos-aquae was reduced by 56, 67, and 67% at the 0.003 nmol/L, respectively relative to the control, and by 80, 86, and 87% at the 30 nmol/L (p < 0.05). The valine, isoleucine, leucine content of A. azotica cells was reduced by 35, 45, and 47% at the 0.003 nmol/L concentration, and by 61, 52, and 55% at the 30 nmol/L concentration. The other amino acids content of A. azotica and A. flos-aquae cells such as glycine, alanine, phenylalanine, serine, threonine, methionine, glutarnine, asparticacid, lysine, and arginine were respectively reduced 8-55% and 67-90% at monosulfuron-ester concentrations 0.003 and 30 nmol/L, and proline, tyrosine, and histidine were below detection at 30 nmol/L. Again A. flos-aquae was the more sensitive of the two.
Discussion
The transient stimulative effect of monosulfuron-ester on algal growth on the second day of this study is similar to the findings of Shen et al. (2005) for butachlor and acetochlor on several Anabaena species.77 Shen JY, Lu YT. Effects of herbicides on biodiversity of rice fields in China. In: Impact Assessment of Farm Chemicals Run Off from Paddy Conference. Japan: National Institute for Agro-Environmental Sciences, Japan (NIAES)/National Institute of Agricultural Science and Technology, Korea (NIAST); 2005:56–67. Our result that the corresponding growth rates of the A. flos-aquae and A. azotica exposed to different concentrations of monosulfuron-ester exhibited wave patterns was similar to the report of Shen et al. (2011).1313 Shen JY, Luo W. Effects of monosulfuron on growth: photosynthesis, and nitrogenase activity of three nitrogen-fixing cyanobacteria. Arch Environ Contam Toxicol. 2011;60(1):34-43. Hormesis, the stimulative effect of a toxin at sub-toxic concentrations, following the application of other herbicides and allelochemicals has been documented.1414 Calabrese EJ. Paradigm lost, paradigm found: the re-emergence of hormesis as a fundamental dose response model in the toxicological sciences. Environ Pollut. 2005;138:379-412.
The study has evaluated the effects of herbicides on metabolic pathways of protein synthesis in non-target organisms.1515 Okmen G, Turkcan O, Erdal P. Effect of herbicides on chlorophyll-a, β-caroten, phycocyanin and allophycocyanin content of Anabaena sp.. J Appl Biol Sci. 2012;7:20-27. Our findings at low monosulfuron-ester concentrations (0.03-3 nmol/L) indicate that the protein content of cells from the two cyanobacteria increased substantially. The increase may have resulted from stimulation in protein synthesis via increased production of RNA.1616 Gruenhagen RD, Moreland DE. Effects of herbicides on ATO levels in excised soybean hypocotyls. Weed Sci. 1971;19:319-323. Alternatively, at lower monosulfuron-ester concentrations, cyanobacterial cells may have assimilated more organic carbon and nitrogen, which interfered with herbicide uptake, due to the formation of an inactive herbicide complex with organic carbon and nitrogen, and thus reduced the herbicide' toxicity; the bacteria may also degrade the herbicide into hydrate of carbon and amino acids metabolites resulting in a lower toxicity.1717 Nagai T, Ishihara S, Yokoyama A, et al. Effects of four rice paddy herbicides on algal cell viability and the relationship with population recovery. Environ Toxicol Chem. 2011;30(8):1898-1905. The lower protein contents of both cyanobacteria subjected to monosulfuron-ester at high concentrations (30-300 nmol/L) may be related to increase of protease activity1818 Bhunia AK, Basu NK, Roy D, Chakrabarti A, Banerjee SK. Growth, chlorophyll a content, nitrogen-fixing ability, and certain metabolic activities of Nostoc muscorum: effect of methylparathion and benthiocarb. Bull Environ Contam Toxicol. 1991;471:43-50.; it may also be the result of inhibition of the ALS activity which interfered with production of the three branched-chain amino acids (valine, leucine, and isoleucine) essential precursors of proteins.1212 Fan ZJ, Ai YW, Qian CF, Li ZM. Herbicide activity of Monosulfuron-ester and its mode of action. J Environ Sci (China). 2005;17:399-403.
The mode of action of the sulfonylurea is inhibition of ALS, which is responsible for catalyzing the biosynthesis of the branch-chain amino acids valine, leucine, and isoleucine.33 Riar DS, Norsworthy JK, Srivastava V, et al. Physiological and molecular basis of acetolactate synthase-inhibiting herbicide resistance in barnyardgrass (Echinochloa crus-galli). J Agric Food Chem. 2012;61(2):278-289. Shen et al. (2009) reported that monosulfuron inhibits vitro ALS activity in three nitrogen-fixing cyanobacteria at higher concentrations.1919 Shen JY, Antonio DT, Shen M, et al. Molecular basis for differential metabolic responses to monosulfuron in three nitrogen-fixing cyanobacteria. Weed Sci. 2009;57(2):133-141. Our study is similar to her results that the ALS activity was more reduced as the concentration monosulfuron-ester increased; IC50 values of 3.3 and 101.3 nmol/L for A. flos-aquae and A. azotica, respectively, have provided further evidences of the negative impact of this herbicide on growth of these cyanobacterial species. The strong inhibition of ALS activity found in both species in vitro confirms that the toxic effects of monosulfuron-ester is indeed of inhibition to ALS as is the case with other sulfonylurea herbicides.2020 Chaleff RS, Mauvais CJ. Acetolactate synthase is the site action of two sulfonylurea herbicides in higher plants. Science. 1984;224:1443-2144. Shen et al. (2009) also pointed out that monosulfuron stimulated the extractable ALS activity in A. azotica, but the specific activity in A. flos-aquae decreased at high concentration of monosulfuron.1919 Shen JY, Antonio DT, Shen M, et al. Molecular basis for differential metabolic responses to monosulfuron in three nitrogen-fixing cyanobacteria. Weed Sci. 2009;57(2):133-141. In our study, with monosulfuron-ester present at 0.03-300 nmol/L, the extractable ALS activity in A. azotica increased by 28-45%, while the specific activity of A. flos-aquae decreased by 3-11%. It is possible that differences in specific activity between in vitro and in vivo responses of the two cyanobacteria were due to the presences of different levels of herbicide detoxifying enzymes (e.g. glutathione S-transferase (GST) and mixed-function oxidase (MFO)).2121 Chen J, Zheng HW, Shong TY, Xi GZ, Tong LF. The genetic diversity of Anabaena azollae based on RAPD analysis. Acta Hydrobiol Sin. 2001;25:531-534. Other possible reasons for the markedly different effects between the cyanobacterial species on the extractable ALS specific activity at the same monosulfuron-ester concentration include absorption of the herbicide and the different degrees of ALS inhibition between the species.
That A. flos-aquae exhibited greater sensitivity to monosulfuron-ester than A. azotica may be related to its greater capacity for taking up the herbicide. Such different responses may be due to different biological properties of the cyanobacteria2222 Cummins I, Wortley DJ, Sabbadin F, et al. Key role for a glutathione transferase in multiple-herbicide resistance in grass weeds. Proc Natl Acad Sci U S A. 2013;110(15):5812-5817. and may be exploited to select for cyanobacteria that are more resistant to monosulfuron-ester in the cropping systems. For instance, the culturing of beneficial nitrogen-fixing cyanobacteria in paddy fields as ‘bio-fertilizers' is a highly desirable option for increasing rice yield and preventing any negative effects of this cropping system on the environment.1010 Shen JY, Lu YT, Cheng G. Effects of chemical herbicides on toxicity of non-target nitrogen-fixing Cyanobacteria in paddy fields. In: 20th Asian-Pacific Weed Sci. Conf. Vietnam: Weed Sci. Soc. Asian-Pacific. 2005:665–670.
Conclusion
Application of monosulfuron-ester at 0.03-300 nmol/L affected the growth rates of A. flos-aquae and A. azotica in the wave patterns, and had an inhibitory effect on protein synthesis at higher concentrations (30-300 nmol/L). The production of amino acids was reduced with increasing monosulfuron-ester concentration. Monosulfuron-ester substantially inhibited in vitro Acetolactate synthase (ALS) activity as evidenced by the IC50 values of 3.3 and 101.3 nmol/L for A. flos-aquae and A. azotica, respectively. The results show that the normal agricultural use of monosulfuron-ester at post-emergence rates of 30-60 nmol/L in rice fields will likely be toxic to the both ubiquitous nitrogen-fixing cyanobacteria; the monosulfuron-ester toxicity was due to its interference with protein metabolism via inhibition of branch-chain amino acid biosynthesis, especially the ALS activity. We also suggest that in rice cropping systems where the new sulfonylurea herbicide monosulfuron-ester is frequently applied, the beneficial nitrogen-fixing cyanobacteria, A. azotica, may be employed as "biofertilizers" since it is more resistant to the herbicide than A. flos-aquae.
Acknowledgment
Funding for this work was provided by the National Natural Science Foundation of China (21377086).
References
-
1Li ZM, Jia GF, Wang LX. Sulfonylurea compounds and its herbicide usage. Chinese patent CNI: ZL94118793. 1994.
-
2Wang W. Application of new sulfonylurea herbicides in rice direct seedling fields. Shanghai Agric Sci 2006;:12-16.
-
3Riar DS, Norsworthy JK, Srivastava V, et al. Physiological and molecular basis of acetolactate synthase-inhibiting herbicide resistance in barnyardgrass (Echinochloa crus-galli). J Agric Food Chem. 2012;61(2):278-289.
-
4Yu Q, Nelson JK, Zheng MQ, et al. Molecular characterisation of resistance to ALS-inhibiting herbicides in Hordeum leporinum, biotypes. Pest Manage Sci. 2007;63(9):918-927.
-
5Reddy KN, Whiting K. Weed control and economic comparisons of glyphosate-resistant, sulfonylurea-tolerant, and conventional soybean (Glycine max) systems. Weed Technol. 2014;14(1):204-211.
-
6Grewe CB, Pulz O. The Biotechnology of Cyanobacteria. Ecology of Cyanobacteria II; 2012:707–739.
-
7Shen JY, Lu YT. Effects of herbicides on biodiversity of rice fields in China. In: Impact Assessment of Farm Chemicals Run Off from Paddy Conference Japan: National Institute for Agro-Environmental Sciences, Japan (NIAES)/National Institute of Agricultural Science and Technology, Korea (NIAST); 2005:56–67.
-
8Vijai KA. Anabaena flos-aquae. Crit Rev Environ Sci Technol 2014;44(18):1995-2037.
-
9Dai G, Deblois CP, Liu S, et al. Differential sensitivity of five cyanobacterial strains to ammonium toxicity and its inhibitory mechanism on the photosynthesis of rice-field cyanobacterium Ge-Xian-Mi (Nostoc). Aquat Toxicol. 2008;89(2):113-121.
-
10Shen JY, Lu YT, Cheng G. Effects of chemical herbicides on toxicity of non-target nitrogen-fixing Cyanobacteria in paddy fields. In: 20th Asian-Pacific Weed Sci. Conf. Vietnam: Weed Sci. Soc. Asian-Pacific 2005:665–670.
-
11Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-254.
-
12Fan ZJ, Ai YW, Qian CF, Li ZM. Herbicide activity of Monosulfuron-ester and its mode of action. J Environ Sci (China). 2005;17:399-403.
-
13Shen JY, Luo W. Effects of monosulfuron on growth: photosynthesis, and nitrogenase activity of three nitrogen-fixing cyanobacteria. Arch Environ Contam Toxicol. 2011;60(1):34-43.
-
14Calabrese EJ. Paradigm lost, paradigm found: the re-emergence of hormesis as a fundamental dose response model in the toxicological sciences. Environ Pollut. 2005;138:379-412.
-
15Okmen G, Turkcan O, Erdal P. Effect of herbicides on chlorophyll-a, β-caroten, phycocyanin and allophycocyanin content of Anabaena sp.. J Appl Biol Sci. 2012;7:20-27.
-
16Gruenhagen RD, Moreland DE. Effects of herbicides on ATO levels in excised soybean hypocotyls. Weed Sci 1971;19:319-323.
-
17Nagai T, Ishihara S, Yokoyama A, et al. Effects of four rice paddy herbicides on algal cell viability and the relationship with population recovery. Environ Toxicol Chem. 2011;30(8):1898-1905.
-
18Bhunia AK, Basu NK, Roy D, Chakrabarti A, Banerjee SK. Growth, chlorophyll a content, nitrogen-fixing ability, and certain metabolic activities of Nostoc muscorum: effect of methylparathion and benthiocarb. Bull Environ Contam Toxicol. 1991;471:43-50.
-
19Shen JY, Antonio DT, Shen M, et al. Molecular basis for differential metabolic responses to monosulfuron in three nitrogen-fixing cyanobacteria. Weed Sci. 2009;57(2):133-141.
-
20Chaleff RS, Mauvais CJ. Acetolactate synthase is the site action of two sulfonylurea herbicides in higher plants. Science 1984;224:1443-2144.
-
21Chen J, Zheng HW, Shong TY, Xi GZ, Tong LF. The genetic diversity of Anabaena azollae based on RAPD analysis. Acta Hydrobiol Sin 2001;25:531-534.
-
22Cummins I, Wortley DJ, Sabbadin F, et al. Key role for a glutathione transferase in multiple-herbicide resistance in grass weeds. Proc Natl Acad Sci U S A. 2013;110(15):5812-5817.
Publication Dates
-
Publication in this collection
Jul-Sep 2017
History
-
Received
16 June 2016 -
Accepted
17 Oct 2016