Abstracts
The first report of a western South Atlantic soft skate, genus Malacoraja Stehmann, 1970, is described as Malacoraja obscura, new species, from the southeastern Brazilian continental slope off the states of Espírito Santo and Rio de Janeiro, in depths ranging from 808-1105 m. The new species is known from five specimens and is distinguished from congeners by its unique dorsal coloration with small, faded white spots on disc and pelvic fins, by retaining in larger specimens an irregular row of thorns along dorsal midline of tail (extending from tail base to two-thirds of tail length in 680 mm total length female), and by presenting a ventral tail midline devoid of small denticles only at base (naked region not extending posterior to pelvic fin rear margin). Further diagnostic characters in combination include the lack of scapular thorns in larger specimens, elevated number of tooth rows (64/62 tooth rows in subadult male of 505 mm TL, and 76/74 in large female of 680 mm TL) and vertebrae (27-28 Vtr, 68-75 Vprd), ventral disc and tail with a uniform dark brown coloration, paired postventral fenestrae on scapulocoracoid, enlarged posterior postventral fenestra, circular foramen magnum and paired internal carotid foramina on braincase floor. Adult males were unavailable for study, but an anatomical description of M. obscura, n. sp., is provided. Comparisons are made with all known material of M. kreffti, literature accounts of M. senta, and with abundant material of South African M. spinacidermis; M. obscura, n. sp., most closely resembles M. spinacidermis from the eastern South Atlantic in squamation, coloration and size. Malacoraja is monophyletic due to its unique squamation and rostral appendices, and apparently comprises two species-groups, one for M. obscura and M. spinacidermis, and the other for M. kreffti and M. senta, but clarification of species-level relationships must await more anatomical information, particularly of the latter two species.
Rajinae; Gurgesiellini; anatomy; taxonomy; systematics; phylogenetic relationships
O primeiro registro para o Atlântico Sul ocidental de uma espécie do gênero Malacoraja Stehmann, 1970 é feita com base na descrição de Malacoraja obscura, espécie nova, proveniente do talude continental do Sudeste brasileiro dos estados do Espírito Santo e Rio de Janeiro em profundidades de 808-1105 m. A espécie nova é conhecida através de cinco exemplares e é distinta de seus congêneres pela sua coloração dorsal composta por numerosas manchas esbranquiçadas e pequenas na região do disco e nadadeiras pélvicas, por apresentar uma fileira irregular de espinhos ao longo da superfície dorsal mediana da cauda a qual persiste em espécimes maiores (desde a base da cauda até dois-terços do seu comprimento numa fêmea de 680 mm de comprimento total, CT) e uma região pequena desprovida de dentículos na base ventral da cauda (estendendo somente até a margem distal da nadadeira pélvica). Outros caracteres diagnósticos em combinação incluem a ausência de espinhos escapulares em indivíduos maiores, número elevado de fileiras dentárias (64/62 fileiras num macho subadulto de 505 mm de CT e 76/74 numa fêmea de 680 mm de CT) e de vértebras (27-28 Vtr, 68-75 Vprd), coloração ventral do disco uniformemente castanha escura, duas fenestras pós-ventrais na cintura escapular, fenestra pós-ventral posterior grande, forame magno circular e dois forames para a carótida interna na placa basal ventral do neurocrânio. Machos adultos não são conhecidos, porém uma descrição anatômica de M. obscura, sp. nov., é fornecida. Comparações são realizadas com todo o material conhecido de M. kreffti, com a literatura sobre M. senta e com material abundante de M. spinacidermis da África do Sul; M. obscura, sp. nov., assemelha-se mais a M. spinacidermis do Atlântico Sul oriental em esqueleto dérmico, coloração e tamanho. Malacoraja é monofilético devido à sua espinulação e apêndices rostrais conspícuos e é aparentemente composta por dois grupos de espécies, um para M. obscura e M. spinacidermis e outro para M. kreffti e M. senta, porém a elucidação das relações filogenéticas entre as espécies necessita de mais informações anatômicas, principalmente das duas últimas espécies.
Description of a new species of skate of the genus Malacoraja Stehmann, 1970: the first species from the southwestern Atlantic Ocean, with notes on generic monophyly and composition (Chondrichthyes: Rajidae)
Marcelo R. de CarvalhoI; Ulisses L. GomesII; Otto B. F. GadigIII
IDepartamento de Biologia (FFCLRP), Universidade de São Paulo, Av. Bandeirantes 3900, 14040-901 Ribeirão Preto, SP, Brazil. e-mail: mrcarvalho@ffclrp.usp.br
IIDepartamento de Zoologia, Instituto de Biologia, Universidade do Estado do Rio de Janeiro, Rua São Francisco Xavier 524, 20559-900 Rio de Janeiro, RJ, Brazil. e-mail: ulisses@uerj.br
IIIUniversidade Estadual Paulista, Campus do Litoral Paulista, Praça Infante Dom Henrique s/n, 11330-900 São Vicente, SP, Brazil. e-mail: gadig@csv.unesp.br
ABSTRACT
The first report of a western South Atlantic soft skate, genus Malacoraja Stehmann, 1970, is described as Malacoraja obscura, new species, from the southeastern Brazilian continental slope off the states of Espírito Santo and Rio de Janeiro, in depths ranging from 808-1105 m. The new species is known from five specimens and is distinguished from congeners by its unique dorsal coloration with small, faded white spots on disc and pelvic fins, by retaining in larger specimens an irregular row of thorns along dorsal midline of tail (extending from tail base to two-thirds of tail length in 680 mm total length female), and by presenting a ventral tail midline devoid of small denticles only at base (naked region not extending posterior to pelvic fin rear margin). Further diagnostic characters in combination include the lack of scapular thorns in larger specimens, elevated number of tooth rows (64/62 tooth rows in subadult male of 505 mm TL, and 76/74 in large female of 680 mm TL) and vertebrae (27-28 Vtr, 68-75 Vprd), ventral disc and tail with a uniform dark brown coloration, paired postventral fenestrae on scapulocoracoid, enlarged posterior postventral fenestra, circular foramen magnum and paired internal carotid foramina on braincase floor. Adult males were unavailable for study, but an anatomical description of M. obscura, n. sp., is provided. Comparisons are made with all known material of M. kreffti, literature accounts of M. senta, and with abundant material of South African M. spinacidermis; M. obscura, n. sp., most closely resembles M. spinacidermis from the eastern South Atlantic in squamation, coloration and size. Malacoraja is monophyletic due to its unique squamation and rostral appendices, and apparently comprises two species-groups, one for M. obscura and M. spinacidermis, and the other for M. kreffti and M. senta, but clarification of species-level relationships must await more anatomical information, particularly of the latter two species.
Key-words: Rajinae, Gurgesiellini, anatomy, taxonomy, systematics, phylogenetic relationships.
RESUMO
O primeiro registro para o Atlântico Sul ocidental de uma espécie do gênero Malacoraja Stehmann, 1970 é feita com base na descrição de Malacoraja obscura, espécie nova, proveniente do talude continental do Sudeste brasileiro dos estados do Espírito Santo e Rio de Janeiro em profundidades de 808-1105 m. A espécie nova é conhecida através de cinco exemplares e é distinta de seus congêneres pela sua coloração dorsal composta por numerosas manchas esbranquiçadas e pequenas na região do disco e nadadeiras pélvicas, por apresentar uma fileira irregular de espinhos ao longo da superfície dorsal mediana da cauda a qual persiste em espécimes maiores (desde a base da cauda até dois-terços do seu comprimento numa fêmea de 680 mm de comprimento total, CT) e uma região pequena desprovida de dentículos na base ventral da cauda (estendendo somente até a margem distal da nadadeira pélvica). Outros caracteres diagnósticos em combinação incluem a ausência de espinhos escapulares em indivíduos maiores, número elevado de fileiras dentárias (64/62 fileiras num macho subadulto de 505 mm de CT e 76/74 numa fêmea de 680 mm de CT) e de vértebras (27-28 Vtr, 68-75 Vprd), coloração ventral do disco uniformemente castanha escura, duas fenestras pós-ventrais na cintura escapular, fenestra pós-ventral posterior grande, forame magno circular e dois forames para a carótida interna na placa basal ventral do neurocrânio. Machos adultos não são conhecidos, porém uma descrição anatômica de M. obscura, sp. nov., é fornecida. Comparações são realizadas com todo o material conhecido de M. kreffti, com a literatura sobre M. senta e com material abundante de M. spinacidermis da África do Sul; M. obscura, sp. nov., assemelha-se mais a M. spinacidermis do Atlântico Sul oriental em esqueleto dérmico, coloração e tamanho. Malacoraja é monofilético devido à sua espinulação e apêndices rostrais conspícuos e é aparentemente composta por dois grupos de espécies, um para M. obscura e M. spinacidermis e outro para M. kreffti e M. senta, porém a elucidação das relações filogenéticas entre as espécies necessita de mais informações anatômicas, principalmente das duas últimas espécies.
Introduction
Skates of the genus Malacoraja Stehmann, 1970 occur mostly in deep waters of the continental slope and are restricted to the Atlantic Ocean. Malacoraja was originally described by Stehmann (1970) as a subgenus of Raja Linnaeus, 1758 to accommodate Raja mollis Bigelow & Schroeder, 1950, a poorly-known, primarily western North Atlantic species of deep-water skate. The genus was diagnosed, as emended by Stehmann (1977), largely on the basis of its unusual squamation composed of small, closely-set dermal denticles which are evenly scattered on dorsal disc and dorsal and ventral tail regions, in conjunction with a general lack of thorns posterior to the scapular area. Malacoraja, colloquially known as "soft skates" (Compagno, 1999), remains taxonomically undiverse including only M. senta (Garman, 1885), M. spinacidermis (Barnard, 1923; with Raja mollis as a putative synonym; Hulley, 1970; cf. Stehmann, 1977, 1993, 1995), and M. kreffti Stehmann, 1977 (McEachran & Dunn, 1998; Compagno, 1999).
Soft skates are relatively uncommon batoids, mostly known from disjunct records. Malacoraja senta has the shallowest distribution of all Malacoraja species, being more abundant at depths between 110 and 457 m (McEachran & Musick, 1975), whereas its congeners are more plentiful below 900 m; M. senta is also the only moderately common species. Species of Malacoraja occur in the western (M. senta and M. spinacidermis) and eastern (M. spinacidermis and M. kreffti) North Atlantic (Bigelow & Schroeder, 1950, 1953, 1954a; Templeman, 1965; Krefft & Lübben, 1966; Stehmann, 1970, 1977, 1993, 1995; Stehmann & Bürkel, 1984; McAllister, 1990; McEachran, 2002; McEachran & Carvalho, 2002; Packer et al., 2003; Schwartz, 2003), with an isolated record of M. spinacidermis from off Western Sahara (Stehmann, 1995), and in the eastern South Atlantic off Namibia and South Africa (M. spinacidermis; Hulley, 1970, 1972a, 1986; Hulley & Stehmann, 1977; Bianchi et al., 1999).
The first western South Atlantic specimens of Malacoraja were collected from the southeastern Brazilian continental slope in 1999 during cruises sponsored by the Brazilian Federal Government to survey potential fisheries resources in its Exclusive Economic Zone (as part of the REVIZEE Program, Central Coast region; Figueiredo et al., 2002). This material was originally documented (as Malacoraja sp.) in an unpublished doctoral thesis revising the Brazilian skate fauna (Gomes, 2002). However, it was only after comparisons with other Malacoraja species in the collections of Hamburg and Cape Town that it became apparent that the Brazilian material, even though very similar in overall morphology to M. spinacidermis, represents a new species of Malacoraja, which we describe below. Species-limits are subtle in Malacoraja, a difficulty compounded by the lack of adult males of the Brazilian form (and of western North Atlantic M. spinacidermis, referred to hereafter as "M. mollis"). Our new species, however, is distinguished on the basis of characters other than those from the clasper.
Material and Methods
Measurements and counts are according to Bigelow & Schroeder (1953) as modified by Hubbs & Ishiyama (1968), with the following distinctions: internarial space is distance between exposed inner margins of nostrils; nasal curtain width is measured at posterior nasal curtain just anterior to mouth opening; base length of dorsal fins excludes their interconnecting membrane (only fleshy part of dorsals covered by denticles was measured); pelvic fin posterior lobe width is measured at its greatest width; and pelvic length was measured from origin of anterior lobe to posterior lobe apex. Institutional abbreviations follow Leviton et al. (1985). X-ray radiographs were taken on Kodak mammography film (Min-R2000). Terminology for skeletal structures follows mostly McEachran & Compagno (1979, 1982), for external clasper components Hulley & Stehmann (1977), for muscles Miyake et al. (1992), and for lateral line canals mostly Chu & Wen (1979; also Maruska, 2001), with the additional subdivision of the scapular canal into anterior and posterior segments. Abbreviations for skeletal structures are explained in the legends, and may differ from those used by the above authors. Skeletal structures were observed from radiographs of all specimens and by gross dissection of the smallest male (primarily to observe sensory canals, ventral gill arches and their associated muscles, scapulocoracoid, and neurocranium). Abbreviations used throughout text include DW for disc width, DL for disc length, TL for total length, Vtr for pretransitional vertebrae (from first complete centrum in synarcual to mono-diplospondyly transition), and Vprd for predorsal caudal vertebrae. Comparative material examined of other Malacoraja species is listed in the AppendixAppendix.
Results
Family Rajidae Blainville, 1816Genus Malacoraja Stehmann, 1970Malacoraja obscura, new species
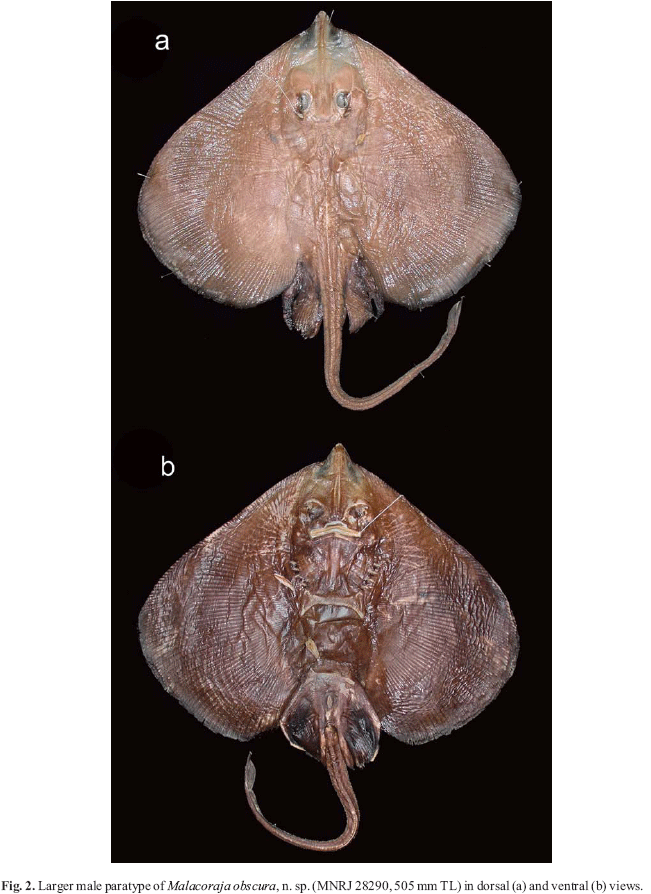
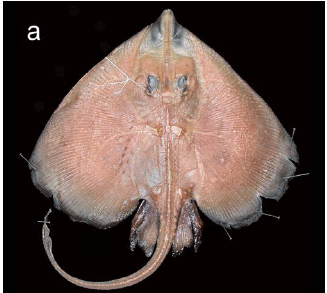
Holotype. MNRJ 28289, adult female, 680 mm TL, 19º39'57"S, 038º38'26"W, state of Espírito Santo (Brazil), 808 m, sta. D-0503, N/O Thalassa, 29 Jun 1999 (Fig. 1). Paratypes. (4 specimens). MNRJ 28290, juvenile male, 505 mm TL, 19º39'57"S, 038º38'26"W, state of Espírito Santo (Brazil), 808 m, sta. D-0503, N/O Thalassa, 29 Jun 1999 (Fig. 2); MNRJ 28291, juvenile male, 355 mm TL, 19º39'57"S, 038º38'26"W state of Espírito Santo (Brazil), 808 m, sta. D-0503, N/O Thalassa, 29 Jun 1999 (Fig. 3a); MNRJ 28292, juvenile male, 295 mm TL, 19º39'57"S, 038º38'26"W, state of Espírito Santo (Brazil), 808 m, sta. D-0503, N/O Thalassa, 29 Jun 1999 (Fig. 3b; dissected for sensory canals, neurocranium, scapulocoracoid and ventral gill arch muscles); MNRJ 28293, juvenile female, 251 mm TL, 21º46'34"S, 039º53'22"W, state of Rio de Janeiro (Brazil), 1105 m, sta. E-547, N/O Thalassa, 6 Jul 2000.
Diagnosis. A species of Malacoraja distinguished from all congeners by presenting small, scattered, and faded whitish spots on dorsal disc and pelvic fins, an irregular row of small thorns along dorsal midline of tail which persists in larger specimens (extending from tail base to two-thirds of tail length), and ventral tail midline devoid of small denticles only at base (naked region not extending beyond pelvic fins). The following characters in combination further distinguish M. obscura, n. sp.: ventral disc and tail with a uniform dark brown coloration, absence of scapular thorns in larger specimens, relatively higher number of tooth rows (64/62 tooth rows in subadult male of 505 mm TL, and 76/74 in large female of 680-mm TL), relatively higher number of vertebrae (27-28 Vtr, 68-75 Vpd), paired postventral fenestrae on scapulocoracoid, enlarged posterior postventral fenestra, circular foramen magnum, and paired internal carotid foramina on neurocranial basal plate. The significance of these features among species of Malacoraja is discussed below.
Description. Proportional morphometrics are presented in Table 1, and counts in Table 2 (where they and compared to other species of Malacoraja). The description below is based on all specimens, but salient features of the holotype are separately mentioned.
External morphology. Disc cordiform, much wider than long (DW 60.3-67.5 % of TL, DL 47.5-53.1 % of TL), with greatest width just posterior to its horizontal midline. Snout extremity not greatly elongated but clearly projecting beyond anterior disc margin. Anterior margin of disc relatively straight from disc apices to snout tip, not convex from level of orbits to snout tip. Disc apices broadly rounded, with widely convex and broad posterior margins. Posterior lobe of disc extending caudally to about one-half of length of posterior lobe of pelvic fin. Smaller specimens with more acute snout angle (100-101º, vs. 105-107º in larger specimens; Figs. 1-3), appearing more triangular in outline. Disc relatively thin and dorsoventrally compressed. First three gill openings slitlike, horizontal wider than both posterior openings (especially fifth); posterior openings semicircular. Gill openings situated just medial to propterygia; fifth gill openings just anterior to coracoid, situated at anterior coracoid corners; distance between fifth openings slightly greater than half of distance between first openings.
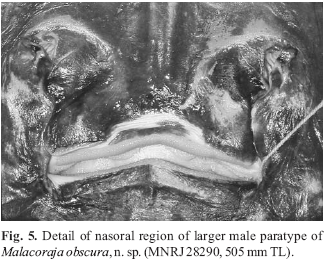
Eyes and spiracles closely set; spiracles positioned near outside margins of eyes (interspiracular distance greater than interorbital distance); spiracles projecting obliquely from midline (Fig. 4). Orbital length greater than spiracle length. Spiracle oval, elongated, extending anteriorly to posterior one-fourth of eye. Nine pseudobranchial folds on anterior spiracular wall in most specimens (holotype with 10); folds knoblike and short. Prenasal distance much shorter than preorbital distance. Preorbital distance just less than one-third of disc length. Nasal curtain covering large nasal apertures extensive, but with only a small, semicircular nostril; nasal flaps small and tube-like, more prominent on lateral (incurrent) nostril margin; nasal flaps around nostril not distally fringed (Fig. 5). In smaller specimens, posterior margin of nasal curtain slightly concave medially, but strongly concave in holotype, exposing upper jaw teeth. Lateral borders of nasal curtain sigmoidal. Posterior lobes of nasal curtain weakly fringed. Nasal curtain length at midline about one-half of greatest width (at posterior corners). Distance between nostrils about equal to distance between mouth corners. Mouth situated at about midorbital level. Mouth opening more or less straight across (more so in females), but upper jaw with slight median indentation which accomodates median projection of lower jaw.
Teeth set in quincunx, with circular to trapezoidal, flattened crowns and weakly pointed cusps; cusps not longer than tooth base length or width (no adult males examined). Crowns with well developed aprons anteriorly, but with weak uvula posteriorly, when present. Roots very large, almost as wide as cusps; tooth base bilobed, with lobes separated by a shallow basal median groove (in some teeth the lobes appear contiguous); basal root ornamentation weakly apparent (tooth morphology indistinct from M. senta; see Herman et al., 1994: plate 22). Teeth covering jaw integument for almost full width of mouth opening; teeth anteroposteriorly arranged in a very slender tooth band (widest at symphysis), arranged in just a few series (in some 10 horizontal rows at symphysis in largest male and in holotype). Tooth rows as follows: 76/74 rows in 680 mm TL holotype (symphysis at row 38/37; some portions of tooth bands missing in holotype, but not affecting counts), 64/62 rows in 505 mm TL male (symphysis 33/31), 57/55 in 355 mm TL male, 54/51 in 295 mm TL male, and 48/44 in 251 mm TL female (Table 2).
Pelvic fins much wider than long, with two very distinct lobes (Fig. 6). No sexual dimorphism in pelvic fin shape (mature males unknown). Greatest pelvic fin width (with expanded anterior lobes) slightly smaller than one-half of disc width. Anterior lobe limblike, slender and greatly elongated; posterior lobe with convex posterior margins and about as wide as nasal curtain width. Origin of anterior lobe slightly anterior and ventral to pectoral fin insertion; anterior lobe with first articulation just posterior to its midlength and second articulation at about onefifth of its length. Posterior lobes extend caudally beyond anterior lobes, inserting on both sides of ventral tail base posterior to cloaca; axils separated by a small space. Outer pelvic fin margins weblike, with greater inflection separating anterior and posterior lobes in holotype and larger juveniles; smallest female with less indented outer pelvic margin.
Claspers in largest male extending posteriorly to pelvic fin posterior margin (adult males unknown; Fig. 6). Claspers dorsoventrally compressed, expanding slightly at clasper glans region, at about clasper midlength (more evident in largest male). Claspers about one-half of length of inner margin of posterior lobe of pelvic fins in males 355 and 295 mm TL, and equal to inner margin of posterior lobe in male 505 mm TL. Clasper groove more or less straight in largest male, curving slightly with expansion of glans distally. Clasper with a very small, distal fleshy projection, pointing slightly outward (as in adults of other species of Malacoraja). Internal clasper components not developed even in largest male, but shield, with slightly folded epithelium, extends from more or less level of hypopyle to distal section of glans. Elongate terminal ridge, possibly representing the incipient dike, about one-third length of glans. Inside of glans pigmented on both internal and external surfaces. Denticles and pseudosiphon absent from clasper.
Tail slender and elongate, clearly demarked from disc, and tapering from pelvic base to extremity. Cloaca to tail length greater than snout to cloaca length, but tail length not as great as disc width. Tail wider than high in cross-section throughout its entire length. Lateral tail folds moderately developed, not flaplike, extending from slightly posterior to tail midlength to distal tip (in holotype and larger male, tailfolds originate at more or less tail midlength). Tail folds resemble ridges anteriorly, and widest close to dorsal fins. Tail more dorsoventrally compressed at extremity compared to origin. Dorsal fins situated on posterior-most aspect of tail, not fleshy and without robust bases. Dorsal fins confluent at bases, low and long, similarly shaped, and broadly sloping posteriorly. Dorsal fin base length variable, more or less equal in length in some specimens. First dorsal fin without conspicuous free posterior lobe. Second dorsal fin slightly greater than first in some specimens, with enlarged posterior free lobe inflecting anteriorly to insert on low caudal fold. Caudal fold very low, with a straight dorsal contour, and higher dorsally than ventrally. Lateral tail folds meet ventrally just posterior to second dorsal fin base to form ventral caudal fold. Distal segment of caudal fold dorsoventrally continuous, wrapping around caudal extremity. Length of caudal fold just greater than length of second dorsal fin base.
Squamation. Almost entire dorsal surface of disc covered by numerous, closely set, more or less uniformly distributed and evenly spaced, minute dermal denticles (denticles slightly more spaced out in larger specimens) (Figs. 4, 6, 7). Minute denticles present on integument over eyes, but absent from inside spiracles (even from integument on anterointernal ledge formed by spiracular cartilages). Denticles on middisc region (close to shoulder girdle) slightly larger and more spaced apart (especially over suprascapulae); minute denticles also present anterior to nasal capsules, but anterior-most tip of projecting snout without denticles in some specimens. Pectoral axils mostly without small denticles. Pelvic fins with anterior lobes completely devoid of denticles; denticles on pelvic fins present only on caudal portion of posterior lobe, but not as numerous as over disc (i. e., only dorsally exposed portions of pelvics with small denticles). Dorsal and lateral tail with numerous denticles (Fig. 7); denticles on lateral tail slightly larger. Dorsal fins covered with small denticles, except membrane between both fins and dorsal posterior bases. Lateral tail fold and caudal fin generally devoid of denticles as well (a few present in holotype).
Few thorns present antero- and posteromedially to eyes, in midline over dorsal disc and tail, and on shoulder girdle in smaller specimens. Most thorns morphologically distinct from smaller denticles, with oval, more pedunculate bases, and thicker crowns and bases (Fig. 7a, b, d, e). Enlarged thorns proximal to eyes and scapular region more evident in smaller specimens; some thorns possibly missing from specimens due to abrasion, especially on disc midline (see Table 2 for thorn counts). In holotype, nuchal thorns on disc midline originate at middistance between spiracles and shoulder girdle. Nuchal thorns few and evenly spaced apart (six in holotype, some missing), extending posteriorly to anterior suprascapular margin. Three midscapular thorns present in holotype. Middisc thorns present just posterior to shoulder girdle, but absent at more or less middisc, reappearing caudally anterior to pelvic girdle. In larger male, caudal midrow of thorns originates posterior to pelvic girdle. Midrow of tail thorns originates with row of isolated larger thorns, interspersed with smaller thorns, and continues caudally with irregular row of larger and smaller thorns (Fig. 7a, b). Larger, more numerous thorns in midrow posterior to pelvic axils, frequently with two or three adjacent thorns (Fig. 7d, e). Midrow thorns reduce in size and merge with smaller denticles as of approximately posterior two-thirds of tail. Smaller specimens with less conspicuous midrow over tail and posterior disc region. Larger male lacking alar and malar thorns, but with larger denticles anterolaterally on disc.
Ventral disc region and pelvic fins mostly smooth. A small patch of distinct denticles on anterior segment of ventral rostrum and in a few rows on anterior disc margins lateral to rostral appendix (more pronounced in holotype, extending posterolaterally to about one-half of rostrum length). Ventral snout denticles more spaced apart and larger than minute denticles on dorsal side. Smallest male without ventral snout denticles, but other males with same pattern as holotype. Ventral snout denticles with star-shaped and slender bases, usually with four basal projections (anterior, posterior and two lateral; the former two usually much longer than the lateral segments); denticle crowns acute and curved, pointing rearward (Fig. 7c).
Minute denticles present on ventral aspect of tail, extending from pelvic fin posteriorly to level of first dorsal fin origin; ventral caudal distal extremity mostly naked. Denticles missing from ventral midtail base region; denticles on ventral tail mid base present as of one-half of length of posterior lobe of pelvic fin in most specimens (in holotype and larger male paratype, central band without denticles on ventral tail extends posteriorly to about posterior margin of pelvics; Fig. 6c). Denticles on lateral aspect of tail larger and more closely grouped together than those on ventral tail region. Denticles on ventral snout region (Fig. 7c) and tail morphologically similar, but former with longer bases and taller crowns.
Sensory canal system. Tubules of ampullary sensory system greater in diameter than canals of lateral line system, and situated slightly ventral to lateral line system on dorsal surface. Ampullary tubules very sinuous, and wider than terminal pores. Lateral line canals relatively straight, more defined, and about as wide as pores.
Dorsal sensory canals of the lateral line system (Fig. 8) with many segments apparent through integument (e. g. Fig. 3b). From the postorbital canal posterior to eyes, the supraorbital canal runs anteriorly parallel and closely adjacent to rostrum on both sides, passing to ventral surface of disc on anterior snout (distal section of canal mostly concealed). At about anterior half of nasal capsules, five to seven branches radiate from supraorbital canal into space anterior to nasal capsules. From junction with supraorbital canal posterior to eyes, the infraorbital canal runs anteriorly, between spiracles and eyes, in a more or less straight line over propterygial radials, passing to ventral side of disc lateral to supraorbital canals. Branches of the infraorbital extend laterally at canal midlength, at mid nasal capsule level; infraorbital pores present even on anterior disc margin (more numerous in holotype). Postorbital canals short, smaller than eye length, extending posteriorly from junction of infra- and postorbital canals to supratemporal canal slightly posterior to spiracles, and ending at level of endo- and perilymphatic duct openings. Supratemporal canal about as wide as interorbital space. Cranial loop (formed by supraorbital, postorbital and supratemporal canals) relatively straight and slender (not indicated in Fig. 8). Hyomandibular and scapular canals relatively wide. Hyomandibular canal extends laterally from supraorbital canal and continues posteriorly to slightly beyond level of shoulder girdle, where it inflects medially to connect with anterior scapular canal, forming dorsal pleural loop. Hyomandibular canal weblike, with branches extending to outer disc margins, spaced apart by every two to three radials elements (some 15 branches present altogether). Anterior scapular canal relatively straight across at level of shoulder girdle, but curving to extend posterolaterally beyond central disc region (pores present distally at outer posterior disc margins). Branches of anterior scapular canal projecting into dorsal pleural loop (some seven branches present in larger male and holotype). Posterior scapular canal straight relative to anterior scapular canal, extending posteriorly and obliquely from lateral line canal to reach posterior disc region (pores extending to outer disc margin). Few, short, and laterally projecting branches present on posterior scapular canal. Posterior lateral line canals extend caudally in a straight line from scapulae to at least base of tail at mid pelvic fin length (canals over tail region difficult to discern due to squamation and dark integument color). Pores of posterior lateral line canals present on tail, well-spaced apart, and extending to region of caudal fold. Posterior lateral line canals with short secondary branches projecting laterally on disc and base of tail region; branches more densely compacted anteriorly.
Ventral canals of lateral line system not observed even after dissection. Ampullary system more apparent through integument (tubules with lighter pigmentation than rest of disc). Numerous hyoidean ampullary tubules radiating laterally in sinuous fashion from propterygium to outer disc; pores and canals more numerous at anterior propterygium. Posterior supraorbital tubules extending anteriorly from nasal capsules to region lateral to rostrum. Inner tubules grouped together anterior to coracoid, not numerous.
Coloration. Preserved specimens with dorsal disc coloration predominantly gray, rusty-gray or brownish-gray (Figs. 1-3); smaller female with a more uniform purplish-brown color. Snout anterior to nasal capsules and in between propterygial raidals yellowsih-brown, lighter than disc, except for area of rostral cartilage. Eyes through integument slightly darker than disc. Integument of anterior spiracular surface pigmented. Dorsal surface of pelvic fins darker brown, clearly darker than disc, especially anterior segment. Pectoral axils, dorsal midline and outer disc regions slightly darker than pectoral fin center in holotype. Tail with coloration similar to disc, but denticles and thorns over tail midline creamy; tail extremity, including dorsal fins slightly darker than most of disc. Small, circular, and somewhat undefined white or creamy spots more or less evenly scattered on disc, more numerous in larger specimens (especially holotype); spots much smaller than spiracles, appearing as faint specks but varying in size (up to a few mm wide in holotype over pelvic fins). Spots clearly faded in preservative in all specimens. Smaller female mostly devoid of small spots. Lateral line canals apparent through skin over most of disc.
Ventral coloration dark brown over disc and tail, without large whitish blotches on disc (Figs. 1-2). Ventral coloration fading slightly in some specimens over mid and lateral disc (notably on coracoid and pelvic girdle) due to preservation. Cloaca and gill slits discreetly lined in creamy white. Ventral ampullary tubules anterior to coracoid highlighted in lighter pigmentation. Anterior snout region in between propterygial radials much lighter than disc. Tooth integument yellowish-white. Posterior nasal curtain border and integument surrounding mouth lighter in color (Fig. 5). Larger denticles on anterior snout region and minute denticles on tail yellowish. Horizontaly elongated but irregular whitish blotches present at ventral tail base, extending to pelvic axils in most specimens (Fig. 6c; more defined in holotype and small female). Extremities of anterior pelvic lobe whitish.
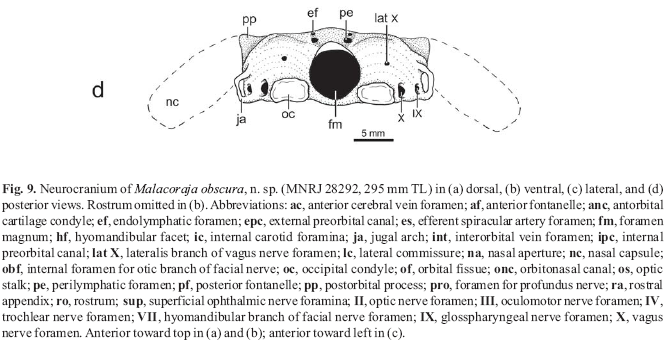
Neurocranium. Neurocranium prismatically calcified throughout. Rostrum slender but firm, tapering anteriorly, and extending anteriorly to disc extremity (Fig. 4). Rostral appendix much longer than wide, extending posteriorly to mid-rostral length (hialine sheet of cartilage appears to extend farther posteriorly to rostral base); rostral node notched on both sides (Fig. 9). Ethmoid region (rostrum and nasal capsules) comprising more than two-thirds of neurocranial length. Neurocranium widest at nasal capsules (about twice width between outer margins of jugal arches). Nasal capsules bulging, very developed, broadly rounded anteriorly and posteriorly, with large and oval nasal apertures. Nasal capsules articulating with sickle-shaped antorbital cartilages; antorbital anterior margin more or less straight, posterior margin strongly concave; antorbital condyle oval, taller than wide. Anterior (precerebral) fontanelle very slender but well defined (even anteriorly), with oval posterior margin, and extending anteriorly to just beyond level of nasal capsules. Epiphysial bar slender. Posterior (frontoparietal) fontanelle shorter than anterior fontanelle, medially constricted in small male (but less so in larger specimens), with oval anterior margin but indented posterior border. Foramen for profundus nerve very small, situated at anterolateral aspect of nasal capsule. External preorbital canal oval and relatively large. Dorsal anterior orbital wall with large internal preorbital canal; orbitonasal canal slightly smaller and ventral to internal preorbital canal. Foramina for the anterior cerebral vein located just posterior and slightly ventral to internal preorbital canal. Optic nerve foramen (II) ventrally placed and anterior to midorbit; trochlear foramen (IV) dorsal and slightly posterior to optic nerve foramen. Oculomotor foramen (III) just anterior to elliptical optic stalk; interorbital vein foramen posterior to optic stalk; foramen for efferent spiracular artery inconspicuous, located beneath optic stalk. Orbital fissure large and oval; hyomandibular branch of facial nerve foramen (VII) large and separated from orbital fissure by stout lateral commissure. Internal foramen for otic branch of facial nerve dorsally situated at posterior orbit. Numerous superficial ophthalmic nerve foramina piercing supraorbital crest. Distinct preorbital process lacking; postorbital process notched for passage of infraorbital lateral line canal. Hyomandibular facet elongate and obliquely positioned on lateral otic region. Endolymphatic foramina smaller than perilymphatic foramina, situated in truncated parietal fossa. Internal carotid foramina very small, paired and situtaed in small depression ventrally. Jugal arches small. Foramen magnum circular. Vagus nerve foramina (X) adjacent to occipital condyles; glosspharyngeal nerve foramina (IX) situated more laterally, underneath jugal arches; lateralis branches of vagus nerves situated above occipital condyles; posterior cerebral vein foramen not observed.
Hyobranchial and axial elements. Jaws very slender and straight, tapering toward midline, and about as wide as nasal capsules (Fig. 10). Hyomandibulae tapering anteriorly. Pseudohyoid bar not observed. Basihyal bar (and first hypobranchial?) wide and slender, arched, and with bifid anterolateral projections (not shown in Fig. 11). Hypobranchials 2 slender, fused to anterior portion of basibranchial copula, and extending anteriorly to close to basihyal bar; hypobranchials 2 curved anteriorly away from midline from basibranchial copula (Fig. 11). Basibranchial copula triangular, tapering posteriorly. Hypobranchials 3 slender and relatively straight, adjacent to basibranchial anterolateral margin, and apparently separate from hypobranchial 4; hypobranchial 5 small and posterior to hypobranchial 4. Ceratobranchials 1-4 overlapping medially; ceratobranchial 5 aritculating directly with basibranchial copula. Other gill arch elements not observed during dissection. Synarcual elongate, about as long as neurocranium without rostrum; longest segment anterior to scapulocoracoid. Synarcual triangular in dorsoventral profile anteriorly, widest at its anterior third (about as wide as otic capsules), and tapering posteriorly. Lateral stays subtriangular. Suprascapulae wing-shaped, wider than synarcual, and with broadly rounded outer margins articulating with scapular processes of shoulder girdle (Fig. 10). Vertebral centra individualized as of posterior third of synarcual; individual centra present posterior to insertion of second dorsal fin; transitional area between mono- and diplospondylous centra caudal to pelvic girdle, at level with posterior basipterygium. Vertebral numbers in Table 2.
Scapulocoracoid. Shoulder girdle with slightly concave anterior and posterior margins. Lateral aspect of scapulocoracoid subrectangular, taller anteriorly at suprascapulae and slightly tapering posteriorly (Fig. 12). Suprascapulae rather wide, but not very tall; rear corner not well defined, anterior ridge present. Four fenestrae present laterally; predorsal fenestra circular to ovoid, much greater than other fenestrae; anterior postventral fenestra (not preventral fenetra) smallest; posterior postventral fenestra slightly larger than postdorsal fenestra; smaller foramina absent. Mesocondyle elongated, greater than circular procondyle and oval metacondyle; condyles equidistant. Median ridge (neopterygial ridge of McEachran & Compagno, 1979) absent. In dorsoventral view, scapulocoracoid widest posteriorly at metacondyles. Propterygium curved, with seven segments; external margins sinuous to articulate with radials; basal-most segment widest, cleaver-shaped, and about as long as second segment; anterior segments very slender. Mesopterygium subtriabgular, with sinuous external margins. Metapterygium curved, divided into two segments; anterior segment with sinuous external margin and very elongate, posterior segment about one-fifth length of anterior segment. Pectoral radials subdivided at sixth or seventh segment away from basals, with a total of some 18-20 segments at middisc; radial segment closest to basals more elongate than remaining segments. Asingle, basally expanded radial element articulating with both pro- and mesopterygium (counted with propterygial radials in Table 2).
Pelvic girdle. Pelvic girdle wide, with slightly concave anterior and posterior margins; posterior margin more strongly concave in males (Fig. 13). Lateral prepelvic processes triangular. Iliac processes medially recurved and elongate, extending to almost anterior puboischiadic margin. Ischial processes triangular, not greatly projecting, articulating with basipterygia. Lateral condyle for articulation with enlarged anterior radial slender. Three pairs of obturator foramina present in anteroposterior series; anterior foramen situated more laterally and anterior to puboischiadic condyle, posterior two formaina caudal to puboischiadic condyle (median foramen obscured by iliac processes). Pelvic anterior lobe with three segments; the first stout, elongate (about twice length of second segment), and with very concave articular surface for puboischiadic condyle. Basipterygia relatively straight, with sinuous external margin, and posteriorly subdi vided. Proximal pelvic radials stout, tapering, and undivided, anterior radials tightly packed together, especially first two, and with first radial articulating directly with pelvic girdle and not basipterygium.
Ventral gill arch muscles. Similar to Leucoraja garmani as described by Miyake (1988) and Miyake et al. (1992), except that coracomandibularis inserts on strong fascia anterior to coracoid bar posteriorly and not directly on coracoarcualis, and the depressor rostri, depressor mandibulae and depressor hyomandibulae are more slender. Depressor mandibulae inserts at junction of Meckel´s cartilage and medial portion of the adductor mandibulae complex; depressor hyomandibulae inserts on proximal segment of hyomandibula through a short tendon. Coracohyoideus muscles insert on basihyal close together; coracobranchiales visible in ventral view slightly lateral to paired coracoarcuales. A small muscle bundle (adductor mandibulae medialis?) runs parallel to lower jaw, inserting on its posterolateral aspect.
Heart valves. Heart valves of the posterior section of the conus arteriosus arranged in two vertical rows, each containing from four to six individual valves (observed without staining; cf. Ishiyama, 1958).
Distribution. Known only from two localities on the continental slope of southeastern Brazil, off the states of Espírito Santo (19º39' S, 038º 38' W) and Rio de Janeiro (21º46' S, 039º53' W), in waters ranging from 808-1105 m in depth (Fig. 14).
Etymology. The specific epithet is derived from the Latin obscurus, meaning dark or indistinct a reference to the subtle nature of the differences separating our new species from its congeners (and among species of Malacoraja in general). Gender feminine.
Proposed common name. Brazilian soft skate.
Comparisons
South American Rajidae.Malacoraja obscura is uniqueamong the known Atlantic skate fauna of South America in lacking thorns posterior to scapular area and in presenting intense squamation on ventral tail region (a newly collected Rajella species, presently under study, also has ventral tail denticles, but these are not identical to M. obscura). The most similar western South Atlantic species is Rajella sadowskii (Krefft & Stehmann, 1974), known from the continental slope of southeastern and southern Brazil in 800-1320 m (Menni & Stehmann, 2000). Rajella sadowskii also has a dense covering of minute denticles (but not on ventral tail), and is similar in disc shape (albeit not as cordiform) and morphology of the oronasal region (but not in its proportions oronasal region much greater in R. sadowskii). Rajella sadowskii is lightly colored ventrally (dark brown in M. obscura), has fewer tooth rows (37-43/34-41 vs. 57-76/55-74 in specimens of M. obscura over 350 mm TL), and presents greatly enlarged thorns along middisc and dorsal tail region (Krefft & Stehmann, 1974), which are absent in M. obscura.
Congeneric species. Species of Malacoraja diverge only slightly in external and internal morphology. Only M. senta is relatively common, but presently there is little information concerning its anatomy (neurocranium and shoulder girdle described by McEachran & Compagno, 1982). Clasper structure is informative to separate Malacoraja from other genera in the tribe Gurgesiellini, and even to identify species of Malacoraja (Stehmann, 1977, 1993), but clasper components of adults are as yet unknown in M. obscura and "M. mollis", and have not been described in detail for M. senta. Characters that in combination distinguish species of Malacoraja include squamation, coloration, and some meristic features. Morphometric proportions are also very similar among species, resulting in few useful distinctions (compare Table 1 to proportions given for M. kreffti and M. spinacidermis in Stehmann, 1977: 82). Stehmann (1977, 1993, 1995) should be consulted for morphological distinctions between M. spinacidermis and "M. mollis".
Dorsal coloration distinguishes M. obscura from all other species of Malacoraja. Malacoraja obscura presents small lighter spots dorsally on disc and pelvic fins, which, even though somewhat faded (probably as a result of preservation), are clearly visible. Other aspects of coloration further separate M. obscura from both M. senta and M. kreffti, as the former species is mostly uniform dark purplish-brown ventrally on disc and tail, even in small juveniles, whereas both M. senta and M. kreffti have whitish ventral disc and tail regions (M. senta occasionally with darker blotches ventrally on disc and darker tail region; McEachran, 2002). Malacoraja kreffti is unique among Malacoraja species in being uniformly pale white or gray both dorsally and ventrally, without any distinctive markings (Stehmann, 1977, 1993; pers. obs.). Malacoraja senta is unique in having a light brown dorsal surface with darker blotches, and juveniles with two lighter crossbands dorsally on tail which are lost with growth (Bigelow & Schroeder, 1953; McEachran, 2002; Schwartz, 2003). In addition, both M. senta and M. kreffti lack whitish streaks over a darker background at tail base, present in M. obscura.
Malacorajaspinacidermis from South Africa is more similar to M. obscura in coloration, presenting irregular whitish blotches ventrally on cloacal and pelvic region (Stehmann & Bürkel, 1984; Hulley, 1986), but these are distinct from the more circumspect white lateral stripe present on ventral tail in some specimens of M. obscura. Ventral coloration of M. spinacidermis is variable, as some specimens also have numerous, usually large, whitish blotches around mouth and nostrils, on ventral gill arch region, and at pectoral axils (more pronounced in SAM 34520), all of which are lacking in M. obscura, which is more uniform dark brown ventrally (but see "Coloration" above for description of small ventral blotches in M. obscura; it must be noted that M. obscura is known from only five individuals and may present variation similar to M. spinacidermis in this regard). Juveniles of M. spinacidermis, however, are much lighter ventrally in comparison to M. obscura (see also Hulley, 1986). Malacoraja spinacidermis from the western North Atlantic ("M. mollis") is reported to be either yellowish or whitish ventrally, sometimes with darker grayish blotches on the posterior portion of the disc, and therefore is also much lighter ventrally than M. obscura (Bigelow & Schroeder, 1953; Templeman, 1965; Krefft & Lübben, 1966; Stehmann, 1977).
As in all other species of Malacoraja, M. obscura has an overall velvety aspect, being covered with small, closelypacked dermal denticles (a synapomorphy of Malacoraja; see below). The larger thorns present on the dorsal tail midline of M. obscura, however, are completely absent from M. kreffti or markedly reduced in adults of other Malacoraja species (however, juveniles usually have a single row on tail midline; Bigelow & Schroeder, 1953; Hulley, 1970; Stehmann, 1977, 1993; Stehmann & Bürkel, 1984; McEachran, 2002). The holotype of M. obscura (680 mm TL) is larger than all specimens of M. spinacidermis examined, and clearly has an irregular row of larger thorns extending posteriorly to two-thirds of tail length. These thorns vary in distribution among specimens of M. obscura, ranging from a single well-defined row in the smallest female (a common condition among small juveniles of Malacoraja) to an irregular row usually with two thorns (and occasionally with three) positioned side-by-side in larger specimens, and sometimes with smaller thorns in between (these larger thorns occur over a band of integument devoid of the smaller, velvety denticles). Smaller specimens are therefore more similar among species of Malacoraja in relation to thorns on the dorsal tail midline, but these are reduced or absent in larger individuals, except in M. obscura. There are exceptions, as a juvenile female of M. spinacidermis from the eastern South Atlantic off Western Sahara (384 mm TL, approx. 188 mm DL and 250 mm DW) lacks even a clearly defined midrow on tail (Stehmann, 1995), but one is present in smaller specimens from off South Africa (e. g. SAM "A6956 059 E24", 214 mm TL, 106 mm DL, 141 mm DW). An adult male from off South Africa, however, has an irregular row of thorns on dorsal tail midline (SAM 32506, 602 mm TL, 345 mm DL, 432 mm DW), similar to the larger male specimen of M. obscura, but this is considered exceptional as SAM 26879 (another adult male reported as 634 mm TL by Hulley & Stehmann, 1977) lacks thorns alltogether on tail midline, as does SAM 33162 (581 mm TL, 323 mm DL, 408 mm DW) and other large females examined. Adults of M. senta have an inconspicuous, irregular, single row of thorns progressively decreasing in size posteriorly as of dorsal tail base, and thorns are missing posterior to tail midlength (Bigelow & Schroeder, 1953; McEachran, 2002). Malacoraja kreffti lacks thorns on disc (and tail) midline posterior to shoulder girdle according to material examined (also Stehmann, 1977, 1993; Stehmann & Bürkel, 1984).
Adult males of M. senta and M. kreffti present significant malar and alar thorns, moderately developed suprascapular, spiracular, nuchal, and orbital thorns (less so in M. kreffti), as well as a clearly demarcated middisc row of thorns extending to anterior third of tail (especially in M. senta; Bigelow & Schroeder, 1953; Stehmann, 1977, 1993; McEachran, 2002). Malacoraja senta additionally has thorns on inner margin of orbit. Adult males are unknown in M. obscura, but our largest male (505 mm TL) is comparable in size to the adult male M. senta described by Bigelow & Schroeder (1953, 520 mm TL) and to the adult males of M. kreffti described by Stehmann (1977, 1993; reported as 515 and 545 mm TL, but measured by the senior author in 2000 as 505 and 539 mm TL, respectively). Our largest male specimen of M. obscura differs from these species by lacking alar and malar thorns completely (but slightly larger denticles are present anterolaterally on disc), as well as lacking thorns on inner orbital margin. Some specimens of M. obscura show signs of abrasion on nuchal region, and small nuchal thorns may have been present in the larger male. These distinctions in squamation (between males of M. obscura and males of M. senta and M. kreffti) are concordant with distinctions between juvenile and adult male skates in general, and corroborate that our new species sexually matures at a larger size than either M. senta or M. kreffti (see below). A late juvenile, almost adult male of M. spinacidermis (SAM 35454, 572 mm TL) has well developed alar, malar and orbital thorns in comparison to the largest male specimen of M. obscura.
Juveniles of M. senta resemble M. obscura in squamation more than do adults, but differ in presenting a greater central region devoid of denticles on ventral tail base, which may increase as maturity approaches (Bigelow & Schroeder, 1953: 265, fig. 57c). According to Hulley (1970: 175), denticles are also missing from "median line of distal two-thirds" of tail in juveniles of South African M. spinacidermis, but these were present in the juveniles examined (e. g. SAM 35517, SAM "A6956 059 E24"), except for a segment of the ventral tail proximal to pelvic fins. This region is devoid of small denticles in small and large specimens of M. obscura as well, but is not as great and does not expand too much with growth (holotype has a small area devoid of denticles reaching posteriorly to pelvic fin rear margin, which is not much greater proportionally than in the small juvenile female). Barnard (1923: 440) credits the "median line of basal two-thirds" as lacking denticles in M. spinacidermis. According to Hulley (1986: 125) and material examined, the distal half of the median ventral tail of larger South African M. spinacidermis lacks small denticles, representing a relatively greater naked region than in M. obscura (in this respect, M. obscura is more similar to "M. mollis"; Bigelow & Schroeder, 1953; Templeman, 1965).
Meristic data may be diagnostic for species of Malacoraja, but information is still limited (Table 2). Vertebral numbers are very similar for M. kreffti and South African M. spinacidermis, and to a lesser degree for M. obscura (data not available for M. senta). Malacoraja obscura has slightly higher counts than M. kreffti and M. spinacidermis, especially for the male and small female specimens (pretransitional centra vary less than predorsal caudal centra), and approaches "M. mollis" in this regard (Stehmann, 1977: 90). Pelvic radial elements are similar in M. kreffti and M. obscura, but the holotype of M. obscura has slightly more radials. Malacoraja obscura has more pectoral radials than M. kreffti, however, and approaches M. spinacidermis (pelvic and pectoral radial counts not available for M. senta). Malacoraja senta has significantly fewer tooth rows than its congeners (reported as 3840/3638 tooth rows for adult males; Bigelow & Schroeder, 1953; McEachran, 2002). The other species are similar in tooth row counts, but M. obscura and M. kreffti have slightly more rows than M. spinacidermis. An adult male 634 mm TL M. spinacidermis is reported to have 56 upper tooth rows (Hulley & Stehmann, 1977), whereas the smaller 505 mm TL male M. obscura has 64/62 rows (similar to the 515 mm TL holotype of M. kreffti; Stehmann, 1993), and the even smaller 355 mm TL male M. obscura has 57/55 rows. The juvenile female M. spinacidermis reported from Western Sahara has 57/60 tooth rows (Stehmann, 1995), well within the range of South African M. spinacidermis (5460/5370; Hulley & Stehmann, 1977) and western North Atlantic "M. mollis" (5463/5564; Templeman, 1965).
In summary, M. obscura more closely resembles M. spinacidermis in coloration, squamation and size than either M. senta or M. kreffti. It is distinguished from M. spinacidermis by presenting small whitish spots dorsally on disc and pelvics (missing in M. spinacidermis), a more uniform ventral coloration (without large whitish blotches, but see above), in retaining in larger specimens a compound midrow of thorns on anterior two-thirds of dorsal tail (a single but irregular row present in juvenile M. spinacidermis but lost in larger individuals), in having a much smaller region devoid of denticles on ventral tail base (reaching to posterior pelvic margin vs. anterior half of tail in adult M. spinacidermis), and in having relatively more tooth rows and vertebral centra (Table 2). Additional anatomical characters that separate both species are discussed below.
Relationships. The monophyly of Malacoraja is supported by: (i) the presence of intense 'micromeric' squamation on ventral tail region (small denticles are also present on dorsal disc and pelvics and dorsal and lateral tail, and naked only on ventral disc and anteriormost ventral tail) (Figs. 6, 7); and (ii) slender, elongate rostral appendices fused to rostrum and extending posteriorly to midlength of rostrum (Figs. 4, 9, 10) (cf. Stehmann, 1970, 1977; Hulley & Stehmann, 1977). Malacoraja is phylogenetically resolved as the most basal genus of the tribe Gurgesiellini, which also includes Neoraja, Gurgesiella, and Fenestraja (McEachran & Dunn, 1998). The subfamily Rajinae is composed of the tribes Gurgesiellini, Amblyrajini and Rajini, but their phylogenetic relationships are presently unresolved (see also McEachran & Aschliman, 2004).
A similar but not indistinguishable micromeric condition on ventral tail region is present but independently derived for Rajella nigerrima, R. fuliginea and possibly R. bathyphila (but not present in other described Rajella spp.; Bigelow & Schroeder, 1954b; Hulley, 1970; Krefft & Stehmann, 1974; McEachran & Miyake, 1984; Stehmann, 1995), and is present to a lesser degree in Neoraja africana (Stehmann, 1995). Many other rajids (mostly rajines) are intensely covered with small denticles, but these are not present ventrally on tail (e. g. most Rajella spp., Breviraja spp., Neoraja stehmanni, Pseudoraja fischeri; Bigelow & Schroeder, 1953, 1954b; Hulley, 1972b; McEachran & Matheson, 1985; McEachran & Miyake, 1987).
McEachran & Compagno (1982) illustrate the neurocranium of M. senta as having short rostral appendices, but these are probably similar to other Malacoraja species as they can be easily missed in dissections and are usually not seen in radiographs (appendices are uncalcified, hialine). The appendices in Malacoraja are unique at least among the Amblyrajini and Gurgesiellini in being firmly attached to rostrum (as also noted by Hulley & Stehmann, 1977), and relatively more slender and elongate. Similar conditions appear to be present in the Rajini (Dipturus, Raja, Okamejei, Cruriraja?; e. g. Ishiyama, 1958; Hulley, 1972a; McEachran & Miyake, 1990), but the appendices do not extend posteriorly to midlength of rostrum as they do in Malacoraja; this character requires further study among rajids as illustrations of neurocrania based solely on radiographs may not represent the appendices correctly.
Additionally, M. obscura closely agrees with its congeners in having disproportionally large and rounded nasal capsules, slender rostral base with a very slender but calcified and unsegmented rostrum extending anteriorly to snout extremity, slender anterior fontanelle and internasal septum, anterior fontanelle extending very little anteriorly beyond nasal capsules, absence of true preorbital processes, laterally narrow interorbital region, narrow basal plate, anteroposteriorly short otic capsules with small jugal arches, elongate iliac processes on pelvic girdle, and poorly developed rear corners on scapulocoracoid (Hulley & Stehmann, 1977; Stehmann, 1977, 1993; McEachran & Compagno, 1982; see Figs. 9, 12, and 13, respectively, of this study). However, some of these characters are present in other Rajinae, particularly in the amblyrajin Rajella (e. g. R. barnardi, R. confundens, R. sadowskii; Stehmann, 1995; pers. obs.) and in the Gurgesiellini (especially Neoraja; McEachran & Compagno, 1982; McEachran & Stehmann, 1984), and are probably primitive for Malacoraja within the phylogenetic context of McEachran & Dunn (1998). Malacoraja and Rajella are phylogenetically resolved in different but closely related tribes in the Rajinae by McEachran & Dunn (1998), but they share morphological similarities in neurocranium, extent of anterior expansion of propterygial radials, jaws, hyomandibulae, pelvic girdle (see Hulley, 1972a; Stehmann, 1995), and to some extent squamation and disc shape, and may eventually prove to be more closely related (cf. Hulley, 1972a; Hulley & Stehmann, 1977). Both genera also occur at great depths (J. D. McEachran, pers. comm.).
As detailed above, M. obscura more closely resembles M. spinacidermis in coloration, squamation and size, but the phylogenetic significance of these features requires corroboration from additional anatomical data, especially for M. kreffti and "M. mollis". The complex shape of the accessory terminal 1 cartilage of the clasper may be derived for M. spinacidermis and M. kreffti (Hulley & Stehmann, 1977; Stehmann, 1977), but data are lacking for M. obscura and M. senta. M. obscura (Fig. 9c) and M. spinacidermis share an elliptical optic stalk situated obliquely within the orbit, which is not present in M. senta (which has a small, rounded optic stalk; McEachran & Compagno, 1982), but is unknown in M. kreffti; this character is presently ambiguous because of varying conditions in the Gurgesiellini, but the optic stalk of M. obscura and M. spinacidermis is probably plesiomorphic. Malacoraja senta has a single opening for the internal carotid artery ventrally on neurocranium (McEachran & Compagno, 1982), while M. obscura has a pair of openings (Fig. 9b), but the condition in other Malacoraja species is unknown and this character is also ambiguous because of great variation among taxa of Rajinae. The foramen magnum of M. obscura is broadly circular (Fig. 9d), but it is subtriangular in M. spinacidermis (Hulley & Stehmann, 1977; foramen magnum not described for M. kreffti, M. senta and M. mollis). The scapulocoracoid of M. obscura (Fig. 12) is more similar to that of M. spinacidermis and differs from M. kreffti in having a single postdorsal fenestra (paired in M. kreffti), but this is probably primitive as well (a single postdorsal fenestra is present in Neoraja, Leucoraja, Breviraja, Fenestraja and even among taxa of the Arhynchobatinae; McEachran & Compagno, 1982; McEachran & Miyake, 1990; McEachran & Dunn, 1998). Malacoraja obscura has paired postventral fenestrae similar to M. kreffti, however (but much larger proportionally), whereas M. senta (McEachran & Miyake, 1990)and "M. mollis" (Stehmann, 1993) have a single postventral fenestra. Even though both conditions occur in outgroups (a single fenestra is present in Neoraja, Breviraja, Leucoraja; paired fenestrae in Gurgesiella, and both states are present in Fenestraja; McEachran & Compagno, 1982; McEachran & Miyake, 1990), and the condition in South African M. spinacidermis is unknown, our data suggest that the anterior postventral fenestra is derived for M. obscura, as it is larger and more anteriorly positioned than in other rajines endowed with paired postventral fenestrae (Fig. 12). However, we believe that proposing a species-level cladogram for Malacoraja is premature without more anatomical data from M. kreffti and "M. mollis".
Biological notes. All male specimens of M. obscura are sexually immature. The largest male is 505 mm in TL and is clearly a juvenile (albeit late stage), and has small claspers that do not reach beyond pelvic fin length, a disc that is not weakly convex as of level of orbits, and lacks alar and malar thorns. The holotype (680 mm TL) is probably an adult female judging by its large size, but conclusive data on sexual maturity are lacking. Males sexually mature at about 575580 mm TL in M. spinacidermis (South African specimens with elongated but not yet fully calcified claspers; SAM 35454, 572 mm TL), probably under 520 mm TL in M. senta (Bigelow & Schroeder, 1953; cf. Packer et al., 2003, who wrongly estimate size at maturity to be about 560 mm TL), and at least at 505 mm TL in M. kreffti (specimens in ZMH; also Stehmann, 1977, 1993). This indicates that M. obscura matures at a rate closer to that of M. spinacidermis (if indeed our largest female is an adult), and also attains greater sizes than either M. senta or M. kreffti (M. spinacidermis reported to reach 640 mm TL; Hulley, 1986). The holotype of M. obscura is the largest Malacoraja specimen examined or reported to date (at 680 mm TL). The juveniles of M. obscura are not newly hatched, and hence size at birth can only be estimated to be smaller than that of the smallest specimen (female, 251 mm TL, 157 mm DW and 127 mm DL).
The only information available concerning population structure is that four of the five specimens of M. obscura were collected during the same trawling effort, which included the smallest male juvenile as well as the largest female (the holotype), possibly indicating that there is no segregation by size in this species.
Identification. Note that "M. mollis" is not distinguished from M. spinacidermis in the following key to species of Malacoraja.
Acknowledgements
For logistic support during visits and access to the collections under their care, the senior author thanks H. Wilkens, M. F. W. Stehmann, and G. Schultze (ZMH, Hamburg), and L. J. V. Compagno and M. Bougaardt (SAM, Cape Town). For hospitality during a visit to Cape Town, the senior author is grateful to Leonard Compagno and Martina Roeleveld. Gilmar de Oliveira (Radiologia, Hospital Central, Universidade de São Paulo, Ribeirão Preto) is sincerely thanked for taking the radiographs of the Brazilian Malacoraja, material of which was generously made available for study by G. W. A. Nunan (MNRJ). John D. McEachran (Texas A & M University) is acknowledged for discussions concerning Malacoraja and skate systematics in general. Marcelo Di Lello Jordão (UERJ) is thanked for his assistance measuring specimens of M. obscura. We are grateful to Camila N. Signori (UERJ) for translating texts from German, and Alexandre C. Ribeiro (Laboratório de Ictiologia de Ribeirão Preto, USP-FFCLRP) for producing the map. Members of the Laboratório de Ictiologia de Ribeirão Preto are acknowledged for support, especially Flávio A. Bockmann and Murilo de Carvalho. This project was completed thanks to funding from a research grant to MRC from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP proc. no. 02/06459-0), and to support from the program Prociência (UERJ/FAPERJ) to ULG.
Literature Cited
Received March 2005
Accepted May 2005
Malacoraja kreffti: ISH 748-74 (holotype), adult male, 505 mm TL, 61º 08'N, 11º 26'W, FFS Walther Herwig, sta. 894/74, 1200 m, 27. xi. 1974; ISH 35-1981, adult male, 539 mm TL, 61º 03.0'N, 11º 11.9'W, FFS Walther Herwig, sta. 575/81, 1000-1010 m, 27. x. 1981; ISH 87-1981, adult female, 492 mm TL, 56º 35.1'N, 09º 38.4'W, FFS Walther Herwig, sta. 608/81, 1500 m, 13. x. 1981.
Malacoraja spinacidermis: (all specimens from the slope area off western South Africa; further data provided upon request): SAM 26879, adult male (head missing due to previous dissection; see Hulley & Stehmann, 1977); SAM 32506, adult male, 602 mm TL; SAM 33162, adult male, 581 mm TL; SAM 34509, adult female, 604 mm TL; SAM 34520, adult male, 623 mm TL; SAM 34930 (2), adult female, 615 mm TL, juvenile male, 389 mm TL; 35453 (2), adult male, 392 mm DW, adult female, 416 mm DW; 35454, late juvenile male, 572 mm TL; 35517 (2), adult female, 620 mm TL, juvenile male, 361 mm TL; 6 additional small juvenile specimens in SAM (some uncatalogued and some from RUSI).
- Barnard, K. H. 1923. Diagnoses of new species of marine fishes from South African waters. Annals of the South African Museum, 13:439-445.
- Bianchi, G., K. E. Carpenter, J. -P. Roux, F. J. Molloy, D. Boyer & H. J. Boyer. 1999. Field guide to the living marine resources of Namibia. FAO species identification guide for fishery purposes. Rome, FAO. 265 p.
- Bigelow, H. B. & W. C. Schroeder. 1950. New and little known cartilaginous fishes from the Atlantic. Bulletin of the Museum of Comparative Zoology, 103(7):385-408, 7 pls.
- Bigelow, H. B. & W. C. Schroeder. 1953. Fishes of the Western North Atlantic. Part 2: Sawfishes, guitarfishes, skates and rays. Memoir of the Sears Foundation for Marine Research, New Haven, 1(2):1-558.
- Bigelow, H. B. & W. C. Schroeder. 1954a. Deep water elasmobranchs and chimaeroids from the northwestern Atlantic slope. Bulletin of the Museum of Comparative Zoology, 112(2):37-87.
- Bigelow, H. B. & W. C. Schroeder. 1954b. A new family, a new genus, and two new species of batoid fishes from the Gulf of Mexico. Breviora, 24:1-15.
- Chu, Y.T. & M. C. Wen. 1979. A study of the lateral-line canal systems and that of lorenzini ampullae and tubules of elasmobranchiate fishes of China. Monograph of Fishes of China 2. Shanghai, Shanghai Science and Technology Press. 132p., 64pls.
- Compagno, L. J. V. 1999. Checklist of living elasmobranches. Pp. 471-498. In: Hamlett, W. C. (Ed.). Sharks, skates, and rays, the biology of elasmobranch fishes. Baltimore, John Hopkins Univ. Press. 515p.
- Figueiredo, J. L., A. P. dos Santos, N. Yamaguti, R. A. Bernardes & C. L. D. B. Rossi-Wongtschowski. 2002. Peixes da Zona Econômica Exclusiva da região sudeste-sul do Brasil. Levantamento com rede de meia água. São Paulo, Editora da Universidade de São Paulo. 242p.
- Gomes, U. L. 2002. Revisão taxonômica da família Rajidae no Brasil (Chondrichthyes, Elasmobranchii, Rajiformes). Unpublished doctoral thesis, Universidade Federal do Rio de Janeiro. 286p., 231 figs.
- Herman, J., M. Hovestadt-Euler, D. C. Hovestadt & M. Stehmann. 1994. Part B: Batomorphii No. 1a: Order Rajiformes Suborder Rajoidei Family: Rajidae genera and subgenera: Anacanthobatis (Schroederobatis), Anacanthobatis (Springeria), Breviraja, Dactylobatus, Gurgesiella (Gurgesiella), Gurgesiella (Fenestraja), Malacoraja, Neoraja and Pavoraja Pp. 165-207. In: Stehmann, M. (Ed.). Contributions to the study of the comparative morphology of teeth and other relevant ichthyodorulites in living supra-specific taxa of chondrichthyan fishes. Bulletin de l´Institut Royal des Sciences Naturelles de Belgique. Biologie, 64.
- Hubbs, C. L. & R. Ishiyama. 1968. Methods for the taxonomic study and description of skates (Rajidae). Copeia, 1968(3):483-491.
- Hulley, P.A. 1970. An investigation of the Rajidae of the west and south coasts of southern Africa. Annals of the South African Museum, 55(4):151-220.
- Hulley, P.A. 1972a. The origin, interrelationships and distribution of southern African Rajidae (Chondrichthyes, Batoidei). Annals of the South African Museum, 60(1):1-103.
- Hulley, P.A. 1972b. A new species of South African brevirajid skate (Chondrichthyes, Batoidei, Rajidae). Annals of the South African Museum, 60(9):253-263.
- Hulley, P.A. 1986. Rajidae. Pp.115-127. In: Smith, M. M. & P. C. Heemstra (Eds.). Smiths' sea fishes. Johannesburg, Southern Book Publishers. 1047p.
- Hulley, P.A. & M. Stehmann. 1977. The validity of Malacoraja Stehmann, 1977 (Chondrichthyes, Batoidea, Rajidae) and its phylogenetic significance. Annals of the South African Museum, 72(12):227-237.
- Ishiyama, R. 1958. Studies on the rajid fishes (Rajidae) found in the waters around Japan. Journal of the Shimonoseki College of Fisheries, 7(2/3):193-394.
- Krefft, G. & C. Lübben. 1966. Raja mollis Bigelow and Schroeder, 1950 (Batoidea, Elasmobranchii, Chondrichthyes), ein Erstfund im Nordost-Atlantik. Zoologischer Anzeiger, 176(6):389-395.
- Krefft, G. & M. Stehmann. 1974. Ergebnisse der Forschungsreisen des FFS "Walter Herwig" nach Südamerika. XXXIII. Raja (Rajella) sadowskii spec. nov. (Chondrichthyes, Batoidea, Rajidae), ein weiterer neuer Roche vom südwestatlantischen Kontinentalabhang. Archiv für Fischereiwissenschaft, 25(1):33-50.
- Leviton, A. E., R. H. Gibbs Jr., E. Heal & C. E. Dawson. 1985. Standards in herpetology and ichthyology: Part I. Standard symbolic codes for institutional resource collections in herpetology and ichthyology. Copeia, 1985(3):803832.
- Maruska, K. P. 2001. Morphology of the mechanosensory lateral line system in elasmobranch fishes: ecological and behavioral considerations. Pp. 47-75. In: Tricas, T. C. & S. H. Gruber (Eds.). The behavior and sensory biology of elasmobranch fishes: an anthology in memory of Donald Richard Nelson. Environmental Biology of Fishes, 60(1-3). Dordrecht, Kluwer Academic Publishers. 320p.
- McAllister, D. E. 1990. A list of the fishes of Canada. Syllogeus No. 64. Ottawa, National Museum of Natural Sciences. 310p.
- McEachran, J. D. 2002. Skates. Family Rajidae. Pp. 60-75. In: Collette, B. B. & G. Klein-MacPhee (Eds.). Bigelow and Schroeder's fishes of the Gulf of Maine. 3rd ed. Washington D.C., Smithsonian Institution Press. 748p.
- McEachran, J. D. & N. Aschliman. 2004. Phylogeny of Batoidea. Pp. 79-113. In: Carrier, J. C., J. A. Musick & M. R. Heithaus (Eds.). Biology of sharks and their relatives. Boca Raton, CRC Press. 596p.
- McEachran, J. D. & M. R. de Carvalho. 2002. Rajidae. Pp. 531-561. In: Carpenter, K. E. (Ed.). The living marine resources of the Western Central Atlantic. Volume 1: introduction, molluscs, crustaceans, hagfishes, sharks, batoid fishes and chimaeras. FAO Species Identification Guide for Fisheries Purposes. Rome, FAO and American Society of Ichthyologists and Herpetologists Special Publication no. 5. 600p.
- McEachran, J. D. & L. J. V. Compagno. 1979. A further description of Gurgesiella furvescens with comments on the interrelationships of Gurgesiellidae and Pseudorajidae (Pisces, Rajoidei). Bulletin of Marine Science, 29(4):530-553.
- McEachran, J. D. & L. J. V. Compagno. 1982. Interrelationships of and within Breviraja based on anatomical struc-tures (Pisces, Rajoidei). Bulletin of Marine Science, 32(2):399-425.
- McEachran, J. D. & K. Dunn. 1998. Phylogenetic analysis of skates, a morphologically conservative clade of elasmobranchs (Chondrichthyes: Rajidae). Copeia, 1998(2):271-290.
- McEachran, J. D. & R. E. Matheson, Jr. 1985. Polychromatism amd polymorphism in Breviraja spinosa (Elasmobranchii, Rajiformes), with description of three new species. Copeia, 1985(4):1035-1052.
- McEachran, J. D. & T. Miyake. 1984. Comments on the skates of the tropical eastern Pacific: one new species and three new records (Elasmobranchii: Rajiformes). Proceedings of the Biological Society of Washington, 97(4):773-787.
- McEachran, J. D. & T. Miyake. 1987. A new species of skate of the genus Breviraja from off Nova Scotia, with comments on the status of Breviraja and Neoraja (Chondrichthyes, Rajoidei). Copeia, 1987(2):409-417.
- McEachran, J. D. & T. Miyake. 1990. Phylogenetic interrelationships of skates: a working hypothesis (Chondrichthyes, Rajoidei). Pp. 285-304. In: Pratt, H. L., S. H. Gruber & T. Taniuchi (Eds.). Elasmobranchs as living resources: advances in the biology, ecology, systematics and the status of the fisheries. NOAA Technical Report 90. 518p.
- McEachran, J. D. & J. A. Musick. 1975. Distribution and relative abundance of seven species of skates (Pisces: Rajidae) which occur between Nova Scotia and Cape Hatteras. United States Fishery Bulletin, 73(1):110-136.
- McEachran, J. D. & M. Stehmann. 1984. A new species of skate, Neoraja carolinensis, from off the southeastern United States (Elasmobranchii: Rajoidei). Proceedings of the Biological Society of Washington, 97(4):724-735.
- Menni, R. C. & M. F. W. Stehmann. 2000. Distribution, environment and biology of batoid fishes off Argentina, Uruguay and Brazil. A review. Revista del Museo Argentino de Ciencias Naturales, n. s., 2(1):69-109.
- Miyake, T. 1988. The systematics of the stingray genus Urotrygon, with comments on the interrelationships within Urolophidae (Chondrichthyes, Myliobatiformes). Vols. I and II. Unpublished PhD thesis, Texas A & M University, College Station. 491p.
- Miyake, T., J. D. McEachran & B. K. Hall. 1992. Edgeworth's legacy of cranial muscle development with an analysis of muscles in the ventral gill arch region of batoid fishes (Chondrichthyes: Batoidea). Journal of Morphology, 212:213-256.
- Packer, D. B., C. A. Zetlin & J. J. Vitaliano. 2003. Smooth skate, Malacoraja senta, life history and habitat characteristics. NOAATechnical Memorandum NMFS-NE-177. 26p.
- Schwartz, F. J. 2003. Sharks, skates, and rays of the Carolinas. Chapel Hill, The University of North Carolina Press. 161p.
- Stehmann, M. 1970. Vergleichend morphologische und anatomische Untersuchungen zur Neuordnung der Systematik der nordostatlantischen Rajidae (Chondrichthyes, Batoidea). Archiv für Fischereiwissenschaft, 21(2):73-164.
- Stehmann, M. 1977. Ein neuer archibenthaler Roche aus dem Nordostatlantik, Raja kreffti spec. nov. (Elasmobranchii, Batoidea, Rajidae), die zweite Spezies im Subgenus Malacoraja Stehmann, 1970. Archiv für Fischereiwissenschaft, 28(2/3):77-93.
- Stehmann, M. 1993. Neufunde eines adulten Pärchens von Malacoraja kreffti (Stehmann, 1970) im Bereich des Rockall-Grabens, Nordostaltaltik (Pisces, Rajiformes, Rajidae). Archiv für Fischereiwissenschaft, 41(3):169-186.
- Stehmann, M. 1995. First and new records of skates (Chondrichthyes, Rajiformes, Rajidae) from the West African continental slope (Western Sahara to South Africa), with descriptions of two new species. Archive of Fishery and Marine Research, 43(1):1-119.
- Stehmann, M. & D. L. Bürkel. 1984. Rajidae. Pp. 163-196. In: Whitehead, P. J. P., M.-L. Bauchot, J.-C. Hureau, J. Nielsen & E. Tortonese (Eds.). Fishes of the North-Eastern Atlantic and Mediterranean, Volume 1. Paris, Unesco. 510p.
- Templeman, W. 1965. Rare skates of the Newfoundland and neighbouring areas. Journal of the Fisheries Research Board of Canada, 22(2):259-279.
Appendix
Publication Dates
-
Publication in this collection
17 Dec 2007 -
Date of issue
June 2005
History
-
Accepted
May 2005 -
Received
Mar 2005