Abstract
This study aimed to obtain sorghum doughs subjected to two fermentation processes (backslopping and spontaneous fermentation techniques) with enhanced biological properties and to assess their influence on the bioaccessibility of phenolic compounds and Ferulic Acid (FA) of cookies formulated from fermented sorghum doughs. The best contents of Total Phenols (TP) (µgGAE/g) were 700.9 ± 7.6/36 h and 484.3 ± 6.1/48 h in sorghum doughs fermented by the backslopping and spontaneous fermentation techniques, respectively. The FA values (µg/g) in sorghum doughs fermented by backslopping techniques were significantly higher than those in spontaneous fermentation (21.2 ± 0.27/20 h versus 18.14 ± 0.12/12 h). Cookies formulated from selected sorghum-fermented doughs showed higher bioaccessibility of TP and FA than cookies formulated from nonfermented doughs. High bioaccessibility values for TP were found in cookie digests formulated from sorghum doughs fermented by backslopping and spontaneous processes at 36 h and 12 h, respectively. In contrast, high bioaccessibility values for FA were found in cookie digests formulated from sorghum doughs fermented by backslopping and spontaneous processes at 20 h and 48 h, respectively. The formulation of cookies with fermented sorghum doughs could be a suitable methodology for the prevention of several chronic nontransmissible diseases.
Keywords:
Functional foods; Antioxidant activity; Phenolic compounds
Resumo
O objetivo deste estudo foi obter massas de sorgo submetidas a dois processos de fermentação (backslopping e espontâneo) com propriedades biológicas aprimoradas e avaliar sua influência na bioacessibilidade de compostos fenólicos e ácido ferúlico de biscoitos formulados a partir das massas de sorgo fermentadas obtidas. Os melhores teores de fenóis totais (FT) (µgGAE/g) foram 700,9 ± 7,6/36 h e 484,3 ± 6,1/48 h nas massas de sorgo fermentadas pelas técnicas de backslopping e fermentação espontânea, respectivamente. Os valores de ácido ferúlico AF (µg/g) nas massas de sorgo fermentadas por técnicas de backslopping foram significativamente superiores aos da fermentação espontânea (21,2 ± 0,27/20 h vs. 18,14±0,12/12 h). Os biscoitos formulados a partir de massas fermentadas de sorgo selecionadas apresentaram maior bioacessibilidade de FT e AF do que os biscoitos formulados a partir de massas não fermentadas. Altos valores de bioacessibilidade para FT foram encontrados em biscoitos digeridos formulados a partir de massas de sorgo fermentadas por backslopping e processos espontâneos em 36 h e 12 h, respectivamente. Por sua vez, altos valores de bioacessibilidade para AF foram encontrados em biscoitos digeridos formulados a partir de massas de sorgo fermentadas por backslopping e processos espontâneos em 20 h e 48 h, respectivamente. A formulação de biscoitos com massa fermentada de sorgo pode ser uma metodologia adequada para a prevenção de diversas doenças crônicas não transmissíveis.
Palavras-chave:
Alimentos funcionais; Atividade antioxidante; Compostos fenólicos
HIGHLIGHTS
• Sorghum doughs fermented by the backslopping method improved their biological potential
• Cookies formulated with fermented sorghum doughs increased their total phenol content
• Cookies formulated with fermented sorghum dough as part of a healthy diet
1 Introduction
The consumption of whole grains has been associated with the prevention of certain chronic diseases, such as cardiovascular disease, diabetes, and some types of cancer (Tieri et al., 2020Tieri, M., Ghelfi, F., Vitale, M., Vetrani, C., Marventano, S., Lafranconi, A., Godos, J., Titta, L., Gambera, A., Alonzo, E., Sciacca, S., Riccardi, G., Buscemi, S., Del Rio, D., Ray, S., Galvano, F., Beck, E., & Grosso, G. (2020). Whole grain consumption and human health: An umbrella review of observational studies. International Journal of Food Sciences and Nutrition, 71(6), 668-677. PMid:31964201. http://dx.doi.org/10.1080/09637486.2020.1715354
http://dx.doi.org/10.1080/09637486.2020....
). This protective effect has been attributed to the presence of phenolic compounds that are found in high concentrations in the outermost layers of the grain (Polonskiy et al., 2020Polonskiy, V., Loskutov, I., & Sumina, A. (2020). Biological role and health benefits of antioxidant compounds in cereals. Biological Communications, 65(1), 53-67. https://doi.org/10.21638/spbu03.2020.105
https://doi.org/10.21638/spbu03.2020.105...
). Sorghum (Sorghum bicolor L.) is a cereal that, in addition to being an important source of nutrients, is considered exceptional due to its diverse phenolic profile, which is predominated mainly by phenolic acids (ferulic, coumaric, caffeic and sinapic acids), which are found in variable amounts depending on the sorghum genotype (Xiong et al., 2019Xiong, Y., Zhang, P., Warner, R. D., & Fang, Z. (2019). Sorghum grain: From genotype, nutrition, and phenolic profile to its health benefits and food applications. Comprehensive Reviews in Food Science and Food Safety, 18(6), 2025-2046. PMid:33336966. http://dx.doi.org/10.1111/1541-4337.12506
http://dx.doi.org/10.1111/1541-4337.1250...
; Girard & Awika, 2018Girard, A. L., & Awika, J. M. (2018). Sorghum polyphenols and other bioactive components as functional and health promoting food ingredients. Journal of Cereal Science, 84, 112-124. http://dx.doi.org/10.1016/j.jcs.2018.10.009
http://dx.doi.org/10.1016/j.jcs.2018.10....
). Sorghum, as can be noted in most whole grains, contains Ferulic Acid (FA) in the insoluble bound form (>80%), covalently bound to cell wall structural components such as cellulose, hemicellulose (e.g., arabinoxylans) and lignin (Acosta Estrada et al., 2014Acosta Estrada, B. A., Gutiérrez Uribe, J. A., & Serna Saldívar, S. O. (2014). Bound phenolics in foods, a review. Food Chemistry, 152, 46-55. PMid:24444905. http://dx.doi.org/10.1016/j.foodchem.2013.11.093
http://dx.doi.org/10.1016/j.foodchem.201...
), and cannot be absorbed through the gastrointestinal tract in this form. Several studies have reported the low bioaccessibility of FA found in cereal products in the human gastrointestinal tract (<5%) (Amaya Villalva et al., 2018Amaya Villalva, M. F., González-Aguilar, G., Sández, O. R., Astiazarán García, H., Ledesma Osuna, A. I., López-Ahumada, G. A., & Robles-Sánchez, R. M. (2018). Bioprocessing of wheat (Triticum aestivum cv. Kronstad) bran from Northwest Mexico: Effects on ferulic acid bioaccessibility in breads. CYTA: Journal of Food, 16(1), 570-579. http://dx.doi.org/10.1080/19476337.2018.1440007
http://dx.doi.org/10.1080/19476337.2018....
; Mateo Anson et al., 2009 Mateo Anson, N., van den Berg, R., Havenaar, R., Bast, A., & Haenen, G. R. M. M. (2009). Bioavailability of ferulic acid is determined by its bioaccessibility. Journal of Cereal Science, 49(2), 296-300. http://dx.doi.org/10.1016/j.jcs.2008.12.001
http://dx.doi.org/10.1016/j.jcs.2008.12....
). The FA can be absorbed predominantly in its free form, but it can also be absorbed in the form of a dimer or in its aglycone form; the relevant thing is that it reaches adequate concentrations in circulation so that it can exert its function (0.1-10 µM). Scientific evidence has shown that the phenolic compound content in sorghum can be improved by different innovative methods, such as extrusion, cooking, and fermentation (Rashwan et al., 2021Rashwan, A. K., Yones, H. A., Karim, N., Taha, E. M., & Chen, W. (2021). Potential processing technologies for developing sorghum-based food products: An update and comprehensive review. Trends in Food Science & Technology, 110, 168-182. http://dx.doi.org/10.1016/j.tifs.2021.01.087
http://dx.doi.org/10.1016/j.tifs.2021.01...
). These processes have been shown to influence the content of phenolic compounds, and significant increases in free phenolic compounds have been observed with respect to unprocessed sorghum and in some cases have been associated with an increase in antioxidant activity. However, limited studies have been carried out with respect to phenolic compounds and antioxidant capacity in in vitro gastrointestinal digestion. Lactic acid fermentation in sorghum grain is carried out to obtain several foods, such as tings, kisra, and porridges (Adebo, 2020Adebo, O. A. (2020). African sorghum-based fermented foods: Past, current and future prospects. Nutrients, 12(4), 1111. PMid:32316319. http://dx.doi.org/10.3390/nu12041111
http://dx.doi.org/10.3390/nu12041111...
). The backslopping procedure is a common practice for obtaining these products, particularly because it has some advantages in spontaneous fermentation, such as reduced fermentation times and higher acidification, among others. This procedure has given good results in terms of the improvement of phenolic compounds and antioxidant capacity in finished products as well as in acceptable sensory profiles. Cookies are confectionary food products that are consumed as snacks by all ages. They are ready to eat, convenient, and inexpensive food products. Cookies from sorghum would be an inexpensive carrier of the aforementioned health benefits, making them a promising functional food. Therefore, this investigation aimed to evaluate two traditional fermentation processes of doughs from whole sorghum grain to identify the one that is most biologically active for the prevention of diseases. In the first stage of this investigation, the contents of phenolic compounds, FA and antioxidant capacity were determined and compared with nonfermented sorghum dough. In the second stage, the influence of fermentation processes on the bioaccessibility of phenolic compounds, FA and antioxidant capacity of cookies from whole sorghum grain was examined.
2 Materials and methods
2.1 Sample preparation
White low-tannin sorghum (0.062 ± 0.007 mgCE/g), was obtained from local growers in Sonora, Mexico. Grains were cleaned manually and milled using a Laboratory Mill 1100 fitted with a 0.5-mm opening screen to produce a whole-grain flour, which was packed and stored at -15 °C prior to usage.
2.2 Preparation of backslopped inoculum
A natural inoculum was prepared by making a dough of milled whole sorghum and water in a ratio of 1:1.06 (flour:water) (Taylor & Taylor, 2002Taylor, J., & Taylor, J. R. (2002). Alleviation of the adverse effect of cooking on sorghum protein digestibility through fermentation in traditional African porridges. International Journal of Food Science & Technology, 37(2), 129-137. http://dx.doi.org/10.1046/j.1365-2621.2002.00549.x
http://dx.doi.org/10.1046/j.1365-2621.20...
). This dough was incubated at 26 °C in a covered container, and the pH was monitored daily until pH fell from approximately 3.5 to 4.0. Five percent of this material was then transferred to fresh sorghum dough using the same ratio of flour to water as before. This was mixed and incubated at 26 °C in a covered container for 7 days, after which the procedure was repeated, thus maintaining a natural inoculum. The natural inoculum was used to inoculate fresh sorghum dough, which was prepared according to the indications mentioned above. The resulting dough was placed in trays of 20 x 30 cm, which were incubated in a fermenting cabinet (NationalMeg.CO. MODEL 505-SS 2/3) at 26 °C. These samples were labeled as BLF (Backslopping Lactic Fermentation). Additionally, fresh sorghum dough without starter inoculum was prepared and fermented under the same conditions. These samples were labeled as SLF (Spontaneous Lactic Fermentation). Nonfermented whole sorghum dough was used as a control (SCD). Ten grams of each sample was taken to determine the pH at intervals of 4 h for 48 h. The remaining fermented sorghum dough samples were lyophilized, including the unfermented dough (zero time), henceforth these samples were called powdered doughs and used to determine the Total Phenols (TP), FA and antioxidant activity. Samples that showed better results with respect to their bioactive potential were used for cookie formulations.
2.3 Preparation of sorghum cookies
Cookies were formulated and baked according to the official AACC 10-53.01 method with slight modifications (American Association of Cereal Chemists, 2000American Association of Cereal Chemists – AACC. (2000). Approved methods of analysis (10th ed.). St. Paul: AACC International.). The cookie formulation was 40 g of sugar, 50 g of commercial white margarine (according to the label, it contains the following: vegetable fats and oils 80%, water, salt, milk solids, emulsifiers monoglyceride, and sunflower seed lecithin, preservative sodium benzoate, butter flavoring, vitamins A, D, and E), 1 g of sodium bicarbonate, 20 g of skimmed milk powder, 1 g of salt, 100 g of each powdered doughs and 20 mL of tap water. White margarine, sugar, sodium bicarbonate and salt were creamed at low speed for 3 min using an electric mixer. The creamed ingredients were then mixed with each powdered doughs, and finally, water was added until homogeneous dough was obtained. The doughs were rolled out to a thickness of 6 mm, and a cookie-cutter mold with a diameter of 25 mm was used and excess dough was removed. The cookies were baked in a preheated oven set at 180 °C for 10 min. The cookies were subsequently cooled, ground, and stored at −20 °C until analysis.
2.4 Methanolic extracts
Methanolic extracts were obtained following the procedure reported by Salazar López et al. (2016). Briefly, 1 g of each of the samples was weighed and mixed with 15 mL of methanol (80%). This mixture was subjected to sonication at room temperature for 60 min. The samples were subsequently centrifuged, and the supernatant was separated from the residue by filtration with Whatman No. 1 paper. This procedure was repeated two more times. The supernatants obtained were evaporated to dryness in a rotary evaporator at 35 °C, and samples were redissolved in 5 mL of 50% methanol and stored at -15 °C.
2.5 Determination of TP
The TP of each of the treatments and the control were quantified following the methodology proposed by Singleton & Rossi (1965)Singleton, V. L., & Rossi, J. A. (1965). Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture, 16(3), 144-158., with modifications to adapt the test to a FluoStar Omega microplate reader (BMG Labtech Inc., Ortenberg, Germany). Briefly, 30 μL of each extract was mixed with 150 μL of the Folin–Ciocalteu reagent (previously diluted 1:10 with deionized water) and 120 μL of sodium carbonate solution (0.075 g/mL), after which they were left to react for 60 min. Changes in absorbance were monitored at 765 nm against a reactive blank. The results were expressed as μg of gallic acid equivalents (GAE)/g of the sample according to a standard gallic acid curve.
2.6 Trolox equivalent antioxidant capacity (TEAC)
The assay is based on the ability of antioxidant molecules to capture the cationic radical ABTS●+. For the activation of the ABTS radical, 0.019 mg of ABTS radical was weighed and dissolved in 5 mL of distilled water. After mixing, 88 μL of potassium persulfate (37.8 mg/mL) was added; the solution was mixed vigorously and then left in the dark for 16-18 h at room temperature. Then, a working solution was prepared by mixing 1 mL of the stock solution with approximately 88 mL of ethanol. In a microplate well, 280 μL of the working solution was mixed with 20 μL of each sample and left to react for 5 min. Changes in absorbance were measured at 734 nm in a microplate reader. The results were expressed as μmol Trolox equivalents per gram of sample, with reference to a Trolox curve (Amaya Villalva et al., 2018Amaya Villalva, M. F., González-Aguilar, G., Sández, O. R., Astiazarán García, H., Ledesma Osuna, A. I., López-Ahumada, G. A., & Robles-Sánchez, R. M. (2018). Bioprocessing of wheat (Triticum aestivum cv. Kronstad) bran from Northwest Mexico: Effects on ferulic acid bioaccessibility in breads. CYTA: Journal of Food, 16(1), 570-579. http://dx.doi.org/10.1080/19476337.2018.1440007
http://dx.doi.org/10.1080/19476337.2018....
).
2.7 Determination of FA
FA was quantified using an Agilent Technologies 1260 UHPLC system (USA) with a Diode Array Detector (DAD). Separation was carried out on a Zorbax Eclipse Plus C18 flash column (50 mm x 2.1 mm id). A binary phase solvent system was used: 0.1% acetic acid dissolved in water; Phase B: 0.1% acetic acid dissolved in methanol. The separation gradient was as follows: 0–11 min, 9–14% B; 11–15 min, 15% B. The same gradient remained for 3 min to equilibrate the column. The column temperature was set at 30 °C, the flow was 0.7 mL/min, and the absorbance readings were taken at 280 nm. The results were expressed in μg/g, according to a calibration curve of a FA standard at concentrations of 1.5–50 μg/mL (Salazar-López et al., 2016Salazar-López, N. J., Loarca-Piña, G., Campos-Vega, R., Gaytán Martínez, M., Morales Sánchez, E., Esquerra-Brauer, J. M., Gonzalez-Aguilar, G. A., & Robles Sánchez, M. (2016). The extrusion process as an alternative for improving the biological potential of sorghum bran: Phenolic compounds and antiradical and anti-Inflammatory capacity. Evidence-Based Complementary and Alternative Medicine, 2016, 8387975. PMid:27738445. http://dx.doi.org/10.1155/2016/8387975
http://dx.doi.org/10.1155/2016/8387975...
).
2.8 Simulated in vitro gastrointestinal digestion
To mimic in vivo gastrointestinal digestion conditions, namely, the oral, gastric, and intestinal phases, the protocols suggested by Salazar-López et al. (2018)Salazar-López, N. J., González Aguilar, G. A., Rouzaud Sández, O., & Robles Sánchez, M. (2018). Bioaccessibility of hydroxycinnamic acids and antioxidant capacity from sorghum bran thermally processed during simulated in vitro gastrointestinal digestion. Journal of Food Science and Technology, 55(6), 2021-2030. PMid:29892102. http://dx.doi.org/10.1007/s13197-018-3116-z
http://dx.doi.org/10.1007/s13197-018-311...
were followed, with slight modifications. Three healthy and fasted volunteers chewed 1 g of each of the experimental cookies for 15 s. Subsequently, the participants expelled each chewed sample into a 50-mL conical tube, rinsed their mouth twice with 5 mL of water for 60 s, and then expelled the liquid into the respective tubes, which stood for 3 min at 37 °C to obtain the mouth digest. The digestion process beyond the mouth (i.e., gastric digestion) was simulated by adding 5 mL of 0.2 M HCl-KCl buffer solution to the digests and adjusting the pH to 1.5. Next, 667 μL of pepsin solution (300 mg/mL) was added, and the tubes were incubated for 1 h in a water bath with constant shaking at 37 °C (Precision Scientific Mod. 66800; Winchester, VA, USA). At the end of the incubation period, 9.0 mL of phosphate solution (0.1 M, pH 7.5) was added, and the pH was adjusted to 7.5. In addition, 1 mL of pancreatin solution (17 mg/mL) and bile salts (80 mg) were added, and the mixture was incubated for 6 h in a shaking water bath (37 °C, 100 rpm) to obtain the intestinal fraction. For this digestion phase, a control was prepared that did not contain a cookie sample but that was subjected to the same simulated digestion conditions. All samples and the control from the intestinal phase were centrifuged (10 min, 1,500 x g, 4 °C), and the recovered supernatants were frozen at −80 °C and subsequently lyophilized. The lyophilized digests were redissolved in 50% methanol, filtered (Econofltr Nylon 0.25 mm 0.45 µm; Santa Clara, CA, United States), and stored at −20 °C in amber vials until analysis. The intestinal fractions were analyzed for TP, FA, and antioxidant capacity using the procedures described above. Bioaccessibility was calculated as the ratio of the concentration of the bioactive component in the intestinal digestion (supernatants) to its respective concentration in the cookies before digestion (Equation 1). The results are expressed as bioaccessibility percentages (B%).
2.9 Statistical analyses
The values are expressed as the means ± SE of three measurements. The data were analyzed by analysis of variance, and Tukey’s test (significance of differences p ≤ 0.05) was used to find significant differences between group means. Statistical analyses were performed using the JMP 5.0.1 program (SAS Institute, Inc., USA).
3 Results and discussion
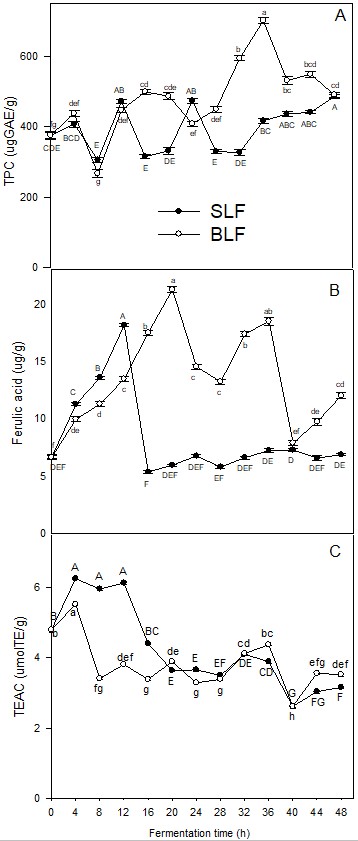
Table 1 shows the changes in pH values during the sorghum dough fermentation time. The pH of the BLF dough decreased in a shorter time than that of the SLF dough. BLF showed a significant pH decrease during the first 4 h of fermentation, whereas SLF dough showed a pH reduction at 12 h of fermentation. Neither of the two fermentation processes could reach pH values <4; however, it should be noted that several studies have shown that a fermentation process can be delayed when the food substrate to be fermented is a whole grain because the seed coat (pericarp), which functions as a grain coat protector, can inhibit the activity of some fermenting microorganisms, resulting in a slower pH decrease (Adebo et al., 2018Adebo, O. A., Njobeh, P. B., & Kayitesi, E. (2018). Fermentation by Lactobacillus fermentum strains (singly and in combination) enhances the properties of ting from two whole grain sorghum types. Journal of Cereal Science, 82, 49-56. http://dx.doi.org/10.1016/j.jcs.2018.05.008
http://dx.doi.org/10.1016/j.jcs.2018.05....
).
Effects of backslopping (BLF) and spontaneous (SLF) lactic acid fermentation time of sorghum doughs on pH.
It is relevant to mention that the change in pH values during the fermentation process is the main indicator of metabolic activity attributed to Lactic Acid Bacteria (LAB), where the products derived from the process may depend on the degree of adaptability of these bacteria to the conditions of the fermentation process. The sorghum grain shows wide variability in the content of TP and antioxidant activity, which has been attributed to the genotype of the grain and also to the type of extraction of phenolic compounds; in our study, the content of TP, FA, and antioxidant activity in whole sorghum flour before dough preparation was determined, the results were as follow: 377.05 ± 23.5 μgGAE/g, 8.05 ± 0.95 μg/g and 6.84 ± 0.18 μmTE/g for TP, FA and antioxidant activity, respectively. These results are within the range of values reported by other authors ranging from 100-9000 μg GAE/g for TP and from 1-180 μmTE/g for antioxidant activity (Dykes et al., 2005Dykes, L., Rooney, L. W., Waniska, R. D., & Rooney, W. L. (2005). Phenolic compounds and antioxidant activity of sorghum grains of varying genotypes. Journal of Agricultural and Food Chemistry, 53(17), 6813-6818. PMid:16104804. http://dx.doi.org/10.1021/jf050419e
http://dx.doi.org/10.1021/jf050419e...
, Salazar López et al., 2017; Ruiz Hernández et al., 2021). Figure 1 A shows the effects of lactic acid fermentation time in sorghum fermented doughs by the backslopping procedure (BLF) and by spontaneous fermentation (SLF) on TP. For both fermentation processes, the TP showed fluctuations during the first 28 hours. After this time, a significant increase was observed in the sorghum fermented doughs (p ≤ 0.05), reaching maximum values of 700.9 ± 7.6 μg GAE/g at 36 h in BLF samples and 484.79 ± 6.1 μg GAE/g in SLF at 48 h, corresponding to increases of 86% and 28% with respect to the unfermented dough (t = 0 h) (384.0 ± 5.7 μgGAE/g). Regarding changes in FA content during fermentation time and comparing with the unfermented sample, significant increases in FA were observed in the BLF and SLF samples during the first hours of fermentation. In the case of the dough with added inoculum (BLF), the FA content increased from 6.66 ± 0.23 µg/g to 21.25 ± 0.27 µg/g at 20 h of fermentation (+219%), whereas for the SLF samples, it reached a maximum FA concentration of 18.14 ± 0.12 µg/g at 12 h of fermentation (+172%). After these times, the concentration of FA decreased considerably in both processes but always maintained the highest values of this phenolic compound in the BLF samples (Figure 1B). Higher values of antioxidant capacity were observed for SLF samples, presenting maximum values at 4, 8 and 12 hours; after this time, the values decreased significantly. For the BLF samples, the antioxidant capacity showed its maximum value at 4 hours of fermentation (5.52±0.03 µmolTE/g); after this time, the activity decreased, showing a behavior very similar to that of the SLF samples, even reaching a value at the end of the fermentation process that was less than that presented in the unfermented dough (SCD). Various studies have shown that the fermentation process associated with LAB promotes structural changes in the food matrix. These changes lead to modifications in the contents of components such as proteins, fats, carbohydrates and other nonnutrient components such as phenolic compounds (Schober et al., 2007Schober, T. J., Bean, S. R., & Boyle, D. L. (2007). Gluten-free sorghum bread improved by sourdough fermentation: Biochemical, rheological, and microstructural background. Journal of Agricultural and Food Chemistry, 55(13), 5137-5146. PMid:17536829. http://dx.doi.org/10.1021/jf0704155
http://dx.doi.org/10.1021/jf0704155...
; Elkhalifa et al., 2005Elkhalifa, A. E. O., Schiffler, B., & Bernhardt, R. (2005). Effect of fermentation on the functional properties of sorghum flour. Food Chemistry, 92(1), 1-5. http://dx.doi.org/10.1016/j.foodchem.2004.05.058
http://dx.doi.org/10.1016/j.foodchem.200...
). These changes can be promoted by enzymes either present in the dough to be fermented or synthesized by the microorganisms themselves (Liukkonen et al., 2003Liukkonen, K. H., Katina, K., Wilhelmsson, A., Myllymaki, O., Lampi, A. M., Kariluoto, S., Piironen, V., Heinonen, S. M., Nurmi, T., Adlercreutz, H., Peltoketo, A., Pihlava, J. M., Hietaniemi, V., & Poutanen, K. (2003). Process-induced changes on bioactive compounds in whole grain rye. The Proceedings of the Nutrition Society, 62(1), 117-122. PMid:12740066. http://dx.doi.org/10.1079/PNS2002218
http://dx.doi.org/10.1079/PNS2002218...
). During the fermentation process, fluctuations in the contents of total and individual phenols and the antioxidant capacity in grains in sorghum and in other cereals, such as wheat, corn, quinoa, and rye, have been observed (Mohapatra et al., 2019Mohapatra, D., Patel, A. S., Kar, A., Deshpande, S. S., & Tripathi, M. K. (2019). Effect of different processing conditions on proximate composition, anti-oxidants, anti-nutrients and amino acid profile of grain sorghum. Food Chemistry, 271, 129-135. PMid:30236657. http://dx.doi.org/10.1016/j.foodchem.2018.07.196
http://dx.doi.org/10.1016/j.foodchem.201...
; Adebo et al., 2018Adebo, O. A., Njobeh, P. B., & Kayitesi, E. (2018). Fermentation by Lactobacillus fermentum strains (singly and in combination) enhances the properties of ting from two whole grain sorghum types. Journal of Cereal Science, 82, 49-56. http://dx.doi.org/10.1016/j.jcs.2018.05.008
http://dx.doi.org/10.1016/j.jcs.2018.05....
; Dordević et al., 2010Dordević, T. M., Šiler-Marinković, S. S., & Dimitrijević-Branković, S. I. (2010). Effect of fermentation on antioxidant properties of some cereals and pseudo cereals. Food Chemistry, 119(3), 957-963. http://dx.doi.org/10.1016/j.foodchem.2009.07.049
http://dx.doi.org/10.1016/j.foodchem.200...
; Dlamini et al., 2007Dlamini, N. R., Taylor, J. R. N., & Rooney, L. W. (2007). The effect of sorghum type and processing on the antioxidant properties of African sorghum-based foods. Food Chemistry, 105(4), 1412-1419. http://dx.doi.org/10.1016/j.foodchem.2007.05.017
http://dx.doi.org/10.1016/j.foodchem.200...
; Katina et al., 2007Katina, K., Laitila, A., Juvonen, R., Liukkonen, K. H., Kariluoto, S., Piironen, V., Landberg, R., Åman, P., & Poutanen, K. (2007). Bran fermentation as a means to enhance technological properties and bioactivity of rye. Food Microbiology, 24(2), 175-186. PMid:17008162. http://dx.doi.org/10.1016/j.fm.2006.07.012
http://dx.doi.org/10.1016/j.fm.2006.07.0...
), which are attributed to various factors, such as the type and cultivar of sorghum to be fermented, the fermentation conditions, and the type of fermentation, among others. The times at which increases in TP, FA and antioxidant capacity in fermented sorghum were observed can be attributed to various mechanisms, such as enzymatic activity of microorganisms (β-glucosidase, decarboxylase, esterases, hydrolase, reductase, among others), cell wall rupture, increased extractability after fermentation, mobilization/release of phenols bound to free forms, and can also be attributed to the synthesis/release of new bioactive compounds. With the above results, it was possible to obtain a multipurpose flour from fermented sorghum doughs that possesses biological potential for the benefit of health maintenance. We selected four formulations for cookie production: The first two formulations corresponded to those with higher content of TP, labeled BLFC36 h and SLFC 48 h; and the last two corresponded to those with highest contents of FA, BLFC20 h and SLFC12 h. Additionally, cookies were formulated from unfermented sorghum powdered dough (CSC) as a control. The TP in the experimental cookies of the BLFC group (before intestinal digestion) did not show significant differences compared to the control cookies (CSC) (p > 0.05). The SLFC cookies presented higher values of TP compared to BLFC and CSC, with the SLFC (48 h) showing a higher content in TP (1344.12 ± 26.1 µg GAE/g), surpassing the CSC cookies by 30%. The FA content increased significantly in all the experimental cookies. FA values ranged from 22.30 ± 0.10 to 26.97 ± 1.70 µg/g. These contents surpassed the approximately 250% quantified in CSCs (Table 2). A study carried out by Michalska et al. (2008)Michalska, A., Amigo-Benavent, M., Zielinski, H., & del Castillo, M. D. (2008). Effect of bread making on formation of Maillard reaction products contributing to the overall antioxidant capacityof rye bread. Journal of Cereal Science, 48(1), 123-132. http://dx.doi.org/10.1016/j.jcs.2007.08.012
http://dx.doi.org/10.1016/j.jcs.2007.08....
attributed the increase in TP in breads formulated from rye flour to the products of the Maillard reaction. This is because these products positively affect the reaction of Folin. However, it is also possible that other events attributed to heat treatment in addition to the Maillard reactions have contributed to the increase in TP and FA, as in the study reported by Dewanto et al. (2002)Dewanto, V., Wu, X., & Liu, R. H. (2002). Processed sweet corn has higher antioxidant activity. Journal of Agricultural and Food Chemistry, 50(17), 4959-4964. PMid:12166989. http://dx.doi.org/10.1021/jf0255937
http://dx.doi.org/10.1021/jf0255937...
, who evaluated the effect of the sweet corn cooking process on the contents of total free phenols and FA. They found that at a cooking temperature of 121 °C/25 min, TP and FA in their free form increased as they decreased in their bound fraction compared to corn without processing. Hithamani & Srinivasan (2014)Hithamani, G., & Srinivasan, K. (2014). Effect of domestic processing on the polyphenol content and bioaccessibility in finger millet (Eleusine coracana) and pearl millet (Pennisetum glaucum). Food Chemistry, 164, 55-62. PMid:24996305. http://dx.doi.org/10.1016/j.foodchem.2014.04.107
http://dx.doi.org/10.1016/j.foodchem.201...
carried out a study on the effects of the application of heat treatments in sorghum on phenolic compounds. Heat treatments such as roasting, pressure cooking, no pressure cooking and microwave heating promoted significant increases in FA, while only roasting (150 °C/20 min) promoted up to a 50% increase in total phenols. The increases were attributed to the release of bound phenols from the cell wall, degradation of conjugated polyphenols and the increasing extractability of phenols after roasting. By another hand, regarding to antioxidant activity in cookies, it is relevant to mention the antioxidant components provided by the white margarine added to the cookie formulation (Vitamins A and E), it did not affect the antioxidant activity values reported for the cookies before and after the intestinal digestion process. This process could have been attributed mainly because the extraction was directed toward water-soluble components and not lipophilic. The values reported as methanolic fractions (before digestion) will not necessarily reflect what may happen when this product is subjected to an in vitro gastrointestinal digestion process since the digestive conditions exert other types of reactions that are very different from those that occur in a chemical extraction, which can lead to the phenolic compounds that were accessible in the food matrix no longer being detected, possibly attributed to their degradation or interaction with other components that prevent their release. Moreover, it is also possible that the digestion conditions can significantly favor the release of phenolic compounds; that is, the enzymatic processes and changes in pH can promote the release of phenolic compounds that were bound in the sample before digestion or that were conjugated or physically entrapped in the food matrix. In accordance with the aforementioned issue, for this study, the effects of the in vitro gastrointestinal digestion process of 1 g of each of the cookies on the contents of TP and FA and on the antioxidant capacity were evaluated. It is worth mentioning that the complete gastrointestinal digestion test was performed to obtain intestinal digests, which are considered the most interesting, since it is at this stage of digestion that the maximum absorption of the digestion products of food occurs (Shahidi & Peng, 2018Shahidi, F., & Peng, H. (2018). Bioaccessibility and bioavailability of phenolic compounds. Journal of Food Bioactives, 4, 11-68. http://dx.doi.org/10.31665/JFB.2018.4162
http://dx.doi.org/10.31665/JFB.2018.4162...
). In Table 2, it is shown that the intestinal digestion process promoted important changes in the contents of TP and FA and in the antioxidant capacity in the cookies; in particular, the TP increased significantly in the digests of the experimental cookies, including the cookie control. The total phenol content in the SLFC (48 h and 12 h) cookie digests was higher than that found in the BLFC (36 h and 20 h) cookie digests. The content of FA present in the digests of the evaluated cookies showed a drastic reduction compared to the samples before digestion. However, the FA content was significantly higher in all the digests of the experimental cookies compared to CSC, with the highest values being observed in cookie digests BLFC20 h, SLFC48 h and SLFC12 h. It is known that the type of food and its processing are two of the main factors that affect the type and phenolic compound content in food. Once ingested, the health benefits derived from phenolic compounds depend on the degree to which they are released from the matrix, absorbed from the gastrointestinal tract, and available for metabolism. Bioaccessibility is the fraction of the compound that is released from the matrix during digestion and that is available for absorption in the small intestine) and the calculation of bioaccessibility in percentage terms can be an indicator of the amount of a bioactive compound that can potentially be absorbed from the intestine (Ribas Agustí et al., 2018Ribas Agustí, A., Martín Belloso, O., Soliva Fortuny, R., & Elez Martínez, P. (2018). Food processing strategies to enhance phenolic compounds bioaccessibility and bioavailability in plant-based foods. Critical Reviews in Food Science and Nutrition, 58(15), 2531-2548. PMid:28609142. http://dx.doi.org/10.1080/10408398.2017.1331200
http://dx.doi.org/10.1080/10408398.2017....
; Alminger et al., 2014Alminger, M., Aura, A. M., Bohn, T., Dufour, C., El, S. N., Gomes, A., Karakaya, S., Martínez-Cuesta, M. C., McDougall, G. J., Requena, T., & Santos, C. N. (2014). In vitro models for studying secondary plant metabolite digestion and bioaccessibility. Comprehensive Reviews in Food Science and Food Safety, 13(4), 413-436. PMid:33412708. http://dx.doi.org/10.1111/1541-4337.12081
http://dx.doi.org/10.1111/1541-4337.1208...
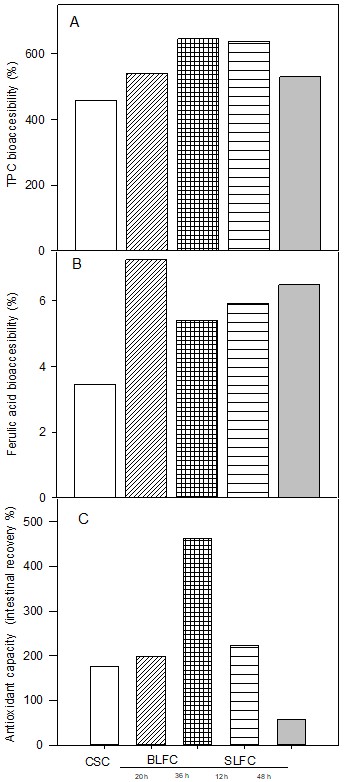
). Particularly when the concentrations of phenolic compounds present in the food matrix prior to digestion are different for each sample evaluated, as in the case of our study, it is necessary to standardize the values in terms of percentage in such a way that the concentrations of both TP and FA and the antioxidant capacity present in the original sample are taken as 100%, that is, the maximum percentage that can be extracted from these components in their free form. Figure 2A shows the bioaccessibility results of the TP present in CSC and the experimental cookies. It is first observed that all the cookies evaluated, including the CSC, exceeded 100% bioaccessibility of TP, that is, that the gastrointestinal digestion process promoted the release of these compounds at a higher percentage than that reported in the samples before digestion. Regarding the effect of the inclusion in the formulation of sorghum doughs subjected to lactic acid fermentation, it was observed that these cookies exceeded the bioaccessibility percentage of the control cookie (CSC). The BLFC36 h cookies showed the highest percentages of bioaccessibility of TP (646%). Regarding the bioaccessibility of FA in cookies (Figure 2B), none of the cookies formulated with BLF samples nor the control cookie managed to exceed 100% bioaccessibility of FA compared to the cookies measured before the process of digestion. However, despite the low values of bioaccessibility, a positive effect was observed in that the BLFC and SLFC cookies showed higher values of bioaccessibility than the control cookie. A presumed mechanism for the fermentation-induced increase in bioaccessibility of phenolic compounds could be that the degrading enzymes present in both grains and microorganisms resulted in the structural breakdown of the cell wall matrix, which increased the accessibility of bound and conjugated phenolic compounds at digestive enzymatic attack (Dordević et al., 2010Dordević, T. M., Šiler-Marinković, S. S., & Dimitrijević-Branković, S. I. (2010). Effect of fermentation on antioxidant properties of some cereals and pseudo cereals. Food Chemistry, 119(3), 957-963. http://dx.doi.org/10.1016/j.foodchem.2009.07.049
http://dx.doi.org/10.1016/j.foodchem.200...
). Bioactive compounds such as phenols exert different effects for the benefit of human health, and the mechanisms to which these effects can be attributed range from antimutagenic, anticancer, and anti-inflammatory activities, among others. One of the most widely studied mechanisms is antioxidant activity, that is, its ability to donate a hydrogen atom or an electron to a free radical and thereby inhibit the oxidation of biologically important oxidizable substrates such as lipids, proteins, carbohydrates, and DNA. In Figure 2C, the results of the intestinal recovery of the antioxidant capacity present in the cookies were determined in the same way as those determined for TP and FA; that is, the value of antioxidant capacity found in the cookie samples before digestion was considered 100%. Moreover, the cookies BLFC (20 h and 36 h) and SLFC (12 h) shower higher intestinal antioxidant capacity recovery than SLFC (48 h); these values were also higher than that presented by control cookies.
Effect of backslopping and spontaneous fermentation on TPC (A), ferulic acid (B) and antioxidant capacity (C) of doughs from whole sorghum grain.
Contents of Total Phenols (TP), Ferulic Acid (FA) and TEAC in cookies from fermented sorghum doughs before and after simulation in vitro gastrointestinal digestion1.
Effects of backslopping (BLFC) and spontaneous SLFC) fermentation on the bioaccessibility of TPC (A), ferulic acid (B) and intestinal antioxidant capacity recovery (C) of cookies from whole sorghum grain.
4 Conclusions
The fermentation processes significantly increased the contents of TP and FA compared with the unfermented doughs, and the highest values corresponded to the doughs treated by lactic acid fermentation (backslopping). Compared to spontaneous fermentation (SLF), fermentation with added inoculum (backslopping) required a shorter dough fermentation time to obtain the highest values of TP, as well as a longer fermentation time to achieve the highest content of FA. The treatments of the different fermentation processes were able to increase the bioaccessibility of FA for all the experimental cookies since all the values were always higher than those of the control cookie.
Acknowledgements
Valenzuela Gutierrez, J.L. received a scholarship from CONACyT (National Research and Technology Council), Mexico.
-
Cite as: Valenzuela Gutiérrez, J. L., Rouzaud Sández, O., González Aguilar, G., López Ahumada, G. A., & Robles Sánchez, M. (2022). Effect of fermentation on biological functionality of cookies from fermented whole grain sorghum. Brazilian Journal of Food Technology, 25, e2021152. https://doi.org/10.1590/1981-6723.15221
-
Funding: None.
References
- Acosta Estrada, B. A., Gutiérrez Uribe, J. A., & Serna Saldívar, S. O. (2014). Bound phenolics in foods, a review. Food Chemistry, 152, 46-55. PMid:24444905. http://dx.doi.org/10.1016/j.foodchem.2013.11.093
» http://dx.doi.org/10.1016/j.foodchem.2013.11.093 - Adebo, O. A. (2020). African sorghum-based fermented foods: Past, current and future prospects. Nutrients, 12(4), 1111. PMid:32316319. http://dx.doi.org/10.3390/nu12041111
» http://dx.doi.org/10.3390/nu12041111 - Adebo, O. A., Njobeh, P. B., & Kayitesi, E. (2018). Fermentation by Lactobacillus fermentum strains (singly and in combination) enhances the properties of ting from two whole grain sorghum types. Journal of Cereal Science, 82, 49-56. http://dx.doi.org/10.1016/j.jcs.2018.05.008
» http://dx.doi.org/10.1016/j.jcs.2018.05.008 - Alminger, M., Aura, A. M., Bohn, T., Dufour, C., El, S. N., Gomes, A., Karakaya, S., Martínez-Cuesta, M. C., McDougall, G. J., Requena, T., & Santos, C. N. (2014). In vitro models for studying secondary plant metabolite digestion and bioaccessibility. Comprehensive Reviews in Food Science and Food Safety, 13(4), 413-436. PMid:33412708. http://dx.doi.org/10.1111/1541-4337.12081
» http://dx.doi.org/10.1111/1541-4337.12081 - Amaya Villalva, M. F., González-Aguilar, G., Sández, O. R., Astiazarán García, H., Ledesma Osuna, A. I., López-Ahumada, G. A., & Robles-Sánchez, R. M. (2018). Bioprocessing of wheat (Triticum aestivum cv. Kronstad) bran from Northwest Mexico: Effects on ferulic acid bioaccessibility in breads. CYTA: Journal of Food, 16(1), 570-579. http://dx.doi.org/10.1080/19476337.2018.1440007
» http://dx.doi.org/10.1080/19476337.2018.1440007 - American Association of Cereal Chemists – AACC. (2000). Approved methods of analysis (10th ed.). St. Paul: AACC International.
- Dewanto, V., Wu, X., & Liu, R. H. (2002). Processed sweet corn has higher antioxidant activity. Journal of Agricultural and Food Chemistry, 50(17), 4959-4964. PMid:12166989. http://dx.doi.org/10.1021/jf0255937
» http://dx.doi.org/10.1021/jf0255937 - Dlamini, N. R., Taylor, J. R. N., & Rooney, L. W. (2007). The effect of sorghum type and processing on the antioxidant properties of African sorghum-based foods. Food Chemistry, 105(4), 1412-1419. http://dx.doi.org/10.1016/j.foodchem.2007.05.017
» http://dx.doi.org/10.1016/j.foodchem.2007.05.017 - Dordević, T. M., Šiler-Marinković, S. S., & Dimitrijević-Branković, S. I. (2010). Effect of fermentation on antioxidant properties of some cereals and pseudo cereals. Food Chemistry, 119(3), 957-963. http://dx.doi.org/10.1016/j.foodchem.2009.07.049
» http://dx.doi.org/10.1016/j.foodchem.2009.07.049 - Dykes, L., Rooney, L. W., Waniska, R. D., & Rooney, W. L. (2005). Phenolic compounds and antioxidant activity of sorghum grains of varying genotypes. Journal of Agricultural and Food Chemistry, 53(17), 6813-6818. PMid:16104804. http://dx.doi.org/10.1021/jf050419e
» http://dx.doi.org/10.1021/jf050419e - Elkhalifa, A. E. O., Schiffler, B., & Bernhardt, R. (2005). Effect of fermentation on the functional properties of sorghum flour. Food Chemistry, 92(1), 1-5. http://dx.doi.org/10.1016/j.foodchem.2004.05.058
» http://dx.doi.org/10.1016/j.foodchem.2004.05.058 - Girard, A. L., & Awika, J. M. (2018). Sorghum polyphenols and other bioactive components as functional and health promoting food ingredients. Journal of Cereal Science, 84, 112-124. http://dx.doi.org/10.1016/j.jcs.2018.10.009
» http://dx.doi.org/10.1016/j.jcs.2018.10.009 - Hithamani, G., & Srinivasan, K. (2014). Effect of domestic processing on the polyphenol content and bioaccessibility in finger millet (Eleusine coracana) and pearl millet (Pennisetum glaucum). Food Chemistry, 164, 55-62. PMid:24996305. http://dx.doi.org/10.1016/j.foodchem.2014.04.107
» http://dx.doi.org/10.1016/j.foodchem.2014.04.107 - Katina, K., Laitila, A., Juvonen, R., Liukkonen, K. H., Kariluoto, S., Piironen, V., Landberg, R., Åman, P., & Poutanen, K. (2007). Bran fermentation as a means to enhance technological properties and bioactivity of rye. Food Microbiology, 24(2), 175-186. PMid:17008162. http://dx.doi.org/10.1016/j.fm.2006.07.012
» http://dx.doi.org/10.1016/j.fm.2006.07.012 - Liukkonen, K. H., Katina, K., Wilhelmsson, A., Myllymaki, O., Lampi, A. M., Kariluoto, S., Piironen, V., Heinonen, S. M., Nurmi, T., Adlercreutz, H., Peltoketo, A., Pihlava, J. M., Hietaniemi, V., & Poutanen, K. (2003). Process-induced changes on bioactive compounds in whole grain rye. The Proceedings of the Nutrition Society, 62(1), 117-122. PMid:12740066. http://dx.doi.org/10.1079/PNS2002218
» http://dx.doi.org/10.1079/PNS2002218 - Mateo Anson, N., van den Berg, R., Havenaar, R., Bast, A., & Haenen, G. R. M. M. (2009). Bioavailability of ferulic acid is determined by its bioaccessibility. Journal of Cereal Science, 49(2), 296-300. http://dx.doi.org/10.1016/j.jcs.2008.12.001
» http://dx.doi.org/10.1016/j.jcs.2008.12.001 - Michalska, A., Amigo-Benavent, M., Zielinski, H., & del Castillo, M. D. (2008). Effect of bread making on formation of Maillard reaction products contributing to the overall antioxidant capacityof rye bread. Journal of Cereal Science, 48(1), 123-132. http://dx.doi.org/10.1016/j.jcs.2007.08.012
» http://dx.doi.org/10.1016/j.jcs.2007.08.012 - Mohapatra, D., Patel, A. S., Kar, A., Deshpande, S. S., & Tripathi, M. K. (2019). Effect of different processing conditions on proximate composition, anti-oxidants, anti-nutrients and amino acid profile of grain sorghum. Food Chemistry, 271, 129-135. PMid:30236657. http://dx.doi.org/10.1016/j.foodchem.2018.07.196
» http://dx.doi.org/10.1016/j.foodchem.2018.07.196 - Polonskiy, V., Loskutov, I., & Sumina, A. (2020). Biological role and health benefits of antioxidant compounds in cereals. Biological Communications, 65(1), 53-67. https://doi.org/10.21638/spbu03.2020.105
» https://doi.org/10.21638/spbu03.2020.105 - Rashwan, A. K., Yones, H. A., Karim, N., Taha, E. M., & Chen, W. (2021). Potential processing technologies for developing sorghum-based food products: An update and comprehensive review. Trends in Food Science & Technology, 110, 168-182. http://dx.doi.org/10.1016/j.tifs.2021.01.087
» http://dx.doi.org/10.1016/j.tifs.2021.01.087 - Ribas Agustí, A., Martín Belloso, O., Soliva Fortuny, R., & Elez Martínez, P. (2018). Food processing strategies to enhance phenolic compounds bioaccessibility and bioavailability in plant-based foods. Critical Reviews in Food Science and Nutrition, 58(15), 2531-2548. PMid:28609142. http://dx.doi.org/10.1080/10408398.2017.1331200
» http://dx.doi.org/10.1080/10408398.2017.1331200 - Ruiz-Hernández, A. A., Cárdenas-López, J. L., Cortez-Rocha, M. O., González-Aguilar, G. A., & Robles-Sánchez, R. M. (2021). Optimization of germination of white sorghum by response surface methodology for preparing porridges with biological potential. CYTA: Journal of Food, 19(1), 49-55. http://dx.doi.org/10.1080/19476337.2020.1853814
» http://dx.doi.org/10.1080/19476337.2020.1853814 - Salazar-López, N. J., Loarca-Piña, G., Campos-Vega, R., Gaytán Martínez, M., Morales Sánchez, E., Esquerra-Brauer, J. M., Gonzalez-Aguilar, G. A., & Robles Sánchez, M. (2016). The extrusion process as an alternative for improving the biological potential of sorghum bran: Phenolic compounds and antiradical and anti-Inflammatory capacity. Evidence-Based Complementary and Alternative Medicine, 2016, 8387975. PMid:27738445. http://dx.doi.org/10.1155/2016/8387975
» http://dx.doi.org/10.1155/2016/8387975 - Salazar-López, N. J., González-Aguilar, G. A., Loarca-Piña, G., Cinco-Moroyoqui, F. J., Rouzaud-Sández, O., Domínguez-Avila, J. A., & Robles-Sánchez, M. (2017). Contribution and interactions of hydroxycinnamic acids found in bran and wholegrain sorghum (Sorghum bicolor L. Moench): Effects on the antioxidant capacity and inhibition of human erythrocyte hemolysis. Oxidative Medicine and Cellular Longevity, 2017, 8219023. PMid:29158873. http://dx.doi.org/10.1155/2017/8219023
» http://dx.doi.org/10.1155/2017/8219023 - Salazar-López, N. J., González Aguilar, G. A., Rouzaud Sández, O., & Robles Sánchez, M. (2018). Bioaccessibility of hydroxycinnamic acids and antioxidant capacity from sorghum bran thermally processed during simulated in vitro gastrointestinal digestion. Journal of Food Science and Technology, 55(6), 2021-2030. PMid:29892102. http://dx.doi.org/10.1007/s13197-018-3116-z
» http://dx.doi.org/10.1007/s13197-018-3116-z - Schober, T. J., Bean, S. R., & Boyle, D. L. (2007). Gluten-free sorghum bread improved by sourdough fermentation: Biochemical, rheological, and microstructural background. Journal of Agricultural and Food Chemistry, 55(13), 5137-5146. PMid:17536829. http://dx.doi.org/10.1021/jf0704155
» http://dx.doi.org/10.1021/jf0704155 - Shahidi, F., & Peng, H. (2018). Bioaccessibility and bioavailability of phenolic compounds. Journal of Food Bioactives, 4, 11-68. http://dx.doi.org/10.31665/JFB.2018.4162
» http://dx.doi.org/10.31665/JFB.2018.4162 - Singleton, V. L., & Rossi, J. A. (1965). Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture, 16(3), 144-158.
- Taylor, J., & Taylor, J. R. (2002). Alleviation of the adverse effect of cooking on sorghum protein digestibility through fermentation in traditional African porridges. International Journal of Food Science & Technology, 37(2), 129-137. http://dx.doi.org/10.1046/j.1365-2621.2002.00549.x
» http://dx.doi.org/10.1046/j.1365-2621.2002.00549.x - Tieri, M., Ghelfi, F., Vitale, M., Vetrani, C., Marventano, S., Lafranconi, A., Godos, J., Titta, L., Gambera, A., Alonzo, E., Sciacca, S., Riccardi, G., Buscemi, S., Del Rio, D., Ray, S., Galvano, F., Beck, E., & Grosso, G. (2020). Whole grain consumption and human health: An umbrella review of observational studies. International Journal of Food Sciences and Nutrition, 71(6), 668-677. PMid:31964201. http://dx.doi.org/10.1080/09637486.2020.1715354
» http://dx.doi.org/10.1080/09637486.2020.1715354 - Xiong, Y., Zhang, P., Warner, R. D., & Fang, Z. (2019). Sorghum grain: From genotype, nutrition, and phenolic profile to its health benefits and food applications. Comprehensive Reviews in Food Science and Food Safety, 18(6), 2025-2046. PMid:33336966. http://dx.doi.org/10.1111/1541-4337.12506
» http://dx.doi.org/10.1111/1541-4337.12506
Edited by
Publication Dates
-
Publication in this collection
18 July 2022 -
Date of issue
2022
History
-
Received
04 Oct 2021 -
Accepted
12 May 2022