Abstract
The anthelminthic activity of the essential oil (EO) of Piper aduncum L. was tested in vitro on eggs and larvae of resistant (Embrapa2010) and susceptible (McMaster) isolates of Haemonchus contortus. The EO was obtained by steam distillation and its components identified by chromatography. EO concentrations of 12.5 to 0.02 mg/mL were used in the egg hatch test (EHT) and concentrations of 3.12 to 0.01 mg/mL in the larval development test (LDT). Inhibition concentrations (IC) were determined by the SAS Probit procedure, and significant differences assessed by ANOVA followed by Tukey’s test. In the EHT, the IC50 for the susceptible isolate was 5.72 mg/mL. In the LDT, the IC50 and IC90 were, respectively, 0.10 mg/mL and 0.34 mg/mL for the susceptible isolate, and 0.22 mg/mL and 0.51 mg/mL for the resistant isolate. The EO (dillapiole 76.2%) was highly efficacious on phase L1. Due to the higher ICs obtained for the resistant isolate, it was raised the hypothesis that dillapiole may have a mechanism of action that resembles those of other anthelmintic compounds. We further review and discuss studies, especially those conducted in Brazil, that quantified the major constituents of P. aduncum-derived EO.
Keywords:
Isolates; Haemonchus contortus; cross resistance; dillapiole; synergy
Resumo
Este estudo avaliou a atividade anti-helmíntica in vitro do óleo essencial (OE) de Piper aduncum L. sobre ovos e larvas de Haemonchus contortus, verificando se um isolado resistente (Embrapa2010), apresentaria o mesmo comportamento que um sensível (McMaster). O OE foi obtido por arraste a vapor e analisado por cromatografia para identificação dos constituintes. O óleo foi avaliado nas concentrações de 12,5 a 0,02 mg/mL no Teste de eclosão dos ovos (TEO) e nas concentrações de 3,12 a 0,01 mg/mL no Teste de desenvolvimento larvar (TDL). As concentrações inibitórias (CI) foram determinadas pelo procedimento Probit do SAS e as diferenças estatísticas geradas pela ANOVA seguida pelo teste de Tukey. Para o isolado sensível obteve-se CI50 de 5,72 mg/mL no TEO. No TDL o óleo apresentou CI50 e CI90 de 0,10 mg/mL e 0,34 mg/mL para o isolado sensível e 0,22 mg/mL e 0,51 mg/mL para o resistente, respectivamente. Demonstrou-se que o OE (dilapiol 76,2%) teve alta eficácia sobre a fase L1. Devido às elevadas CIs obtidas para o isolado resistente, levantou-se a hipótese de que o dilapiol talvez possua um mecanismo de ação semelhante a algum grupo anti-helmíntico. O artigo faz uma revisão e discute estudos de quantificação dos constituintes majoritários do OE de P. aduncum, destacando os realizados no Brasil.
Palavras-chave:
Isolados; Haemonchus contortus; resistência cruzada; dilapiol; sinergismo
Introduction
The nematode Haemonchus contortus parasitizes the gastrointestinal tract of ruminants, causing anemia, weight loss, and, in severe infections, death (URQUHART et al., 1998). Haemonchus infections affect hundreds of millions of small ruminants worldwide causing estimated losses of US$ 10 billion per year (WALLER & CHANDRAWATHANI, 2005; ROEBER et al., 2013). The use of synthetic anthelmintic substances constitutes the main course of action against gastrointestinal parasitism (FREEMAN et al., 2003). However, resistance to currently available anthelmintic drugs drastically reduces the effects of parasite control programs (JAMES et al., 2009; SUTHERLAND & LEATHWICK, 2011). Therefore, phytotherapeutic strategies have become the target of researchers aiming at developing environmentally-friendly tools for the control of nematodes in small ruminants.
Secondary metabolites probably mediate many of the biological activities of medicinal plants. These metabolites have become an alternative treatment, especially in organic farming where regulation forbids the routine use of synthetic anthelmintic drugs (CHAGAS, 2004; LISONBEE et al., 2009). Among such compounds, essential oils (EOs) represent an important category. They can be obtained from hydro- or dry distillation of plant material (ISO, 2013), and consist of a mixture of volatile, lipophilic, often odoriferous and liquid substances. Interaction among these substances may interfere in nematode metabolism, inhibiting and disorganizing vital functions since the early stages of development (OKA et al., 2000).
Some plant species, when processed, yield large amounts of EOs, especially those in the Apiaceae, Asteraceae, Cupressaceae, Hypericaceae, Clusiaceae, Lamiaceae, Lauraceae, Fabaceae, Liliaceae, Myrtaceae, Pinaceae, Piperaceae, Rosaceae, Rutaceae, Santalaceae, Zingiberaceae and Zygophyllaceae families (HUSNU et al., 2007; FIGUEIREDO et al., 2008). Specifically, Piperaceae family EOs have been tested in many different contexts worldwide (GUPTA, 1995; OVIEDO-RONDON et al., 2006; SRINIVASAN, 2007; SCOTT et al., 2008; GUERRINI et al., 2009; SILVA et al., 2009; SINGH et al., 2009; PINO et al., 2011; ARAUJO et al., 2012). The biological activity of the Piper genus, native to tropical and subtropical regions (JARAMILLO & MANOS, 2001), has been more extensively studied. Within this genus, the spiked pepper Piper aduncum L. produces a biodegradable EO with high commercial value due to its fungicidal, bactericidal, molluscicidal, acaricidal, insecticidal and larvicidal activities (MAIA et al., 1998). The species has a high tolerance to different weather conditions and can be cultured in a wide range of areas (LORENZI & MATOS, 2002; SOUSA et al., 2008). Finally, spiked pepper cultures may represent a crucial stepping stone for regional economic development based on a renewable source of chemical raw materials, which can be processed in large or small scales (FAZOLIN et al., 2006).
The distinct biological activities attributed to the varying chemical composition of P. aduncum EO fuel studies on the potential veterinary applications of this species. Thus, the present work aims at assessing the in vitro anthelmintic activity of P. aduncum EO on eggs and larvae of resistant and susceptible isolates of H. contortus. Because these parasites had never been exposed to the plant extract, we assessed if the resistant isolate would present similar response that of susceptible isolate.
Materials and Methods
P. aduncum cultivation and EO extraction
P. aduncum plants were cultivated at Embrapa Amazônia Ocidental, located in the city of Manaus, state of Amazonas, Brazil, in 2014. The cultivation took place in field plots with soil classified as Dystrophic Yellow Latosol, which was fertilized with chicken manure (1.0 kg m2) added before cultivation. The field plots were located at the geographic coordinates 03º 06’ 23.04” S and 60º 01’ 35.14” W, at an average altitude of 50 m, average temperature of 25.6 °C, and average annual rainfall of 2,200 mm. The equatorial climate is characterized as Af, according to the world map of the Köppen-Geiger climate classification. Samples of P. aduncum were deposited in the EAFM herbarium of the Amazon Federal Institute under the number 10.480.
After harvest, in July 2014, leaves were separated from branches and shade-dried, in a barn. After drying for one week, leaves were taken to the laboratory where extraction was conducted by hydro-distillation in a modified Clevenger-type apparatus.
EO chromatographic analyses
Chemical composition analysis of the EO was conducted at Embrapa Agroindústria de Alimentos, Rio de Janeiro, Brazil, with gas chromatography coupled to mass spectrometry (GC-MS) in an Agilent 5973 N system (Agilent Technologies, DE, USA) equipped with a HP-5MS capillary column (5%-diphenyl, 95%-dimethyl-silicone, 30 m × 0.25 mm; film thickness 0.25 μm). Helium was used as the carrier gas (1.0 mL min-1). A total volume of 1.0 μL of a 1% solution of the oil in dichloromethane was injected into an injector heated to 250 °C, operating in split mode (split ratio 1:100). Oven temperature was raised from 60 °C to 240 °C, at a rate of 3 °C min-1. The mass detector was operated in electronic ionization mode (70 eV) with the mass analyzer maintained at 150 °C, the ionization source at 220 °C, and the transfer line at 260 °C. Linear retention indices were calculated by injection of a series of n-alkanes (C7-C26) in the same column and conditions as above. Identification of the essential oil components was done by comparison of both mass spectra and retention indices with the Wiley sixth edition spectral database and other data from the literature (ADAMS, 2007).
For quantification, the essential oil was analyzed in an Agilent 7890A chromatograph equipped with a flame ionization detector (FID) kept at 280 °C and fitted with an HP-5MS capillary column (5% diphenyl-95%-dimethyl-silicone; 30 m× 0.25 mm; film thickness 0.25 μm). The same injection and chromatographic conditions described above were applied, but hydrogen was used as the carrier gas at 1.5 mL min-1. Results were expressed as relative area (percentage area).
Donor animals and isolates of H. contortus
Four Santa Inês animals were obtained from the ovine herd of Embrapa Pecuária Sudeste (CPPSE), São Carlos/SP. These animals had between three and four months of age, and weighed an average of 30 kg. They were initially treated with Zolvix® (monepantel, 2.5 mg/kg body weight) for the removal of natural parasitic infections. Seven and 14 days after deworming, we performed fecal counts of eggs per gram (EPG) to be sure the animals were clean of nematodes. Fecal material was collected directly from the rectum and eggs were enumerated using the McMaster x 50 technique (ROBERTS & O'SULLIVAN, 1950). Then, two animals were mono-specifically infected with 4000 L3 of the isolate H. contortus McMaster and the other two with 4000 L3 the isolate H. contortus Embrapa2010. Each pair of animals was placed in separate stalls, supplemented ad libitum with corn silage, water and mineral salt. All procedures involving donor animals were approved by the Committee on Animal Research and Ethics of the CPPSE (Protocol no. 06/2012).
H. contortus McMaster displays susceptibility to numerous chemical groups and has no history of exposure to anthelminthic agents (GILL et al., 1995). H. contortus Embrapa2010 was originally isolated from the CPPSE herd, and cryopreserved since 2006. The isolate was registered in the anthelmintic resistance consortium SNPs-parasite isolate database (CARS). It has displayed resistance to benzimidazole (20% efficacy), ivermectin (52%), moxidectin (85%), levamisole (93%) and high susceptibility to closantel (100%) and monepantel (100%) (CHAGAS et al., 2013).
In vitro tests
The P. aduncum EO was assessed in the egg hatch test (EHT) in 12 concentrations (0.02, 0.04, 0.09, 0.19, 0.39, 0.78, 1.56, 3.12, 6.25, 12.5, 25 and 50 mg/mL) and in the larval development test (LDT) in seven concentrations (0.04, 0.09, 0.19, 0.39, 0.78, 1.56 and 3.12 mg/mL). To improve EO emulsification in water, Tween 80 at 2% was added in the EHT and DMSO at 0.5% in the LDT and the solutions were homogenized in a vortex mixer until oil, solvent and water formed a stable emulsion.
Recovery of H. contortus eggs
Fresh feces from the donor animals described above were obtained for the isolation of nematode eggs, according to the method described by Coles et al. (1992), and adapted by Chagas et al. (2011). In summary, approximately 5 g of feces, directly collected from the rectum, were mixed with tepid water (37 °C) and filtered through sieves with mesh sizes of 1 mm, 106 µm, 53 µm and 25 µm. The last and finer mesh retained the eggs. Recovered eggs were added to a saturated NaCl solution, centrifuged at 3000 rpm for 5 min, then floating eggs were collected using a 25 µm sieve and washed with distilled water. Eggs were separated and quantified.
EHT
One hundred eggs in 10 µL of distilled water were added to each well of a 24-well microplate. Then the EO, water (negative control 1), 2% Tween 80 (negative control 2) or thiabendazole (Sigma, T8904) at 60 µg/mL (positive control) was added to the wells. Each EO concentration and each control had six repetitions performed in three independent experiments, making 18 wells for each treatment or approximately 1800 eggs evaluated. Each line/treatment of 24-well plates was identified. Then the plate was sealed with PVC film and incubated at 27 °C and ≥ 80% relative humidity for 24 h. After this time, eggs and L1 larvae were counted with an inverted microscope to calculate the egg hatching inhibition (CHAGAS et al., 2011).
LDT
As described in Chagas et al. (2011), 100 eggs were added to each well of a 24-well microplate with distilled water for a total volume of 250 µL in each well. The plates were incubated for 24 h at 27 °C and ≥80% relative humidity to obtain L1 larvae. After this time, nutritive medium was added to each well according to Hubert & Kerboeuf (1992). All concentrations of essential oils, water (negative control 1), DMSO at 0.5% (negative control 2), and ivermectin (Sigma, I8898) at 10 µg/mL (positive control) were tested in six repetitions performed in three independent experiments (approximately 1,800 L1 evaluated/treatment).The plates were incubated under the same conditions for six days, when each well was analyzed with an inverted microscope and all L3 and undeveloped larvae were counted, to estimate larval development inhibition.
Statistical analysis
In the EHT and LDT, the percentage of inhibition or efficacy of each treatment was determined based on the arithmetic mean (X) of the egg hatching or larval development, according to the following equation (COLES et al., 1992):
where, XTest represents the number of unhatched eggs in the EHT, or the number of larvae that did not develop until L3 in the LDT, and Xtotal corresponds to the total number of eggs + L1 in the EHT, or the number of L1+ L2+ L3 in the LDT.
The efficacy data were subjected to a variance analysis by the GLM procedure of SAS following a randomized experimental design with two isolates (resistant and susceptible) and 13 levels of concentration, describing a factorial 2 x 13, with 6 repetitions. We studied the effect of isolate, concentration and their interaction “ISOxCONC”. Then it was performed a Tukey’s multiple comparison test (SAS 9.0 for Windows) in order to detect significant statistical differences (p ≤ 0.01). The calculation of the inhibition concentrations (ICs) was performed by the SAS Probit procedure to estimate the IC50 and IC90.
Results
We obtained an average yield of P. aduncum EO of 3.6% (v/w). The EO chemical composition and retention times are indicated in Table 1. We identified a total of 36 substances, representing 99.1% of the oil extracted from the leaves. Dillapiole represented the major compound (76.2%), followed by (E)-caryophyllene (3.8%) and myristicin (3.6%).
Composition of the essential oil of Piper aduncum L. assessed by GC/MS, retention time (tR subst) and linear retention index available in the literature (LRI).
Results from the EHT indicated that the EO had better effect on the susceptible isolate. At the two lowest concentrations, the EO was not efficacious and results were statically similar to negative controls (2% tween 80 and distilled water). On the other hand, better effects were seen in the highest concentrations; at 0.39, 0.78, 1.56 and, 3.12 mg/mL results of egg inhibition were statistically similar. In the case of the resistant isolate Embrapa2010, the highest EO effect was seen in the concentration 12.5 mg/mL, which was statistically similar to the concentrations 6.25 and 3.12 mg/mL. Again, the EO results were statically similar to negative controls at the two lowest concentrations. When the isolates were compared in the same concentration, all results were significantly different (p ≤ 0.01) (Table 2). The highest EO concentration (12.5 mg/mL) inhibited hatching by 55.80% of the susceptible eggs, and by 14.68% of the resistant eggs. The dose-response was linear for susceptible eggs, and the IC50 was calculated at 5.72 mg/mL (Table 3). Because of the relatively low effect of the EO on egg hatching, we could not define the ICs for the resistant isolate, or the IC90 for the susceptible one.
Inhibition efficacy (mean percentage ± S.E.) of the essential oil extracted from Piper aduncum L. (mg/mL) on hatching of eggs from susceptible McMaster and resistant Embrapa2010 isolates of Haemonchus contortus in the egg hatch test (EHT). Also shown are controls containing water, Tween 80 and thiabendazole.
IC50, IC90 (mg/mL) and confidence intervals obtained in the egg hatch test (EHT) and larval development test (LDT) using essential oil extracted from Piper aduncum L. against susceptible McMaster and resistant Embrapa2010 isolates of Haemonchus contortus.
The percentage inhibitions in larval development from L1 to L3 mediated by the EO are displayed on Table 4. At the three highest concentrations, the EO was highly efficacious, inhibiting larval development of the two H. contortus isolates by more than 99%. There was no significant difference among concentrations from 0.37 to 3.12 mg/mL in the McMaster isolate. The same occurred among concentrations from 0.78 to 3.12 mg/ml to the Embrapa2010 isolate. LDT results were also significantly different between the two isolates (p ≤ 0.01) at every concentration except for the three highest ones. A dose-response relation was observed for both isolates (Table 4).
Inhibition efficacy (mean percentage ± S.E.) of the essential oil extracted from Piper aduncum L. (mg/mL) on larval development of susceptible McMaster and resistant Embrapa2010 isolates of Haemonchus contortus in the larval development test (LDT). Also shown are controls containing water, DMSO and ivermectin.
The ICs for both isolates calculated from the LDT are summarized in Table 3. The IC50 for the susceptible isolate was 0.10 mg/mL, and it reached an approximately twofold higher value (0.22 mg/mL) for the resistant isolate.
Discussion
An estimated 500 thousand plant species exist in the world, and 16% of them are in the Amazon forest in Brazil. The diversity of this country’s flora represents an enormous reservoir of mostly unexplored plant secondary compounds. Despite increasing research efforts to unveil new species and understand their roles over the past two decades, our knowledge remains scant (FAZOLIN et al., 2007). Nevertheless, different industrial sectors have increasingly sought Amazon plant compounds, including the agro-business sector.
Few reports discuss the potential of EOs derived from P. aduncum L. against nematodes that parasitize ruminants. In the present study, EO anti-hatching activity was only evident against susceptible eggs at the highest concentration (55.8% inhibition with 12.5 mg/mL), which was statistically different of the other concentrations on McMaster isolate, as well as at the same concentration (12.5 mg/mL) on the Embrapa2010 isolate. Other work using the EHT showed that the EO from P. aduncum L. was 95% efficacious at 12 mg/mL against H. contortus eggs (OLIVEIRA et al., 2014). We believe this divergent results stem from the chemical composition of EOs, which in the previous study primarily contained 1,8-cineole (55.8% against 0% dillapiole), whereas in the present study 76.2% of the oil was composed of dillapiole with no 1,8-cineole. Qualitative and quantitative differences in plant composition result from several factors including genetic background and plant variety (BARBOSA et al., 2007; HABER, 2008), site of cultivation (HABER, 2008; FURLAN et al., 2010), climatic and agricultural conditions (MARCO et al., 2007), harvest season and timing (BLANK et al., 2007; FURLAN et al., 2010), as well as plant developmental stage (LAHLOU & BERRADA, 2003; LEAL et al., 2003). The extraction method (e.g., super-citric fluid, steam distillation, solvent) also affects oil composition (QUISPE-CONDORI et al., 2008), much like drying (BLANK et al., 2007; BARBOSA et al., 2008) and storage conditions (GUIMARÃES et al., 2008).
In contrast to the mild effects on egg hatching, the EO significantly inhibited larval development of both isolates in a dose-dependent manner. Eggs in their hard and resistant shell usually resist chemicals to a larger extent than L1 larvae (KATIKI et al., 2011). At the four highest concentrations the EO was more than 99% efficacious in inhibiting larval development of the susceptible isolate and, at three highest concentrations of the resistant isolate. For both isolates the results were statistically similar at those concentrations. When isolates were compared at the same concentration, the larval development inhibition was statistically similar at 0.78, 1.56 and 3.12 mg/mL. The IC50 represented low EO doses for both isolates, but the dose required to kill 50% of the resistant larvae was twofold greater than required for the susceptible ones. This in vitro effect observed with the LDT may be related to the arylpropanoid dillapiole, to which many of the pharmacological activities of the EO extracted from P. aduncum L. have been attributed (MAIA et al., 1998; POHLIT et al., 2006). In fact, dillapiole has been shown to have acaricidal (SILVA et al., 2009; PINO et al., 2011; ARAÚJO et al., 2012), anti-bacterial (LARA-JÚNIOR et al., 2012; BRAZÃO et al., 2014), anti-fungal (LARA-JÚNIOR et al., 2012; GUERRINI et al., 2009), leishmanicidal (PARISE-FILHO et al., 2012), anti-inflammatory (PARISE-FILHO et al., 2011) and insecticidal activities (BELZILE et al., 2000; SOUTO, 2006; LING et al., 2009). When 99% pure, this compound has been evaluated and identified as the mediator of many of the biological activities above (ALMEIDA, 2004).
Dillapiole may also associate with other minor bioactive compounds in the EO potentiating their action. For example, myristicin, present at 3.6% in the tested EO, sarisan (BIZZO et al., 2001) or safrole (HUANG et al., 1999), all of which have a methylenedioxyphenyl group in their structure are natural insecticides that could be the target of such association. Brazão et al. (2014), when assessing the EO extracted from P. aduncum against susceptible and resistant isolates of Staphylococcus spp., also showed the combined bactericidal action of dillapiole and myristicin (1 to 2%), lending further support to our conclusions.
Previous work revealed dillapiole as the major component of the EO of different varieties of P. aduncum collected in the Amazon, such as P. aduncum L. (MAIA et al., 1998; ALMEIDA et al., 2009), P. aduncum var. aduncum and P. aduncum var. cordulatum (GOTTLIEB et al., 1981). However, individuals from this same species collected in other regions have other major constituents, including 1,8-cineole (LARA-JÚNIOR et al., 2012; OLIVEIRA et al., 2013), (E)-nerolidol (MESQUITA et al., 2005; OLIVEIRA et al., 2006) and nerolidol (NAVICKIENE et al., 2006), asaricine (FERREIRA, 2011; POTZERNHEIM et al., 2012) and piperitone (POTZERNHEIM et al., 2012). Table 5 summarizes the major components found in leaf EOs from P. aduncum L., highlighting the results of studies conducted in Brazil. The Piperaceae taxonomy is very complex (KATO & FURLAN, 2007). For different reasons, P. aduncum L. was botanically described in the Neotropical region by several authors in distinct times and places. In Brazil, there also were diverging points of view as to its taxonomic identity, and two varieties or subspecies were described by Gottlieb et al. (1981): P. aduncum var. aduncum and P. aduncum var. cordulatum. Recently, a single nomenclature – P. aduncum var. aduncum – was adopted (GUIMARÃES et al., 2015).
Major chemical compounds making up the essential oils from Piper aduncum L. leaves, as reported by different authors.
The chemical variation observed in the EO of P. aduncum L. may result from the predominance of different biosynthetic pathways in two chemotypes (ALMEIDA et al., 2009). In the Amazon forest the shikimate pathway took precedence, producing the phenylpropanoid dillapiole, whereas in the Atlantic forest chemotype, the mevalonate/acetate pathway was favored, generating the terpenoids (E)-nerolidol and linalool. These authors showed the occurrence of both chemotypes in both ecosystems, with their individual predominance depending on environmental factors. In addition, Andrade et al. (2009) conducted a hierarchical analysis based on EO chemical composition and clustered 21 P. aduncum specimens into four groups, from A to D. The EO examined in the present study fits the A chemical profile of the Amazon P. aduncum variety. This complex taxonomy and classification system indicates that differences in results may well stem from misclassifications at the subspecies level. Moreover, in face of such complexity, the phytochemical study of each oil’s efficacy against parasites becomes of paramount importance. Every pharmaceutic and veterinary product must be chemically qualified, i.e., the EOs must have known compositions. Bioactive markers must be established and, for any given use, the EOs must have these markers within the appropriate range to elicit the expected effects. Thus, we propose that the stages of taxonomic identification, extraction and phytochemistry should be clearly developed for P. aduncum to further define its anthelminthic activity, as shown in Figure 1.
Required steps in the research of essential oils derived from Piper aduncum L. as anthelmintic agent against Haemonchus contortus.
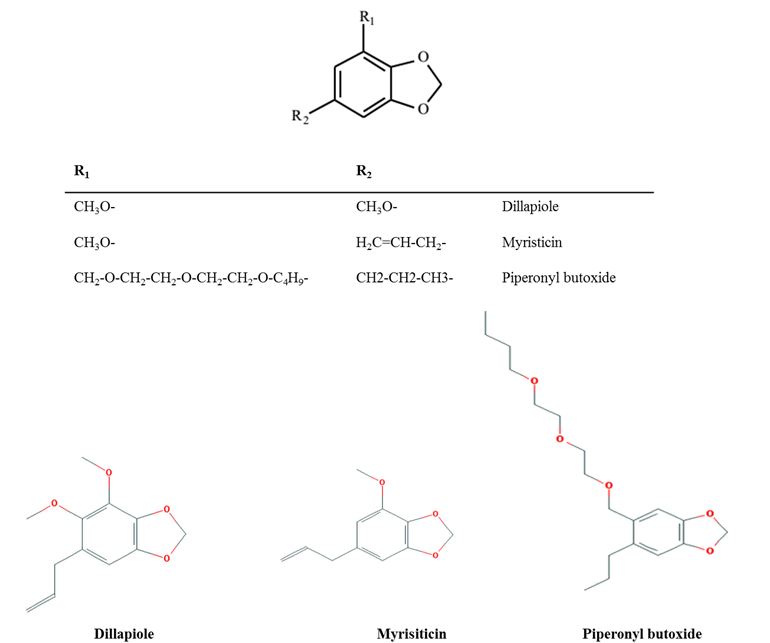
The action of P. aduncum extracts on H. contortus may be explained at the molecular level as an effect of the methylenedioxyphenyl group present in dillapiole (Figure 2) and in other substances of the Piperaceae-derived EOs (FAZOLIN et al., 2007). This group represents an important inhibitor of cytochrome P450-dependent monooxygenases (MUKERJEE et al., 1979; BERNARD et al., 1990; FAZOLIN et al., 2014). Monooxygenase inhibition reduces the detoxification capacity of insects, which are then poisoned by plant compounds that would otherwise be gradually eliminated (BERNARD et al., 1995). According to Kotze et al. (2006), piperonyl butoxide, which is related to dillapiole, acts synergistically with insecticides by inhibiting cytochrome P450-mediated metabolism of the insecticide. In the same study, the anti-helminthic properties of rotenone and its activity in combination with piperonyl butoxide, were examined in vitro. Rotenone was toxic to larvae of H. contortus and Trichostrongylus colubriformis, with IC50 values in the LDT of 0.54 and 0.64 µg/mL, respectively. The compound also caused complete cessation of movement in adult H. contortus at 20 µg/mL. Rotenone toxicity towards larvae and adults increased in the presence of piperonyl butoxide. Thus, the significant synergism observed suggests that these two nematode species use a cytochrome P450 pathway to detoxify rotenone, and indicates that a role may exist for cytochrome P450 inhibitors, such as piperonyl butoxide, to potentiate the action of anthelminthic substances that undergo oxidative metabolism within the nematode (KOTZE et al., 2006). The role of oxidative pathways in the metabolism of benzimidazoles in susceptible and resistant nematodes is unknown. Thus, the ability of a synergist to increase the toxicity of this chemical group towards both susceptible and resistant strains remains to be determined (KOTZE et al., 2006). Resistance to macrocyclic lactones does not involve enhanced oxidative metabolism and will likely require other types of assessments to have its mechanism determined (KOTZE, 2000).
Major aryl-propanoids present in the EO of Piper aduncum, highlighting the presence of a methylenedioxyphenyl group and its structural analogy to piperonyl butoxide.
In conclusion, the EO was highly efficacious against L1 but not against egg hatching. The action of this plant species against H. contortus is likely associated with dillapiole, the major EO component. Thus, the results obtained in the present study suggest that the EO derived from P. aduncum causes nematode toxicity via dillapiole-mediated cytochrome P450 inhibition in both isolates of H. contortus. We started from the premise that this EO, specifically dillapiole, could provide a natural source of anthelminthic agents. However, the differences observed in ICs indicate that the development of anti-parasitic drugs based on dillapiole, or other chemicals containing the methylenedioxyphenyl group, may be of little value. While the results indicate there may be a relationship between resistance status to commercially available chemicals and the plant-derived compound described in the present study, further research should expand the number of resistant and susceptible populations assessed in the bioassay.
Acknowledgements
We thank Embrapa Pecuária Sudeste, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-CAPES, project CAPES/MES-CUBA 952/2013, and the Fundação de Amparo à Pesquisa do Estado do Amazonas (FAPEAM), project 062.02821/2014, for financial support. We also thank CAPES, process 952/2013, for the master’s degree scholarship granted to Y. A. Gaínza. Finally, we thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), process 134470/2014-1, for the master’s degree scholarship granted to R. R. Fantatto, and Dr. Waldomiro Barioni Júnior for the statistical support.
References
- Adams RP. Identification of essential oils components by gas chromatography/mass spectrometry. 4th ed. Illinois: Allured Publishing Corporation, Carol Stream; 2007.
-
Almeida RRP, Souto RNP, Bastos CN, Silva MHL, Maia JGS. Chemical variation in Piper aduncum and biological properties of its dillapiole-rich essential oil. Chem Biodivers 2009; 6(9): 1427-1434. PMid:19774604. http://dx.doi.org/10.1002/cbdv.200800212
» http://dx.doi.org/10.1002/cbdv.200800212 - Almeida RRP. Isolamento, purificação e isomerização do dilapiol, componente majoritário do óleo essencial de P. aduncum para comprovação de sua atividade biológica [Dissertation]. Belém: Universidade Federal do Pará; 2004.
- Andrade EHA, Guimarães EF, Maia JGS. Variabilidade química em óleos essenciais de espécies de Piper da Amazônia. 22nd ed. Belém: FEQ/UFPA; 2009.
-
Araújo MJC, Câmara CAG, Born FS, Moraes MM, Badji CA. Acaricidal activity and repellency of essential oil from Piper aduncum and its components against Tetranychus urticae.Exp Appl Acarol 2012; 57(2): 139-155. PMid:22415244. http://dx.doi.org/10.1007/s10493-012-9545-x
» http://dx.doi.org/10.1007/s10493-012-9545-x -
Arze JBL, Collin G, Garneau FX, Jean FI, Gagnon H. Essential oils from Bolivia. VIII. Piperaceae: Piper heterophyllum Ruiz et Pavón, L. P. aduncumJ Ess Oil Bearing-Pl 2008; 11(1): 53-57. http://dx.doi.org/10.1080/0972060X.2008.10643597
» http://dx.doi.org/10.1080/0972060X.2008.10643597 -
Barbosa LCA, Demuner AJ, Clemente AD, Paula VF, Ismail FMD. Seasonal variation in the composition of volatile oils from raddi. Schinus terebinthifoliusQuim Nova 2007; 30(8): 1959-1965. http://dx.doi.org/10.1590/S0100-40422007000800030
» http://dx.doi.org/10.1590/S0100-40422007000800030 -
Barbosa LCA, Pereira UA, Martinazzo AP, Maltha CRA, Teixeira RR, Melo EC. Evaluation of the chemical composition of Brazilian commercial (D.C.) stapf samples. Cymbopogon citratusMolecules 2008; 13(8): 1864-1874. PMid:18794790. http://dx.doi.org/10.3390/molecules13081864
» http://dx.doi.org/10.3390/molecules13081864 -
Belzile AS, Majerus SL, Podeszfinski C, Guillet G, Durst T, Arnason JT. Dillapiol derivatives as synergists: structure-activity relationship analysis. Pestic Biochem Physiol 2000; 66(1): 33-40. http://dx.doi.org/10.1006/pest.1999.2453
» http://dx.doi.org/10.1006/pest.1999.2453 -
Bernard CB, Arnason JT, Philogene BJR, Lam J, Waddell T. In vivo effect of mixtures of allelochemicals on the life cycle of the European corn borer, Ostrinia nubilalis.Entomol Exp Appl 1990; 57(1): 17-22. http://dx.doi.org/10.1111/j.1570-7458.1990.tb01411.x
» http://dx.doi.org/10.1111/j.1570-7458.1990.tb01411.x -
Bernard CB, Krishanmurty HG, Chauret D, Durst T, Philogène BJR, Sánchez-Vindas P, et al. Insecticidal defenses of Piperaceae from the neotropics. J Chem Ecol 1995; 21(6): 801-814. PMid:24234319. http://dx.doi.org/10.1007/BF02033462
» http://dx.doi.org/10.1007/BF02033462 -
Bizzo HR, Lopes D, Abdala RV, Pimentel FA, Souza JA, Pereira MVG, et al. Sarisan from leaves of C. DC (long pepper). Piper affinis hispidinervumFlavour Fragrance J 2001; 16(2): 113-115. http://dx.doi.org/10.1002/ffj.957
» http://dx.doi.org/10.1002/ffj.957 -
Blank AF, Costa AG, Arrigoni-Blank MF, Cavalcanti SCH, Alves PB, Innecco R, et al. Influence of season, harvest time and drying on ( Jowitt) volatile oil. Java citronellaCymbopogon winterianusRev Brasil Farmacogn 2007; 17(4): 557-564. http://dx.doi.org/10.1590/S0102-695X2007000400014
» http://dx.doi.org/10.1590/S0102-695X2007000400014 - Brazão MAB, Brazão FV, Maia JGS, Monteiro MC. Antibacterial activity of the oil and dillapiole, its main constituent, against multidrug-resistant strains. Piper aduncumBol Latinoam Caribe Plantas Med Aromat 2014; 13(6): 517-526.
-
Chagas ACS, Katiki LM, Silva IC, Giglioti R, Esteves SN, Oliveira MCS, et al. Haemonchus contortus: a multiple-resistant Brazilian isolate and the costs for its characterization and maintenance for research use. Parasitol Int 2013; 62(1): 1-6. PMid:22809891. http://dx.doi.org/10.1016/j.parint.2012.07.001
» http://dx.doi.org/10.1016/j.parint.2012.07.001 - Chagas ACS, Niciura SCM, Molento MB. Manual prático: metodologias de diagnóstico da resistência e de detecção de substâncias ativas em parasitas de ruminantes. Brasília: Embrapa Informação Tecnológica; 2011.
- Chagas ACS. Controle de parasitas utilizando extratos vegetais. Rev Bras Parasitol Vet 2004; 13(S1): 156-160.
- Cicció JF, Ballestero CM. Constituyentes volátiles de las hojas y espigas de (Piperaceae) de Costa Rica. Piper aduncumRev Biol Trop 1997; 45(2): 783-790.
-
Coles GC, Bauer C, Borgsteede FHM, Geerts S, Klei TR, Taylor MA, et al. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Vet Parasitol 1992; 44(1-2): 35-44. PMid:1441190. http://dx.doi.org/10.1016/0304-4017(92)90141-U
» http://dx.doi.org/10.1016/0304-4017(92)90141-U -
Fazolin M, Estrela JLV, Catani V, Alécio MR, Lima MS. Propriedade inseticida dos óleos essenciais de . Piper hispidinervum C. DC.; Piper aduncum L. e Tanaecium nocturnum (Barb. Rodr.) Bur. & K. Shum sobre Tenebrio molitor L., 1758Cienc Agrotec 2007; 31(1): 113-120. http://dx.doi.org/10.1590/S1413-70542007000100017
» http://dx.doi.org/10.1590/S1413-70542007000100017 - Fazolin M, Estrela JLV, Catani V, da Costa CR. Potencialidades da pimenta-de-macaco (Piper aduncum L.): características gerais e resultados de pesquisa. Rio Branco: Embrapa Acre; 2006. vol. 103. Série Documentos.
- Fazolin M, Estrela JLV, Yamaguchi KKL, Pieri FA, Veiga-Junior VF. Amazon Piperaceae with potential insecticide use. In: Gupta VK. Medicinal plants: phytochemistry, pharmacology and therapeutics. New Delhi: Daya Publishing House; 2014. p. 423-439. vol. 3.
- Ferreira MI. Trocas gasosas, biomassa, teor e composição do óleo essencial de folhas e raízes de Piper aduncum L. sob diferentes níveis de luminosidade [Dissertation]. Botucatu: Faculdade de Ciências Agronômicas de Botucatu, Universidade Estadual Paulista “Júlio de Mesquita Filho”; 2011.
-
Figueiredo AC, Barroso JG, Pedro LG, Scheffer JJC. Factors affecting secondary metabolite production in plants: volatile components and essential oils. Flavour Fragrance J 2008; 23(4): 213-226. http://dx.doi.org/10.1002/ffj.1875
» http://dx.doi.org/10.1002/ffj.1875 -
Freeman AS, Nghiem C, Li J, Ashton FT, Guerrero J, Shoop WL, et al. Amphidial structure of ivermectin-resistant and susceptible laboratory and field strains of Haemonchus contortus.Vet Parasitol 2003; 110(3-4): 217-226. PMid:12482650. http://dx.doi.org/10.1016/S0304-4017(02)00321-7
» http://dx.doi.org/10.1016/S0304-4017(02)00321-7 -
Furlan MR, Martins RCC, Rodrigues E, Scalco N, Negri G, Lago JHG. Variação dos teores de constituintes voláteis de Cymbopogon citratus (DC) Staf, Poaceae, coletados em diferentes regiões do Estado de São Paulo. Rev Bras Farmacogn 2010; 20(5): 686-691. http://dx.doi.org/10.1590/S0102-695X2010005000026
» http://dx.doi.org/10.1590/S0102-695X2010005000026 -
Gill JH, Redwin JM, van Wyk JA, Lacey E. Avermectin inhibition of larval development in Haemonchus contortus - Effects of ivermectin resistance. Int J Parasitol 1995; 25(4): 463-470. PMid:7635622. http://dx.doi.org/10.1016/0020-7519(94)00087-5
» http://dx.doi.org/10.1016/0020-7519(94)00087-5 - Gottlieb OR, Koketsu M, Magalhães MT, Maia JGS, Mendez PH, da Rocha AI, et al. Óleos essenciais da Amazônia VII. Acta Amazon 1981; 11(1): 143-148.
-
Guerrini A, Sacchetti G, Rossi D, Paganetto G, Muzzoli M, Andreotti E, et al. Bioactivities of Piper aduncum L. and Piper obliquum Ruiz & Pavon (Piperaceae) essential oils from Eastern Ecuador. Environ Toxicol Pharmacol 2009; 27(1): 39-48. PMid:21783920. http://dx.doi.org/10.1016/j.etap.2008.08.002
» http://dx.doi.org/10.1016/j.etap.2008.08.002 -
Guimarães EF, Carvalho-Silva M, Monteiro D, Medeiros ES, Queiroz GA. Piperaceae. In: Jardim Botânico do Rio de Janeiro – JBRJ. Lista de espécies da flora do Brasil [online]. Rio de Janeiro: JBRJ; 2015 [cited 2015 August 31]. Available from: http://reflora.jbrj.gov.br/jabot/floradobrasil/FB12738
» http://reflora.jbrj.gov.br/jabot/floradobrasil/FB12738 -
Guimarães LG, Cardoso MG, Zacaroni LM, Lima RK, Pimentel FA, Morais AR. Influência da luz e da temperatura sobre a oxidação do óleo essencial de capim-limão (Cymbopogon citratus (DC.) Stapf). Quim Nova 2008; 31(6): 1476-1480. http://dx.doi.org/10.1590/S0100-40422008000600037
» http://dx.doi.org/10.1590/S0100-40422008000600037 - Gupta MP, Arias TD, Smith RM. The composition of the essential oil of Piper aduncum L. from Panama. Rev Latinoamer Quim 1983; 14(1): 35-36.
- Gupta MP. 270 Plantas Medicinales Iberoamericanas. Santafé de Bogotá: Talleres de Editorial Presencia; 1995.
- Haber LL. Caracterização da diversidade genética via marcador microssatélite, e constituintes do óleo essencial de Lychnophora pinaste MART [Thesis]. Botucatu: Faculdade de Ciências Agronômicas de Botucatu, Universidade Estadual Paulista “Júlio de Mesquita Filho”; 2008.
-
Huang Y, Ho SH, Kini M. Bioactivities of safrole and isosafrole on Sitophilus zeamais (Coleoptera: Curculionidae) and Tribolium castaneum (Coleoptera: Tenebrionidae). J Econ Entomol 1999; 92(3): 676-683. http://dx.doi.org/10.1093/jee/92.3.676
» http://dx.doi.org/10.1093/jee/92.3.676 -
Hubert J, Kerboeuf D. A microlarval development assay for the detection of anthelmintic resistance in sheep nematodes. Vet Rec 1992; 130(20): 442-446. PMid:1621342. http://dx.doi.org/10.1136/vr.130.20.442
» http://dx.doi.org/10.1136/vr.130.20.442 -
Husnu K, Başer KHC, Demirci F. Chemistry of essential oils. In: Berger RG. Flavours and fragrances chemistry, bioprocessing and sustainability. Berlin: Springer; 2007. p. 43-86. http://dx.doi.org/10.1007/978-3-540-49339-6_4
» http://dx.doi.org/10.1007/978-3-540-49339-6_4 - International Organization for Standardization – ISO. ISO 9235:2013: aromatic natural raw materials: vocabulary. Geneva: ISO; 2013.
-
James CE, Hudson AL, Davey MW. Drug resistance mechanisms in helminths: is it survival of the fittest? Trends Parasitol 2009; 25(7): 328-335. PMid:19541539. http://dx.doi.org/10.1016/j.pt.2009.04.004
» http://dx.doi.org/10.1016/j.pt.2009.04.004 -
Jantan BI, Ahmad AR, Ahmad AS, Ali NA. A comparative study of the essential oils of five species from Peninsular Malaysia. PiperFlavour Fragrance J 1994; 9(6): 339-342. http://dx.doi.org/10.1002/ffj.2730090611
» http://dx.doi.org/10.1002/ffj.2730090611 -
Jaramillo MA, Manos PS. Phylogeny and patterns of floral diversity in the genus Piper (Piperaceae). Am J Bot 2001; 88(4): 706-716. PMid:11302858. http://dx.doi.org/10.2307/2657072
» http://dx.doi.org/10.2307/2657072 -
Katiki LM, Chagas ACS, Bizzo HR, Ferreira JFS, Amarante AFT. Anthelmintic activity of Cymbopogon martinii, Cymbopogon schoenanthus and Mentha piperita essential oils evaluated in four different tests. in vitroVet Parasitol 2011; 183(1-2): 103-108. PMid:21820807. http://dx.doi.org/10.1016/j.vetpar.2011.07.001
» http://dx.doi.org/10.1016/j.vetpar.2011.07.001 -
Kato MJ, Furlan M. Chemistry and evolution of the Piperaceae. Pure Appl Chem 2007; 79(4): 529-538. http://dx.doi.org/10.1351/pac200779040529
» http://dx.doi.org/10.1351/pac200779040529 -
Kotze AC, Dobson RJ, Chandler D. Synergism of rotenone by piperonyl butoxide in Haemonchus contortus and Trichostrongylus colubriformis in vitro: Potential for drug-synergism through inhibition of nematode oxidative detoxification pathways. Vet Parasitol 2006; 136(3-4): 275-282. PMid:16325340. http://dx.doi.org/10.1016/j.vetpar.2005.11.001
» http://dx.doi.org/10.1016/j.vetpar.2005.11.001 -
Kotze AC. Oxidase activities in macrocyclic-resistant and -susceptible Haemonchus contortus.J Parasitol 2000; 86(4): 873-876. PMid:10958478. http://dx.doi.org/10.1645/0022-3395(2000)086[0873:OAIMRA]2.0.CO;2
» http://dx.doi.org/10.1645/0022-3395(2000)086[0873:OAIMRA]2.0.CO;2 -
Lahlou M, Berrada R. Composition and niticidal activity of essential oils of three chemotypes of L. acclimatized in Morocco. Rosmarinus officinalisFlav Frag J 2003; 18(2): 124-127. http://dx.doi.org/10.1002/ffj.1160
» http://dx.doi.org/10.1002/ffj.1160 - Lara-Júnior CR, Oliveira GL, Mota BCF, Fernandes MFG, Figueiredo LS, Martins ER, et al. Antimicrobial activity of essential oil of Piper aduncum L. (Piperaceae). J. Med. Plants Res 2012; 6(21): 3800-3805.
- Leal TCAB, Freitas SP, Silva JF, Carvalho AJC. Produção de biomassa e óleo essencial em plantas de capim cidreira [ (Dc.). Stapf] em diferentes idades. Cymbopogon citratusRev Bras Pl Med 2003; 5(2): 61-64.
- Ling IA, Sulaiman S, Othman H. Evaluation of Linn. essential oil (Fam: Piperaceae) against . Piper aduncumPeriplaneta americana (L.)Iran J Arthropod-Borne Dis 2009; 3(2): 1-6. PMid:22808375.
-
Lisonbee LD, Villalba JJ, Provenza FD, Hall JO. Tannins and self-medication: implications for sustainable parasite control in herbivores. Behav Processes 2009; 82(2): 184-189. PMid:19576969. http://dx.doi.org/10.1016/j.beproc.2009.06.009
» http://dx.doi.org/10.1016/j.beproc.2009.06.009 - Lorenzi H, Matos FJA. Plantas medicinais no Brasil: nativas e exóticas. Nova Odessa: Instituto Plantarum; 2002.
-
Maia JGS, Zohhbi MGB, Andrade EHA, Santos AS, Silva MHL, Luz AIR, et al. Constituents of the essential oil of L. growing wild in the Amazon region. Piper aduncumFlavour Fragrance J 1998; 13(4): 269-272. http://dx.doi.org/10.1002/(SICI)1099-1026(1998070)13:4<269::AID-FFJ744>3.0.CO;2-A
» http://dx.doi.org/10.1002/(SICI)1099-1026(1998070)13:4<269::AID-FFJ744>3.0.CO;2-A -
Marco CA, Innecco R, Mattos SH, Borges NSS, Nagao EO. Características do óleo essencial de capim-citronela em função de espaçamento, altura e época de corte. Hortic Bras 2007; 25(3): 429-432. http://dx.doi.org/10.1590/S0102-05362007000300020
» http://dx.doi.org/10.1590/S0102-05362007000300020 -
Mesquita JMO, Cavaleiro C, Cunha AP, Lombardi JA, Oliveira AB. Estudo comparativo dos óleos voláteis de algumas espécies de Piperaceae. Rev Bras Farm 2005; 15(1): 6-12. http://dx.doi.org/10.1590/S0102-695X2005000100003
» http://dx.doi.org/10.1590/S0102-695X2005000100003 -
Mukerjee SK, Saxena VS, Tomar SS. New methylenedioxyphenyl synergists for pyrethrins. J Agric Food Chem 1979; 27(6): 1209-1211. http://dx.doi.org/10.1021/jf60226a033
» http://dx.doi.org/10.1021/jf60226a033 -
Navickiene HMD, Morandim AA, Alécio AC, Regasini LO, Bergamo DCB, Telascrea M, et al. Composition and antifungal activity of essential oils from and Piper aduncum, Piper arboreumPiper tuberculatum.Quim Nova 2006; 29(3): 467-470. http://dx.doi.org/10.1590/S0100-40422006000300012
» http://dx.doi.org/10.1590/S0100-40422006000300012 -
Oka Y, Nacar S, Putievsky E, Ravid U, Yaniv Z, Spiegel Y. Nematicidal activity of essential oils and their components against the root-knot nematode. Phytopathology 2000; 90(7): 710-715. PMid:18944489. http://dx.doi.org/10.1094/PHYTO.2000.90.7.710
» http://dx.doi.org/10.1094/PHYTO.2000.90.7.710 -
Oliveira GL, Moreira DL, Mendes ADR, Guimarães EF, Figueiredo LS, Kaplan MAC, et al. Growth study and essential oil analysis of L. from two sites of Cerrado biome of Minas Gerais State, Brazil. Piper aduncumRev Bras Farmacogn 2013; 23(5): 743-753. http://dx.doi.org/10.1590/S0102-695X2013000500005
» http://dx.doi.org/10.1590/S0102-695X2013000500005 -
Oliveira GL, Vieira TM, Nunes VF, Ruas MO, Duarte ER, Moreira DL, et al. Chemical composition and efficacy in the egg-hatching inhibition of essential oil of against from sheep. Piper aduncumHaemonchus contortusRev Bras Farmacogn 2014; 24(3): 288-292. http://dx.doi.org/10.1016/j.bjp.2014.07.004
» http://dx.doi.org/10.1016/j.bjp.2014.07.004 -
Oliveira JCS, Dias IJM, Camara CAG, Schwartz MOE. Volatile constituents of the leaf oils of L. from different regions of Pernambuco (Northeast of Brazil). Piper aduncumJ Essent Oil Res 2006; 18(5): 557-559. http://dx.doi.org/10.1080/10412905.2006.9699166
» http://dx.doi.org/10.1080/10412905.2006.9699166 -
Oviedo-Rondón EO, Clemente-Hernández S, Salvador F, Williams P, Losa R. Essential oils on mixed Coccidia vaccination and infection in broilers. Int J Poult Sci 2006; 5(8): 723-730. http://dx.doi.org/10.3923/ijps.2006.723.730
» http://dx.doi.org/10.3923/ijps.2006.723.730 -
Parise-Filho R, Pasqualoto KFM, Magri FMM, Ferreira AK, Silva BAVG, Damião MCFCB, et al. Dillapiole as antileishmanial agent: discovery, cytotoxic activity and preliminary SAR studies of dillapiole analogues. Arch Pharm 2012; 345(12): 934-944. PMid:22996811. http://dx.doi.org/10.1002/ardp.201200212
» http://dx.doi.org/10.1002/ardp.201200212 -
Parise-Filho R, Pastrello M, Camerlingo CEP, Silva GJ, Agostinho LA, Souza T, et al. The anti-inflammatory activity of dillapiole and some semisynthetic analogues. Pharm Biol 2011; 49(11): 1173-1179. PMid:22014265. http://dx.doi.org/10.3109/13880209.2011.575793
» http://dx.doi.org/10.3109/13880209.2011.575793 -
Pino JA, Marbot RA, Bello A, Urquiola A. Essential oils of (L.) Miq. and L. from Cuba. Piper peltataPiper aduncumJ Essent Oil Res 2004; 16(2): 124-126. http://dx.doi.org/10.1080/10412905.2004.9698670
» http://dx.doi.org/10.1080/10412905.2004.9698670 - Pino O, Sánchez Y, Rodríguez H, Correa TM, Demedio J, Sanabria JL. Caracterización química y actividad acaricida del aceite esencial de subsp. frente a Piper aduncumossanumVarroa destructor.Rev Protección Veg 2011; 26(1): 52-61.
- Pohlit AM, Pinto ACS, Mause R. Piper aduncum L.: pluripotente plant and important phytochemical substance source. Rev Fitos 2006; 2(1): 7-18.
-
Potzernheim MCL, Bizzo HR, Silva JP, Vieira RF. Chemical characterization of essential oil constituents of four populations of L. from Distrito Federal, Brazil. Piper aduncumBiochem Syst Ecol 2012; 42(1): 25-31. http://dx.doi.org/10.1016/j.bse.2011.12.025
» http://dx.doi.org/10.1016/j.bse.2011.12.025 -
Quispe-Condori S, Foglio MA, Rosa PTV, Meireles MAA. Obtaining β-caryophyllene from de Candolle by supercritical fluid extraction. Cordia verbenaceaJ Supercrit Fluids 2008; 46(1): 27-32. http://dx.doi.org/10.1016/j.supflu.2008.02.015
» http://dx.doi.org/10.1016/j.supflu.2008.02.015 -
Rali T, Wossa SW, Leach DN, Waterman PG. Volatile chemical constituents of Piper aduncum L. and . Piper gibbilimbum C. DC (Piperaceae) from Papua New GuineaMolecules 2007; 12(3): 389-394. PMid:17851397. http://dx.doi.org/10.3390/12030389
» http://dx.doi.org/10.3390/12030389 -
Roberts FHS, O’Sullivan JP. Methods for egg counts and larval cultures for strongyles infesting the gastro-intestinal tract of cattle. Aust J Agric Res 1950; 1(1): 99-102. http://dx.doi.org/10.1071/AR9500099
» http://dx.doi.org/10.1071/AR9500099 -
Roeber F, Jex AR, Gasser RB. Next-generation molecular-diagnostic tools for gastrointestinal nematodes of livestock, with an emphasis on small ruminants: a turning point? Adv Parasitol 2013; 83(1): 267-333. PMid:23876874. http://dx.doi.org/10.1016/B978-0-12-407705-8.00004-5
» http://dx.doi.org/10.1016/B978-0-12-407705-8.00004-5 -
Scott IM, Jensen HR, Philogène BJR, Arnason JT. A review of spp. (Piperaceae) phytochemistry, insecticidal activity and mode of action. PiperPhytochem Rev 2008; 7(1): 65-75. http://dx.doi.org/10.1007/s11101-006-9058-5
» http://dx.doi.org/10.1007/s11101-006-9058-5 -
Silva WC, Martins JRS, Souza EM, Heizen H, Cesio MV, Mato M, et al. Toxicity of Piper aduncum L. (Piperales: Piperaceae) from the Amazon forest for the cattle tick Rhipicephalus (Boophilus) (Acari: Ixodidae). microplusVet Parasitol 2009; 164(2-4): 267-274. PMid:19573994. http://dx.doi.org/10.1016/j.vetpar.2009.06.006
» http://dx.doi.org/10.1016/j.vetpar.2009.06.006 -
Singh TU, Kumar D, Tandan SK, Mishra SK. Inhibitory effect of essential oils of Allium sativum and Piper longum on spontaneous muscular activity of liver fluke, Fasciola gigantica.Exp Parasitol 2009; 123(4): 302-308. PMid:19679128. http://dx.doi.org/10.1016/j.exppara.2009.08.002
» http://dx.doi.org/10.1016/j.exppara.2009.08.002 - Smith RM, Kassim H. Essential oil of from Fiji. Piper aduncumNew Zeal J Sci 1979; 2(1): 127-128.
-
Sousa PJC, Barros CAL, Rocha JCS, Lira DS, Monteiro GM, Maia JGS. Avaliação toxicológica do óleo essencial de Piper aduncum L. Rev Bras Farmacogn 2008; 18(2): 217-221. http://dx.doi.org/10.1590/S0102-695X2008000200013
» http://dx.doi.org/10.1590/S0102-695X2008000200013 - Souto RNP. Atividades repelente e inseticida de óleos essenciais de Piper spp Linnaeus da Amazônia em Anopheles marajoara Galvão e Damasceno, Stegomyia aegypti Linnaeus (Díptera: Culicidae) e Solenopsis saevissima Fr. Smith (Hymenoptera: Formicidae) [Thesis]. Belém: Universidade Federal do Pará e Museu Paraense Emílio Goeldi; 2006.
-
Srinivasan K. Black pepper and its pungent principle-piperine: a review of diverse physiological effects. Crit Rev Food Sci Nutr 2007; 47(8): 735-748. PMid:17987447. http://dx.doi.org/10.1080/10408390601062054
» http://dx.doi.org/10.1080/10408390601062054 -
Sutherland IA, Leathwick DM. Anthelmintic resistance in nematode parasites of cattle: a global issue? Trends Parasitol 2011; 27(4): 176-181. PMid:21168366. http://dx.doi.org/10.1016/j.pt.2010.11.008
» http://dx.doi.org/10.1016/j.pt.2010.11.008 - Urquhart GM, Armour J, Duncan JL, Dunn AM, Jennings FW. Parasitologia veterinária. 2nd ed. Rio de Janeiro: Guanabara Koogan; 1998.
-
Vila R, Tomi F, Mundina M, Santana AI, Solis PN, Arce JBL, et al. Unusual composition of the essential oils from the leaves of Piper aduncum.Flav Frag J 2005; 20(1): 67-69. http://dx.doi.org/10.1002/ffj.1369
» http://dx.doi.org/10.1002/ffj.1369 - Waller PJ, Chandrawathani P. Haemonchus contortus: parasite problem no. 1 from tropics - Polar Circle. Problems and prospects for control based on epidemiology. Trop Biomed 2005; 22(2): 131-137. PMid:16883278.

 Piper aduncum against Haemonchus contortus isolates: cross resistance and the research of natural bioactive compounds
Piper aduncum against Haemonchus contortus isolates: cross resistance and the research of natural bioactive compounds